修回日期: 2017-03-21
接受日期: 2017-04-05
在线出版日期: 2017-05-08
肠道内短链脂肪酸(short-chain fatty acids, SCFAs)浓度很高. 他们是微生物自身以及宿主肠上皮细胞(intestinal epithelial cells, IESs)的能量来源, 促进细胞生长, 降低结肠内环境pH值, 减少有害菌生长. 近年研究证实, SCFAs能够调节宿主肠道免疫力, 降低结肠炎症反应; 抑制结肠肿瘤细胞增殖、诱导肿瘤细胞分化和凋亡、影响原癌基因表达. 本综述将详述SCFAs通过G蛋白偶联受体激活途径和组蛋白去乙酰化酶抑制途径, 引起中性粒细胞和调节性T细胞应答, 降低结肠炎; 增强IESs屏障功能; 抑制结肠肿瘤增殖; 治疗非酒精性脂肪性肝病和肥胖.
核心提要: 肠道内短链脂肪酸(short-chain fatty acids, SCFAs)是由未消化吸收的食物残渣中的碳水化合物经结肠内厌氧菌酵解产生. SCFAs具有重要的生理功能, 可以降低肠道炎症反应, 提高肠道上皮屏障功能, 对结肠肿瘤、非酒精性脂肪性肝病、肥胖都有一定的治疗效果. 总而言之, SCFAs在维持机体稳态及生理代谢正常中发挥着不可替代的作用.
引文著录: 王璐璇, 刘玥宏, 朱继开, 钟煜, 李利生, 徐敬东. 短链脂肪酸在疾病治疗中的研究进展. 世界华人消化杂志 2017; 25(13): 1179-1186
Revised: March 21, 2017
Accepted: April 5, 2017
Published online: May 8, 2017
Short chain fatty acids (SCFAs) are found in the intestine at high concentrations. In addition to acting as local substrates for energy production to promote cell growth, reduce environmental pH value in the colon, and reduce the growth of harmful bacteria, SCFAs can also regulate host physiology and immunity, suppress colon neoplasm cell proliferation by inducing their apoptosis and differentiation, and affect proto-oncogene expression. In this review, we discuss how SCFAs interact with G protein-coupled receptors to inhibit histone deacetyltransferase and thereby cause response of neutrophils and regulatory T cells to regulate intestinal immune responses and host physiological function. SCFAs can strengthen the epithelial barrier, suppress colon neoplasm cell proliferation, and be used to treat nonalcoholic fatty liver disease and obesity.
- Citation: Wang LX, Liu YH, Zhu JK, Zhong Y, Li LS, Xu JD. Role of short-chain fatty acids in disease treatment. Shijie Huaren Xiaohua Zazhi 2017; 25(13): 1179-1186
- URL: https://www.wjgnet.com/1009-3079/full/v25/i13/1179.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v25.i13.1179
哺乳动物体内存在复杂的微生物群落. 经长期共生适应, 宿主已可以通过协调和整合代谢信息、微生物感应、免疫应答途径确保自身与微生物和谐共存. 宿主与微生物群落间的动态调节对实现和维持机体稳态至关重要, 当动态调节失衡, 宿主就会产生疾患. 微生物代谢产物内短链脂肪酸(short-chain fatty acids, SCFAs)在人类某些疾病的发生发展中起着至关重要的作用. 研究SCFAs通过何种机制影响机体免疫应答与代谢反应对理解宿主-微生物共生并找到全新有效的疾病治疗途径具有建设性意义.
