修回日期: 2016-01-18
接受日期: 2016-01-25
在线出版日期: 2016-03-08
目的: 探讨GLP-2类似物替度鲁肽治疗大鼠非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)的作用.
方法: 30只SD大鼠随机分为正常饮食组和高脂饮食组, 至第12周末时两组分别取6只大鼠判断NAFLD造模成功与否. 第13周起, 以剩余正常饮食组大鼠为对照(n = 6), 高脂饮食组随机分为NAFLD组(n = 6)和GLP-2组(n = 6), 其中GLP-2组注射替度鲁肽液, 对照组和NAFLD组注射生理盐水, 7 d后眼眶取血并处死取材, 检测NAFLD相关生化指标和病理观察.
结果: NAFLD模型成功构建, 高脂饮食组肝脏匀浆的总甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)及肝脏活动度积分(NAFLD activity score, NAS)均高于正常饮食组(P<0.05); 且高脂饮食组十二指肠肠道黏膜上皮细胞排列疏松、间隙变大, 黏膜Claudin-2蛋白表达增多(P<0.05). 给药GLP-2后, 肝脏匀浆的TG、TC及肝脏NAS均低于NAFLD组(P<0.05); 且改善了肠道黏膜上皮细胞的排列, 减小了细胞间隙和黏膜Claudin-2蛋白的表达(P<0.05).
结论: NAFLD发病可导致肠道黏膜细胞间隙增大, Claudin-2蛋白表达升高, 替度鲁肽可能通过降低NAFLD大鼠肠道黏膜Claudin-2蛋白的表达, 从而改善NAFLD的肝脏病变.
核心提示: 近年来, 非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)与肠道黏膜屏障的相关性研究已成为热点, 本文发现NAFLD发病可导致肠道黏膜细胞间隙增大, Claudin-2蛋白表达升高, 替度鲁肽可能通过降低NAFLD大鼠小肠黏膜Claudin-2蛋白的表达, 从而改善NAFLD的肝脏病变.
引文著录: 胡银霞, 李兰, 袁瑜, 吴礼浩, 何兴祥. 替度鲁肽对大鼠非酒精性脂肪性肝病的治疗作用. 世界华人消化杂志 2016; 24(7): 1009-1016
Revised: January 18, 2016
Accepted: January 25, 2016
Published online: March 8, 2016
AIM: To assess the therapeutic effect of glucagon-like peptide (GLP-2) analogue teduglutide on non-alcoholic fatty liver disease (NAFLD) in rats.
METHODS: Thirty SD rats were randomized into a normal diet group and a high-fat diet group. After feeding for 12 weeks, six rats were respectively selected from the two groups to determine whether the NAFLD model was successfully established. From the 13th week, the rest rats in the normal diet group served as controls (n = 6), and the rest rats of the high-fat diet group were randomized into a NAFLD group (n = 6) and a GLP-2 group (n = 6). The rats in the GLP-2 group were injected with teduglutide and the other two groups were injected with normal saline for 7 d. Then blood samples were collected from the ocular veniplex and rats were sacrificed. NAFLD related biochemical indicators were determined and pathological results were observed.
RESULTS: The NAFLD model was successfully established. Compared to the normal group, triglyceride (TG) and total cholesterol (TC) levels in liver homogenate and NAFLD activity score (NAS) were significantly higher in the high-fat diet group (P < 0.05). Moreover, duodenal mucosal epithelial cells were loosely arranged, and intercellular space and Claudin-2 protein expression were increased (P < 0.05). After treatment with GLP-2, TG and TC levels in liver homogenate and liver NAS were significantly lower than those of the NAFLD group (P < 0.05). Accordingly, the arrangement of intestinal epithelial cells was improved, and intercellular space and Claudin-2 protein expression were decreased (P < 0.05).
CONCLUSION: NAFLD can cause the loose of intestinal mucosal cells and the increase of Claudin-2 protein expression. Teduglutide might exert its therapeutic effect on NAFLD by decreasing the expression of Claudin-2 protein.