SCFAs又称为挥发性脂肪酸, 是由1-6个碳原子组成的有机脂肪酸. SCFAs的主要产生部位是结肠, SCFAs多是由未消化吸收的食物残渣中的碳水化合物经结肠内厌氧菌酵解产生, 主要包括乙酸、丙酸、丁酸(表1)[1,2], 其在结肠内的浓度约为20-140 mmol/L[3]. SCFAs的种类、数量主要取决于肠道内菌群组成、消化的时间、宿主-微生物代谢通量以及宿主食物中的纤维含量. 发酵所产生的SCFAs在人体内参与不同器官的代谢, 发挥不同的功效. 细菌发酵产生的乙酸可被宿主吸收利用, 是宿主能量的重要来源, 提供人体日总能量的约10%; 丙酸经血液吸收后在肝脏中分解代谢, 参与丙酮酸逆转化为葡萄糖的过程, 同时可能抑制脂肪的合成; 丁酸主要被上皮细胞利用, 是上皮细胞的主要能量来源[1,4].
| 细菌类型 | 代谢产物 |
| 拟杆菌类 | 乙酸、丁酸、琥珀酸 |
| 乳杆菌类 | 乳酸 |
| 链球菌类 | 乙酸、乳酸 |
| 真杆菌类 | 乙酸、丁酸、乳酸 |
| 双歧杆菌类 | 甲酸、乙酸、乳酸 |
| 消化链球菌类 | 乙酸、乳酸 |
| 梭菌类 | 乙酸、丙酸、丁酸、乳酸 |
| 瘤胃球菌类 | 乙酸 |
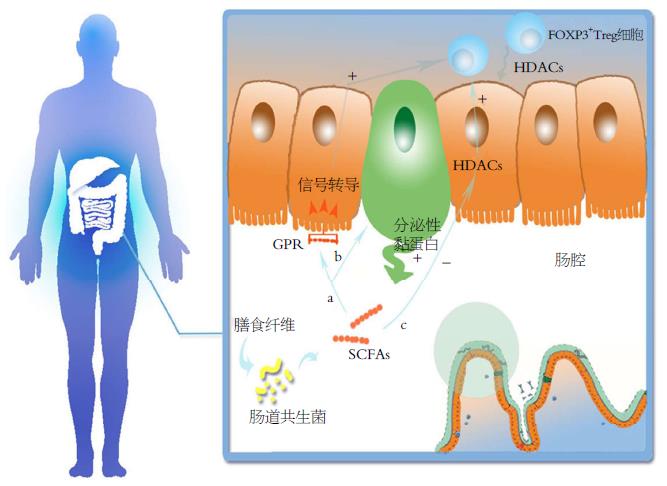
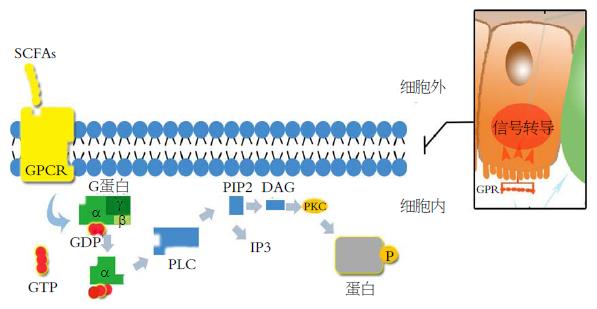
有研究[5]表明, 连续5 wk给予慢性辐射性直肠炎患者含SCFAs的灌肠液后, SCFAs灌肠组患者与对照组相比直肠出血天数以及出血范围减少, 血红蛋白量增加, 慢性辐射性直肠炎有所康复[5,6]. Scheppach对溃疡性结肠炎患者给予SCFAs灌肠8 wk后, 肠道炎症得到改善[7]. 可见, SCFAs具有降低肠道炎症反应的作用. SCFAs通过G蛋白偶联受体(G protein-coupled receptors, GPCRs)激活途径和组蛋白脱乙酰基酶(histone deacetylases, HDACs)抑制途径两条信号通路发挥抗炎作用[8,9](图1, 2). SCFAs既可以直接影响HDACs, 也可以通过与GPCRs作用间接影响HDACs.
SCFAs是HDACs的天然抑制剂. SCFAs对HDACs的抑制作用取决于SCFAs的浓度, 只有高浓度的SCFAs才能抑制HDACs. 例如乳酸作为合成丁酸的底物, 在高浓度下能抑制HDACs的活性(半数抑制浓度IC50为40 mmol/L), 但只有在高强度训练下的肌细胞中乳酸才能达到这一浓度, 因此肠道内的乳酸不能抑制HDACs; 同样, 其他SCFAs或细菌代谢产物, 如丙酮酸, 尚未达到有效抑制上皮细胞内HDACs功能的浓度[10]. Cox等[11]发现, SCFAs若能发挥抗炎作用, 其浓度必须要达到毫摩尔级. 正常情况下, 血液循环中的SCFAs水平很低, 不能达到毫摩尔级, 丁酸和丙酸的浓度只有微摩尔级, 无法起到抗炎作用[12]. 但机体产生炎症后, 体内某些致病菌可以产生SCFAs, 使感染位点的SCFAs浓度提升到毫摩尔级, 启动免疫细胞的抗炎作用[12,13].
中性粒细胞是向炎症性部位聚集的第一种效应细胞, 他们内吞并杀死细菌和真菌. 中性粒细胞在炎症应答中产生肿瘤坏死因子-α(tumor necrosis factor alpha, TNF-α)、白介素(interleukin, IL)-1β、IL-8, 调整炎症应答的多个环节[14]. 中性粒细胞被脂多糖激活后释放TNF-α, 有研究[3]证实SCFAs抑制HDACs活性, 从而降低脂多糖应答反应, 中性粒细胞激活受到抑制, TNF-α释放量减少, 从而起到抗炎作用. SCFAs还可以抑制核因子-κB(nuclear factor-κB, NF-κB)通路阻滞炎症细胞分泌IL-2、IL-6、TNF-α等炎性因子[15,16]. 因此, SCFAs引起的外周血单核细胞和中性粒细胞应答包括: 灭活NF-κB, 下调多种炎症因子.
有研究[17]表明给小鼠饲喂含SCFAs的饮食可提高FOXP3+Treg细胞(regulatory T cells, Treg cell)的抑制功能, 从而能抑制结肠炎症的发生. Treg细胞表达多种HDACs, 其中HDAC9对调节依赖FOXP3+的免疫抑制功能最为重要[18]. 抑制HDAC9会引起FOXP3+Treg细胞数量增加, 增强FOXP3+Treg细胞的免疫功能, 降低小鼠内Treg细胞介导的结肠炎[18](图1). 研究[19-21]已证明特定的SCFAs可以调整结肠的FOXP3+Treg细胞的数量和功能, 保持结肠稳态. 产妇摄入富含SCFAs的食物可将抑制效应传递到子代, 说明了SCFAs在免疫系统发展和预防疾病方面具有一定遗传性[22].
给予含有SCFAs的饮用水可增加野生型小鼠体内诱导型FOXP3+Treg细胞的数量和功能从而减弱小鼠的病情, 但Gpr43−/−小鼠的病情没有减弱[20,23], 说明SCFAs降低肠道炎症需要GPCRs介导. GPCRs由人类染色体基因19q13.1表达[12]. 现已发现SCFAs的特异性受体有GPR41/43以及GPR109A. 许多类型的细胞都会表达GPCRs. GPR41/43主要在肠内分泌细胞、脂肪细胞、多形核细胞和巨噬细胞表达[9,24], GPR109A在结肠细胞、脂肪细胞和肝细胞表达[9,25]. GPR43与SCFAs结合后可引起中性粒细胞向炎症部位趋化[6]并影响Treg细胞的增生和功能[20]. G蛋白偶联烟酸盐受体(G-protein coupled nicotinate receptor, GPR109A)是烟酸和丁酸的受体, SCFAs与GPCR109A结合后可诱导Treg细胞和分泌IL-10的T细胞分化, 从而抑制结肠炎的发生[25,26]; 还会引起肠细胞内环磷酸腺苷水平下降, 减少对电解质和水的吸收, 减轻炎症性肠病的腹泻症状[27]. 有文献报道SCFAs可与肠上皮细胞(intestinal epithelial cells, IESs)GPR43和GPR109A结合促进IL-18的分泌[28,29], 减轻DSS致损伤模型和T细胞转移结肠炎模型中结肠炎的病理变化[29]; 还可促进肠道上皮细胞K+外流, 肠道上皮细胞膜发生超极化, 进而激活NLRP3炎症小体, 起到减轻肠炎的作用[9,30]. 可见, SCFAs及其受体在抗炎过程中起着重要的调节作用.