- Citation: Hu YX, Li L, Yuan Y, Wu LH, He XX. Therapeutic effect of teduglutide on non-alcoholic fatty liver disease in rats. Shijie Huaren Xiaohua Zazhi 2016; 24(7): 1009-1016
- URL: https://www.wjgnet.com/1009-3079/full/v24/i7/1009.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v24.i7.1009
非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)已成为世界上最常见的慢性肝病病因, 其在世界各国的发病率约为10%-30%, 且每年发病率都在上升[1,2]. Yan等[3]和Fung等[4]发现我国部分地区的发病率已超30%, 香港人群的NAFLD发病率甚至高达42%. 目前临床上治疗NAFLD并无特异性的方法, 其治疗多以调整饮食及运动、改善胰岛素抵抗、药物降脂和护肝为主. 近年来, NAFLD与小肠黏膜屏障的关系已成为研究热点, 如Miele等[5]早期研究表明NAFLD患者发病与小肠黏膜屏障通透性增加有关, 但是通过保护肠黏膜治疗NAFLD的机制仍不明确[6,7]. 据报道[8-11], GLP-2类似物替度鲁肽可以修复肠道黏膜屏障, 从而使短肠综合征患者获益, 然而通过替度鲁肽保护肠道黏膜用以治疗NAFLD并未见报道. 本研究通过高脂饮食造模、腹腔注射替度鲁肽, 检测相关生化指标, 观察大鼠肝脏、小肠黏膜形态学变化和肠黏膜紧密连接(tight junctions, TJs)蛋白Claudin-2的表达, 从而探讨替度鲁肽对NAFLD的治疗作用.
SPF级别SD大鼠, 体质量180-220 g, 购自南方医科大学实验动物中心. 饲料均购自广东省医学实验动物中心, 其中高脂饲料中碳水化合物、脂肪和蛋白质分别占总热量的53.0%、27.4%和19.6%. 替度鲁肽(上海吉尔生化有限公司); 兔抗Claudin-2多克隆抗体(武汉博士德公司); 山羊抗兔IgG(H+L)(北京中杉金桥公司).
1.2.1 造模: 30只大鼠适应性喂养1 wk后分为正常(饮食)组和高脂(饮食)组, 自由进食至第12周末, 从两组中分别随机取6只大鼠, 处死、取材判断NAFLD造模成功. 以剩余正常组大鼠为对照(n = 6), 取高脂组大鼠随机分为NAFLD组(n = 6)和GLP-2组(n = 6)后饮食同前, GLP-2组注射替度鲁肽液(250 μg/kg), 2次/d, 正常组和NAFLD组分别腹腔注射等体积生理盐水, 注射7 d后取血并全部处死, 取血清和肝脏匀浆测定总甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)水平, 取肝脏做HE染色、十二指肠做HE染色和Claudin-2蛋白免疫组织化学染色. 免疫组织化学参照Shimizu等[12]的标准进行半定量评分. 生化检测: 采用日立HITACHI系列全自动生化分析仪检测血清、肝脏组织匀浆中TG、TC水平.
1.2.2 肝脏病理: 取肝右叶0.5 cm×0.5 cm组织用40 g/L甲醛固定后脱水、包埋、切片、苏木素-伊红染色后镜下观察. 肝脏组织病理采用NAFLD活动度积分(NAFLD activity score, NAS)评分[13]. 十二指肠病理: 取近端十二指肠0.2 cm用于石蜡包埋, 切片、苏木素-伊红染色后镜下观察.
1.2.3 免疫组织化学检测十二指肠Claudin-2蛋白表达: 取上述十二指肠蜡块, 切片脱蜡至水, 3%H2O2灭活内源酶, pH 8.0 EDTA修复抗原, 5%牛血清蛋白封闭, 滴加抗Claudin-2一抗(1:750), 4 ℃过夜, PBS漂洗3次, 每次2 min, 滴加二抗(1:500), 37 ℃孵育30 min, PBS漂洗2 min×3次, 室温下进行二氨基联苯显色反应. 以PBS代替一抗作为阴性对照.
统计学处理 采用SPSS19.0软件进行统计分析, 数据采用mean±SD表示, 多组间比较采用ANOVA分析, 两两比较采用t检验, P<0.05为差异具有统计学意义.
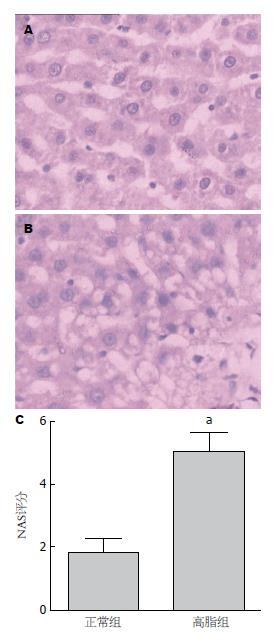
2.1.1 肝脏大体及组织学病变: 实验过程中两组大鼠均无死亡, 12 wk高脂组大鼠平均体质量较高, 但与正常组间无显著差异(P>0.05, 表1). 肉眼观察见正常组肝脏多呈暗红色; 高脂组肝脏多为奶黄色, 有油腻感, 包膜稍紧张, 边缘圆钝. 肝脏病理切片观察: 正常组肝小叶结构清晰, 肝索排列有序, 肝细胞质呈细密颗粒状, 门管区无炎细胞浸润(图1A); 高脂组大鼠多数呈现脂肪肝状态, 可见肝窦狭窄变性、肝细胞肿胀及肝细胞气球样改变, 部分可见炎性细胞浸润及局部灶状坏死(图1B); 高脂组肝脏NAS评分高于正常组(P<0.05, 图1C).
2.1.2 血清和肝脏匀浆生化: 12 wk时各组大鼠血清TG、TC水平无显著异性, 而高脂组大鼠的肝脏TG、TC水平显著高于正常组(表1).
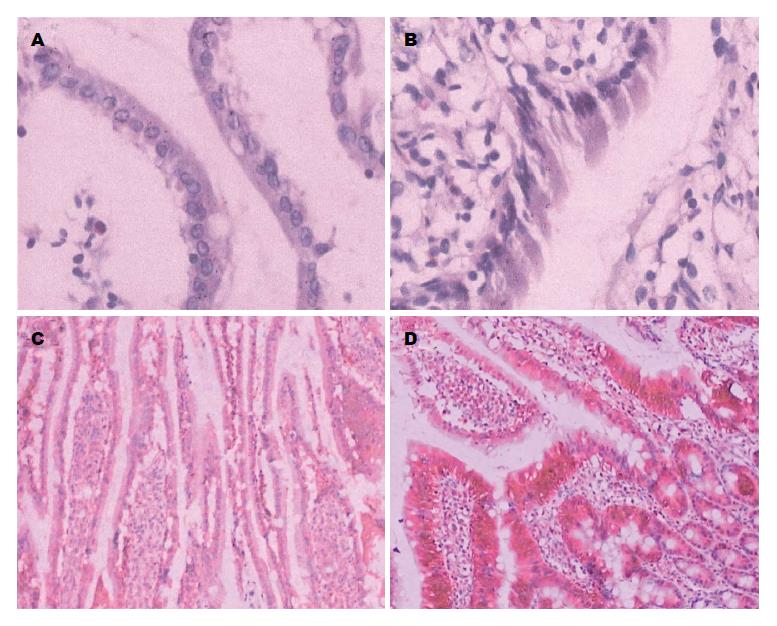
2.1.3 十二指肠HE染色和Claudin-2蛋白免疫组织化学: 12 wk时HE染色显示正常组十二指肠黏膜上皮细胞排列紧密, 未见细胞间隙增加(图2A); 而高脂组十二指肠黏膜上皮细胞排列疏松, 细胞间隙明显增加(图2B). 免疫组织化学显示十二指肠黏膜上皮Claudin-2蛋白呈棕褐色信号, 从隐窝到绒毛表面分布逐渐减少, 主要集中于隐窝处. 正常组和高脂组的Claudin-2蛋白表达量分别为2.50±0.55和5.17±0.75, 高脂组十二指肠黏膜隐窝及黏膜绒毛处阳性表达都较正常组多且均匀(P<0.05, 图2C, D).