无菌小鼠内接种多形拟杆菌和普氏粪杆菌会诱导杯状细胞分化并产生大量黏液[31]. 肠黏膜的黏液层是抵御肠腔内容物的机械性、化学性和微生物性攻击的第一道屏障. 黏液主要是由黏蛋白多聚糖组成的, 黏蛋白由MUC基因(mucin genes)编码. 至今为止, 已经在人体内至少发现了15种不同的MUC基因. 在大肠内, 黏蛋白主要由MUC2基因编码, MUC1、MUC3、MUC4基因也有少量表达[32,33]. MUC2基因编码结肠内主要的分泌性黏蛋白, 而MUC1、MUC3、MUC4基因主要编码位于膜上的黏蛋白[34]. 有研究[35,36]显示, 在富含葡萄糖的培养基中, 丁酸可以明显提高MUC3和MUC5的表达; 在葡萄糖缺乏的培养基中, 丁酸是唯一可获得能源的物质, MUC3、MUC5、MUC5AC和MUC2的表达量均大幅提高. 这项研究还证明丁酸可以在转录水平提高结肠杯状细胞黏蛋白的分泌量(图1).
动物实验证实, 在全肠外营养大鼠模型中, 静脉给予SCFAs(每1000 mL完全肠外培养液中含乙酸钠36 mmoL、丙酸钠15 mmoL、丁酸钠9 mmoL)可以有效维持肠绒毛高度、宽度、隐窝深度、黏膜厚度, 增加肠黏膜细胞增殖能力, 减轻三磷酸吡啶核苷酸所致的肠黏膜萎缩, 维护肠黏膜形态, 维持IESs完整性[37]. 这是因为SCFAs是肠黏膜营养物质之一, 为IESs提供60%-70%能量, 可促进IESs增生从而修复肠黏膜. 实验证实无菌鼠肠道细胞明显能量供应不足, 甚至出现自噬现象, 破坏肠道上皮的完整性, 但对小鼠饲喂含丁酸的食物后, 肠道上皮细胞的形态和结构恢复正常[38]. SCFAs还可以促进肠细胞分泌胰高血糖素样肽2(glucagon-like peptide-2, GLP-2), GLP-2是一种肠上皮特异性生长因子, 能促进肠黏膜的生长及修复肠上皮损伤, 提高肠黏膜屏障功能[39].
此外, SCFAs可以促使细胞机械屏障分子ZO-1蛋白和occludin蛋白-5表达增加, 促进IESs间紧密连接的形成加强IESs屏障功能, 降低肠道通透性, 减少有害物质(如脂多糖LPS)入血. 出血性大肠杆菌侵袭Caco-2细胞时, 乙酸可维持上皮的完整性[40], 肠道内双歧杆菌株产生的醋酸有助于抵御大肠杆菌O157:H7感染[41], 这都与SCFAs增强IESs屏障的完整性并抑制毒素吸收有关.
大量研究证实SCFAs能抑制结肠肿瘤细胞增殖分化、诱导肿瘤细胞凋亡. 陈尔真等[42]对体外培养的已建株的人结肠癌细胞株SWIII6给予不同浓度的丁酸处理, 结果显示丁酸能抑制SWIII6的增殖, 随着丁酸浓度的增加和培养时间的延长, 抑制作用增强; 丁酸还能使SWIII6细胞的癌胚抗原和碱性磷酸酶表达量增加, 随着丁酸浓度的增加和培养时间的不断延长这两种表达物的增加量更为显著.