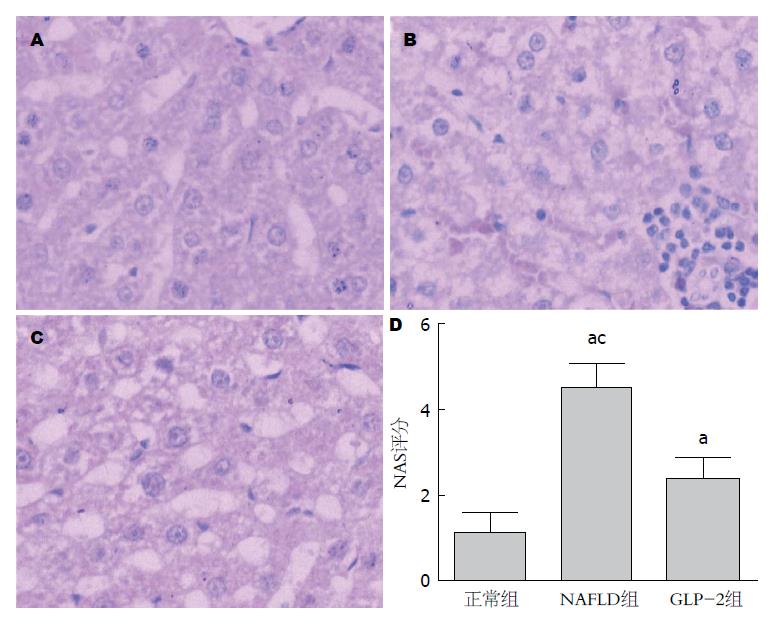
2.2.1 肝脏大体及组织学病变: 13 wk NAFLD组和GLP-2组大鼠平均体质量较高, GLP-2组较正常组间有显著差异(P<0.05, 表2). 正常组和NAFLD组大鼠的肝脏性状各与其12 wk鼠大致相同; 而GLP-2组略呈浅黄色, 包膜略紧张, 边缘略圆钝. 肝脏病理切片观察: 正常组肝小叶结构同12 wk正常组大致相似, 门管区无炎细胞浸润(图3A); NAFLD组大鼠均呈现脂肪肝状态, 肝窦狭窄、肝细胞肿胀及肝细胞气球样改变明显可见, 肝小叶内局部可见炎性细胞浸润及灶状坏死, 部分切片可见门管区炎细胞浸润及纤维增多(图3B); GLP-2组大鼠可见脂肪肝样改变, 但肝窦变性、肝细胞变性都不及NAFLD组明显, 且肝炎性细胞浸润不多见(图3C). 肝脏NAS评分表明NAFLD和GLP-2组的评分都高于正常组(P<0.05), 且GLP-2组评分低于NAFLD组(P<0.05, 图3D).
2.2.2 血清和肝脏匀浆生化: 13 wk NAFLD组和GLP-2组大鼠的血清和肝脏匀浆的TG、TC水平均显著高于正常组(P<0.05); GLP-2组血清TG和肝脏TG、TC水平均低于NAFLD组, 但没有显著性差异(表2).
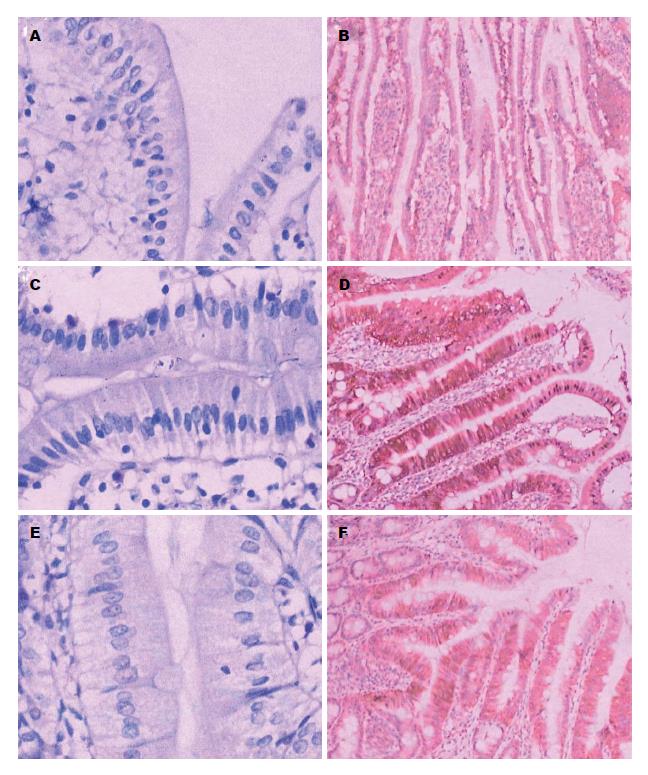
2.2.3 十二指肠HE染色和Claudin-2蛋白免疫组织化学: 13 wk HE染色显示正常组、GLP-2组十二指肠黏膜上皮细胞排列紧密, 未见细胞间隙明显增加(图4A, E); 然而NAFLD组十二指肠黏膜上皮细胞排列疏松, 细胞间隙明显增加(图4C). 正常组、NAFLD组和GLP-2组的十二指肠黏膜上皮Claudin-2蛋白表达量分别为2.83±0.75、5.50±1.05和4.33±0.82, NAFLD组和GLP-2组Claudin-2蛋白阳性表达均较正常组多(P<0.05), GLP-2组Claudin-2蛋白表达少于NAFLD组(P<0.05), 正常组表达最少(图4B, D, F).
本文NAFLD大鼠成功建模后, 发现小肠黏膜上皮细胞间隙增大, 表明肠道屏障与NAFLD的发病发展密切相关, 这与Sellmann等[14]提出的长期高糖或高脂饮食会改变肠道屏障功能从而导致NAFLD的发生、发展结论相符合. 肠道屏障据功能可归纳分为机械屏障、化学屏障、免疫屏障、生物屏障四大类型, 其中机械屏障主要指肠道黏膜上皮细胞间TJs. TJs常位于上皮细胞顶端侧面, 环绕整个细胞, 呈带状连接从而起到屏障和篱笆的双重作用[15]. TJs是一种由多种蛋白构成的复杂复合体, 其可能含有"孔通道"和"漏通道": 孔通道由高容量-高电荷-高分子选择性的Claudins组成, 主要调节离子和小分子的通透性; 漏通道由低容量-低电荷-低分子选择性的occludin和ZO-1组成, 主要调节大分子的通透性[16]. Claudins家族是肠道黏膜上皮细胞TJs的重要蛋白, 主要调节离子和小分子的通透性[16,17], 目前认为其有27种亚型[18]. 许多研究表明, Claudins家族与炎症性肠病等肠道病变有相关性, 如Schumann等[19]发现在乳糜泻活动期, 十二指肠的Claudin-2和15表达会显著增加; Yuan等[20]亦发现Claudin-1、Claudin-2表达的增加与结肠黏膜损伤的关系. 此外, 在克罗恩病活动期乙状结肠中, 多数Claudins不可检测或未发生改变, 但Claudin-5和8都减少并移位, Claudin-2表达急剧上调[21]. 本研究发现NAFLD小肠黏膜上皮细胞间隙变大, 再次确认了NAFLD发病导致肠道通透性增加的现象, 这与同类文献报道[6,22-24]一致, NAFLD患者或动物的肠道通透性变大, 肠道黏膜紧密连接改变, 从而导致相关蛋白的减弱甚至缺失. NAFLD小肠黏膜上皮隐窝和绒毛的Claudin-2蛋白明显增多, 据此推测, 长期的高脂饮食可能会导致Claudin-2蛋白表达的增加, 从而导致上皮细胞间的TJs功能失调, 破坏了肠道黏膜的通透性.
替度鲁肽是GLP-2类似物, 目前已被美国食品和药物管理局(Food and Drug Administration, FDA)和欧洲药品管理局(EMA)批准用于治疗短肠综合征患者, 其药理机制研究可见于Jeppesen等[8]的报告: GLP-2加强肠道上皮屏障功能是通过经细胞和经细胞旁途径共同实现的, 其信号通路可能包括蛋白激酶A(protein kinase A, PKA)/PKB/糖原合成酶激酶-3(glycogen synthesis kinanse-3, GSK-3)、细胞外调节蛋白激酶1/2(extracellular regulated protein kinases 1/2, ERK1/2)和p90核糖体S6激酶(p90RSK)和丝裂原激活蛋白激酶(mitogen-activated protein kinase, MAPK)信号通路等[25-27]. 本研究运用替度鲁肽显著改善了肝脏生理结构和生化指标(图3, 表2), 且改善了肠道黏膜上皮细胞的排列, 减小了细胞间隙和调节黏膜Claudin-2蛋白的表达. 其原因可能如下: (1)GLP-2主要由哺乳动物肠内分泌L细胞分泌, 可以抑制胃酸分泌和胃排空, 促进肠黏膜生长, 增加肠道血流, 从而保护肠道黏膜屏障、促进肠道吸收营养物质[28,29]; (2)替度鲁肽减少NAFLD肠道黏膜TJs中的Claudin-2蛋白的表达, 恢复了上皮细胞间TJs的功能, 从而改善肠道屏障的的通透性(图4); (3)由于二肽基肽酶Ⅳ(DPP-Ⅳ)的降解作用, GLP-2在体内产生后半衰期较短(7 min左右), 而替度鲁肽可以抵抗DPP-IV, 显著提高了GLP-2的半衰期、延长其体内活性, 从而增强了其黏膜保护作用[30].