研究已证实丁酸拮抗HDACs, 使染色质乙酰化水平上调, 处于高转录活性的开放状态, 从而增加基因表达量. 用丁酸预处理结肠癌细胞系后, 发现研究的588个基因中有23个表达量明显上调, 其中P21基因转录水平增加4倍以上, 可知丁酸通过上调组蛋白乙酰化水平而提高P21基因表达量, 使细胞周期停滞在G0期, 抑制结肠癌细胞增殖[43]. Mandal等[44]将3 mmoI/L浓度的丁酸分别与人结直肠癌细胞株SW480、HCTll6、DiFi共同培养, 结果发现3种癌细胞中的凋亡细胞数均较未用丁酸处理组明显增加.
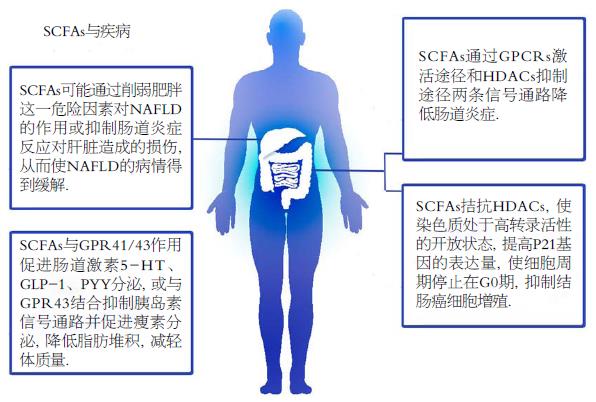
非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)是与肥胖和代谢综合征密切相关的代谢应激性肝病. 肥胖是NAFLD最重要的危险因素[45], SCFAs能够促进胰岛素分泌并发挥降血糖作用, 增加机体能量消耗, 减轻体质量, 削弱肥胖这一危险因素对NAFLD的作用, 可能会改善NAFLD病情. SCFAs也可能通过抑制肠道炎症反应, 减轻微生物细胞成分和其他代谢产物经肝门静脉对肝脏造成的损伤, 从而使NAFLD病情得到缓解[9]. 也有研究证实SCFAs结合白色脂肪细胞的GPR43, 调节机体能量摄取, 间接影响肝脏能量消耗[9,46]. 有研究[47]显示, NAFLD模型组大鼠肠道菌群多样性指数明显低于正常组大鼠, 且多种细菌比例也有所改变. 众所周知, 肝脏是门静脉血流的重要解毒器官, 与肠道菌群在解剖及功能上均有着密切联系, 因此肝病的发生与发展很可能与肠道菌群失调导致的肠道菌群代谢产物的含量变化有关,如SCFAs.
肥胖患者粪便中SCFAs总量较体质量正常者高且各种SCFAs比例改变, 体质量指数低者粪便中乙酸盐比例相对高[48,49]. 研究显示超重人群长期服用丙酸盐后体质量减轻, 脂肪堆积减少. Pirlich等[50]给予GPR43缺乏的小鼠正常饮食, 该小鼠有可能发展为肥胖; 相比之下, 脂肪组织高表达GPR43的小鼠无论摄入多少能量, 体型均能维持正常. 研究证实这是因为SCFAs与肠细胞表面受体GPR41/43作用, 促进肠道激素分泌, 如5-羟色胺(5-hydroxytryptamine, 5-HT)、GLP-1和酪酪肽(peptide tyrosine tyrosine, PYY). 5-HT调节胃肠道运动和分泌, 改变肠壁通透性, 减少胃肠道从食物中吸收能量; GLP-1可以促进机体分泌胰岛素并增强机体对胰岛素的敏感性, 继而增强线粒体功能, 增加能量消耗, 促进过氧化物酶体增殖物激活受体γ, 辅激活因子1α的表达, 促进脂肪酸氧化; PYY可以调节肠道运动减缓胃排空, 提升饱腹感, 减少机体摄入食物[49]. SCFAs与GPR43结合还可抑制胰岛素信号通路, 使磷脂酰肌醇依赖性激酶的激活受到抑制, 蛋白激酶B不能被激活, 细胞凋亡相关的BAD蛋白不被磷酸化而具有活性, 从而使脂肪细胞凋亡, 降低脂肪堆积[50]. 位于脂肪细胞上的GPR43与SCFAs结合后促使细胞分泌瘦素, 瘦素使机体摄食减少, 能量释放增加, 脂肪细胞合成减弱, 进而使体质量减轻[49].