本研究发现NAFLD组大鼠十二指肠黏膜上皮细胞间的间隙增大、紧密连接蛋白Claudin-2表达增多, 这也许是导致脂肪肝发生、发展的先决条件. 肠道屏障损坏可能导致过多的内毒素、能量物质尤其是脂质进入门脉系统从而引起肝脏炎症应激反应及过度的脂质沉积. 而使用替度鲁肽干预后, 高脂饮食大鼠十二指肠黏膜上皮细胞间隙未见明显增大、Claudin-2蛋白增多不如盐水干预组显著. 我们据此推测替度鲁肽可能通过降低肠道黏膜Claudin-2蛋白表达而修复肠道黏膜, 减少了内毒素及脂质等的吸收, 从而改善NAFLD肝脏病变. 由于替度鲁肽用于治疗NAFLD的作用机制不明确, 仍需进一步研究, 故其可行性也有待进一步的临床研究证实.
非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)已成为最常见的慢性肝病病因, 但目前仍无特异性的治疗方法. 明确NAFLD与肠道黏膜屏障的相关性也许可以为完善临床治疗提供保障. 替度鲁肽是肠道特异性修复剂, 目前用于治疗短肠综合征, 能否用于治疗NAFLD还有待进一步证实.
展玉涛, 主任医师, 首都医科大学附属北京同仁医院消化科
至今治疗NAFLD仍无特异性方法, 且其与肠道黏膜屏障紧密连接蛋白(tight junctions, TJs)改变的相关性仍未被完全认识. 明确肠道黏膜TJs蛋白与NAFLD发病的相关性, 有助于指导临床进一步诊疗工作.
本文发现NAFLD发病时小肠黏膜TJs蛋白中的Claudin-2表达增加, 使用替度鲁肽干预后Claudin-2表达可回到正常水平、肝脏脂肪病减轻, 进一步证实了NAFLD发病与肠道黏膜屏障的相关性, 且为替度鲁肽用于治疗NAFLD提供了实验依据.
明确NAFLD与肠道黏膜屏障的相关性, 有助于指导临床特异性治疗. 肠道特异性修复药物替度鲁肽除了可用于治疗短肠综合征, 也许将来也能运用于炎症性肠病、NAFLD等肠道发生改变的疾病治疗中.
Claudin-2: Claudins家族是肠道黏膜上皮细胞TJs的重要蛋白, 主要调节离子和小分子的通透性, 目前研究发现炎症性肠病等肠道疾病发病时小肠黏膜Claudin-2蛋白表达升高.
肠黏膜通透性异常在NAFLD发病机制及防治中的研究相对较少, 本文从NAFLD肠黏膜通透性的角度探讨其防治具有一定的创新性和科学性, 所用研究方法基本可靠, 所得结论有新意, 论文可读性强.