SCFAs是具有短半衰期并能快速代谢的挥发性化合物. 高毫摩尔浓度的SCFAs可有效抑制HDACs功能, 当然, SCFAs也能通过结合GPCRs间接抑制HDACs, 进而诱导吞噬细胞分泌趋化因子、抗炎因子, 阻滞吞噬细胞释放肿瘤坏死因子, 促进T淋巴细胞增殖分化, 达到治疗肠炎的效果. SCFAs也能促进IESs增生并分泌黏液, 加强IESs间的紧密连接, 可用于治疗肠上皮损伤. SCFAs有较好的抑癌作用, 可抑制结肠癌细胞增殖分化, 促进癌细胞凋亡, 在治疗癌症方面有可观的应用前景. 目前的研究显示NAFLD的发病机制很大可能与SCFAs有关, 所以SCFAs有望成为新的NAFLD的治疗靶点. 此外, SCFAs还能促进胰岛素分泌, 提高机体对胰岛素的敏感性, 增加能量消耗, 减少脂肪堆积, 或许可用于治疗肥胖症患者以及Ⅱ型糖尿病患者(图3).
微生物代谢产物SCFAs和人体间的相互作用机制非常复杂, 并受很多环境因素的影响, 他们之间有直接作用, 也有间接作用. SCFAs对机体免疫功能有重要的调节作用, 越来越被认为是人类生存必不可少的部分. 微生物-宿主共代谢在人类健康和疾病中起到作用已得到广泛认可. 因此, 对未来的研究而言, 短链脂肪酸是如何影响宿主免疫细胞亚群和他们的功能的以及在疾病发生发展转化过程中的作用机制仍需广大科研工作者进一步研究.
内短链脂肪酸(short-chain fatty acids, SCFAs)是由1-6个碳原子组成的有机脂肪酸, 主要包括乙酸、丙酸、丁酸. 其在结肠内的浓度约为20-140 mmol/L. SCFAs在人体内参与不同的代谢, 发挥不同的功能. 其中, 乙酸是宿主细胞能量的重要来源, 约提供人体日总能量的10%; 丙酸通过血液循环进入肝脏, 在肝脏中分解代谢, 参与丙酮酸逆转化为葡萄糖的过程; 丁酸是上皮细胞的主要能量来源.
研究证实SCFAs能够改善肠道炎症, 提高肠上皮屏障功能, 抑制结肠肿瘤、非酒精性脂肪性肝病、肥胖等疾病. 这些发现很大程度上拓宽了人们对于SCFAs的认识, 为研究者提供了新思路. 有关SCFAs在疾病治疗方面的研究已成为目前的研究热点, 有助于加深科研工作者们对于宿主-微生物共生的认识. 其中的一些细节问题如SCFAs在疾病发生发展转化过程中的作用机制仍需广大科研工作者进一步研究.
SCFAs在维持机体稳态及正常代谢方面具有不可替代的作用. SCFAs可降低肠道炎性反应, 提高肠上皮屏障功能. "抑制HDAC9会引起FOXP3+Treg细胞数量增加, 增强FOXP3+Treg细胞的免疫功能, 降低小鼠内Treg细胞介导的结肠炎"、"SCFAs可提高肠上皮细胞(intestinal epithelial cells, IESs)屏障功能来维持黏膜免疫, 引起肠上皮的杯状细胞内黏蛋白基因的转录增强"发表于Nature Reviews Immunology 2016期刊上.
本文对SCFAs降低肠道炎症反应, 提高肠上皮屏障功能, 抑制结肠肿瘤、非酒精性脂肪性肝病、肥胖等方面进行了较为完整的叙述, 并配图对重点机制进行了阐述. 本文将SCFAs与疾病联系在一起, 侧重于叙述SCFAs的疾病治疗机制, 并对SCFAs在疾病治疗方面的应用进行了展望. 如SCFAs可促进胰岛素分泌, 提高机体对胰岛素的敏感性, 增加能量消耗, 减少脂肪堆积, 或许可用于治疗肥胖症患者以及Ⅱ型糖尿病患者.
SCFAs可以直接或间接抑制组蛋白脱乙酰基酶, 进而诱导吞噬细胞分泌趋化因子、抗炎因子, 阻滞吞噬细胞释放肿瘤坏死因子, 促进T淋巴细胞增殖分化, 达到治疗肠炎的效果. SCFAs也能促进IESs增生并分泌黏液, 加强IESs间的紧密连接, 可用于治疗肠上皮损伤. 另外, SCFAs有较好的抑癌作用, 可抑制结肠癌细胞增殖分化, 促进癌细胞凋亡, 在治疗癌症方面有可观的应用前景. 目前的研究显示NAFLD的发病机制很大可能与SCFAs有关, 所以SCFAs有望成为新的NAFLD的治疗靶点. 此外, SCFAs还能促进胰岛素分泌, 提高机体对胰岛素的敏感性, 增加能量消耗, 减少脂肪堆积, 或许可用于治疗肥胖症患者以及Ⅱ型糖尿病患者.
非酒精性脂肪性肝病: 一种无过量饮酒史, 由各种原因引起的肝细胞内脂肪堆积, 以肝细胞脂肪变性和脂质蓄积为主要特征的临床病理综合征. 其病理变化随病程的进展而表现有单纯性脂肪肝、高脂血症、高血压, 并被认为是代谢综合征在肝脏的一种病理表现. 是一种与胰岛素抵抗和遗传易感性密切相关的代谢应激性肝损伤;
短链脂肪酸(short-chain fatty acids): 又称挥发性脂肪酸, 依据碳链中碳原子的多少, 把碳原子数为1-6的有机脂肪酸成为短链脂肪酸. 由肠道细菌分解食物残渣产生, 肠迅速吸收, 既储存了能量又降低了渗透压, 并且短链脂肪酸对于维持大肠的正常功能和结IESs的形态和功能具有重要作用.
刘鹏飞, 主任医师, 东南大学医学院附属江阴医院消化内科; 庹必光, 教授, 遵义医学院附属医院消化科; 郑曙云, 副教授, 主任医师, 南京医科大学第三临床医学院(南京医科大学附属南京医院)重症医学科
本文聚焦短链脂肪酸对宿主肠道免疫及肠上皮屏障功能的影响作了较为系统的表述, 文笔流畅, 层次结构清楚, 对了解短链脂肪酸与宿主肠道免疫及肠上皮屏障功能之间关系最新进展有一定的帮助.