编辑: 于明茜 电编: 都珍珍
| 1. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [PubMed] [DOI] |
| 2. | Pallayova M, Taheri S. Non-alcoholic fatty liver disease in obese adults: clinical aspects and current management strategies. Clin Obes. 2014;4:243-253. [PubMed] [DOI] |
| 3. | Yan J, Xie W, Ou WN, Zhao H, Wang SY, Wang JH, Wang Q, Yang YY, Feng X, Cheng J. Epidemiological survey and risk factor analysis of fatty liver disease of adult residents, Beijing, China. J Gastroenterol Hepatol. 2013;28:1654-1659. [PubMed] [DOI] |
| 4. | Fung J, Lee CK, Chan M, Seto WK, Lai CL, Yuen MF. High prevalence of non-alcoholic fatty liver disease in the Chinese - results from the Hong Kong liver health census. Liver Int. 2015;35:542-549. [PubMed] [DOI] |
| 5. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [PubMed] [DOI] |
| 6. | Volynets V, Küper MA, Strahl S, Maier IB, Spruss A, Wagnerberger S, Königsrainer A, Bischoff SC, Bergheim I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2012;57:1932-1941. [PubMed] [DOI] |
| 7. | Yang DH, Ye ZY, Xie YJ, He XJ, Xu WJ, Zhou WM. Effect of salvianolate on intestinal epithelium tight junction protein zonula occludens protein 1 in cirrhotic rats. World J Gastroenterol. 2012;18:7040-7047. [PubMed] [DOI] |
| 8. | Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O'keefe SJ, Forbes A, Heinze H, Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473-1481.e3. [PubMed] [DOI] |
| 9. | Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902-914. [PubMed] [DOI] |
| 10. | Jeppesen PB. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Therap Adv Gastroenterol. 2012;5:159-171. [PubMed] [DOI] |
| 11. | Berg JK, Kim EH, Li B, Joelsson B, Youssef NN. A randomized, double-blind, placebo-controlled, multiple-dose, parallel-group clinical trial to assess the effects of teduglutide on gastric emptying of liquids in healthy subjects. BMC Gastroenterol. 2014;14:25. [PubMed] [DOI] |
| 12. | Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol. 1990;21:607-612. [PubMed] [DOI] |
| 13. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] [DOI] |
| 14. | Sellmann C, Priebs J, Landmann M, Degen C, Engstler AJ, Jin CJ, Gärttner S, Spruss A, Huber O, Bergheim I. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J Nutr Biochem. 2015;26:1183-1192. [PubMed] [DOI] |
| 15. | Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741-754. [PubMed] [DOI] |
| 16. | Liang GH, Weber CR. Molecular aspects of tight junction barrier function. Curr Opin Pharmacol. 2014;19:84-89. [PubMed] [DOI] |
| 17. | Watson AJ. Claudins and barrier dysfunction in intestinal inflammation: cause or consequence? Gut. 2015;64:1501-1502. [PubMed] |
| 18. | Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606-612. [PubMed] [DOI] |
| 19. | Schumann M, Günzel D, Buergel N, Richter JF, Troeger H, May C, Fromm A, Sorgenfrei D, Daum S, Bojarski C. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220-228. [PubMed] [DOI] |
| 20. | Yuan B, Zhou S, Lu Y, Liu J, Jin X, Wan H, Wang F. Changes in the Expression and Distribution of Claudins, Increased Epithelial Apoptosis, and a Mannan-Binding Lectin-Associated Immune Response Lead to Barrier Dysfunction in Dextran Sodium Sulfate-Induced Rat Colitis. Gut Liver. 2015;9:734-740. [PubMed] [DOI] |
| 21. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61-72. [PubMed] |
| 22. | Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, Grieco A, Alisi A, Nobili V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556-560. [PubMed] [DOI] |
| 23. | Xin D, Zong-Shun L, Bang-Mao W, Lu Z. Expression of intestinal tight junction proteins in patients with non-alcoholic fatty liver disease. Hepatogastroenterology. 2014;61:136-140. [PubMed] |
| 24. | Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br J Nutr. 2015;114:1745-1755. [PubMed] [DOI] |
| 25. | Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112-119. [PubMed] [DOI] |
| 26. | Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab. 2007;292:E281-E291. [PubMed] [DOI] |
| 27. | Dong CX, Zhao W, Solomon C, Rowland KJ, Ackerley C, Robine S, Holzenberger M, Gonska T, Brubaker PL. The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology. 2014;155:370-379. [PubMed] [DOI] |
| 28. | Sigalet DL, de Heuvel E, Wallace L, Bulloch E, Turner J, Wales PW, Nation P, Wizzard PR, Hartmann B, Assad M. Effects of chronic glucagon-like peptide-2 therapy during weaning in neonatal pigs. Regul Pept. 2014;188:70-80. [PubMed] [DOI] |
| 29. | Drucker DJ. Glucagon-like peptide 2. J Clin Endocrinol Metab. 2001;86:1759-1764. [PubMed] [DOI] |
| 30. | Marier JF, Beliveau M, Mouksassi MS, Shaw P, Cyran J, Kesavan J, Wallens J, Zahir H, Wells D, Caminis J. Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon-like peptide-2 (GLP-2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol. 2008;48:1289-1299. [PubMed] [DOI] |