手稿来源: 自由投稿
学科分类: 胃肠病学和肝病学
手稿来源地: 北京市
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
编辑: 马亚娟 电编:李瑞芳
| 3. | Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221-1227. [PubMed] [DOI] |
| 5. | Pinto A, Fidalgo P, Cravo M, Midões J, Chaves P, Rosa J, dos Anjos Brito M, Leitão CN. Short chain fatty acids are effective in short-term treatment of chronic radiation proctitis: randomized, double-blind, controlled trial. Dis Colon Rectum. 1999;42:788-795; discussion 795-796. [PubMed] [DOI] |
| 7. | Scheppach W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig Dis Sci. 1996;41:2254-2259. [PubMed] [DOI] |
| 8. | Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [PubMed] [DOI] |
| 9. | 梁 荫基, 林 琛莅, 王 少娜, 张 玉佩, 韩 莉, 王 观龙, 李 媛媛, 邓 远军, 何 毅芳, 何 亲羽. 肥胖相关非酒精性脂肪性肝病防治的新靶点: 短链脂肪酸及其受体信号通路的保护作用. 重庆医科大学学报. 2016;41:628-631. |
| 10. | Schilderink R, Verseijden C, de Jonge WJ. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol. 2013;4:226. [PubMed] [DOI] |
| 11. | Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol. 2009;15:5549-5557. [PubMed] [DOI] |
| 13. | Garland SH. Short chain fatty acids may elicit an innate immune response from preadipocytes: a potential link between bacterial infection and inflammatory diseases. Med Hypotheses. 2011;76:881-883. [PubMed] [DOI] |
| 15. | Park JS, Lee EJ, Lee JC, Kim WK, Kim HS. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 2007;7:70-77. [PubMed] [DOI] |
| 16. | Zhang WH, Jiang Y, Zhu QF, Gao F, Dai SF, Chen J, Zhou GH. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br Poult Sci. 2011;52:292-301. [PubMed] [DOI] |
| 17. | Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. [PubMed] [DOI] |
| 18. | Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299-1307. [PubMed] [DOI] |
| 19. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [PubMed] [DOI] |
| 20. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [PubMed] [DOI] |
| 21. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [PubMed] [DOI] |
| 22. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [PubMed] [DOI] |
| 23. | Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5-9. [PubMed] [DOI] |
| 24. | Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286. [PubMed] [DOI] |
| 25. | Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128-139. [PubMed] [DOI] |
| 26. | Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1-8. [PubMed] [DOI] |
| 27. | Field M, Rao MC, Chang EB. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989;321:800-806. [PubMed] [DOI] |
| 28. | Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. [PubMed] [DOI] |
| 29. | Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745-757. [PubMed] [DOI] |
| 30. | Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22:849-855. [PubMed] [DOI] |
| 31. | Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. [PubMed] [DOI] |
| 32. | Dekker J, Rossen JW, Büller HA, Einerhand AW. The MUC family: an obituary. Trends Biochem Sci. 2002;27:126-131. [PubMed] [DOI] |
| 33. | Porchet N, Buisine MP, Desseyn JL, Moniaux N, Nollet S, Degand P, Pigny P, Van Seuningen I, Laine A, Aubert JP. [MUC genes: a superfamily of genes? Towards a functional classification of human apomucins]. J Soc Biol. 1999;193:85-99. [PubMed] [DOI] |
| 34. | Williams SJ, Munster DJ, Quin RJ, Gotley DC, McGuckin MA. The MUC3 gene encodes a transmembrane mucin and is alternatively spliced. Biochem Biophys Res Commun. 1999;261:83-89. [PubMed] [DOI] |
| 35. | Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168-G1174. [PubMed] [DOI] |
| 36. | Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442-1447. [PubMed] [DOI] |
| 38. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [PubMed] [DOI] |
| 39. | Mangian HF, Tappenden KA. Butyrate increases GLUT2 mRNA abundance by initiating transcription in Caco2-BBe cells. JPEN J Parenter Enteral Nutr. 2009;33:607-617; discussion 617. [PubMed] [DOI] |
| 40. | Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045-1050. [PubMed] [DOI] |
| 41. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [PubMed] [DOI] |
| 43. | Chen YX, Fang JY, Lu J, Qiu DK. [Regulation of histone acetylation on the expression of cell cycle-associated genes in human colon cancer cell lines]. Zhonghua Yixue Zazhi. 2004;84:312-317. [PubMed] [DOI] |
| 44. | Mandal M, Wu X, Kumar R. Bcl-2 deregulation leads to inhibition of sodium butyrate-induced apoptosis in human colorectal carcinoma cells. Carcinogenesis. 1997;18:229-232. [PubMed] [DOI] |
| 45. | Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679-689. [PubMed] [DOI] |
| 46. | Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092-5099. [PubMed] [DOI] |
| 48. | Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18:190-195. [PubMed] [DOI] |
| 49. | 周 达, 范 建高. 肠道菌群-SCFAs在代谢性疾病中的作用研究. 胃肠病学和肝病学杂志. 2016;25:330-332. |