修回日期: 2015-11-12
接受日期: 2015-11-23
在线出版日期: 2016-01-08
目的: 评估18F-脱氧葡萄糖(18F-fluorodeoxyglucose, 18F-FDG)正电子发射计算机断层显像(positron emission tomography, PET)及PET/计算机断层扫描(computed tomography, CT)在胰腺癌(pancreatic cancer)中的诊断价值.
方法: 计算机检索2015-04-01之前在Pubmed、EMBASE、Web of science、Cochrane协作网数据库、中国知网、谷歌学术搜索、万方及维普等数据库公开发表的有关18F-FDG PET及18F-FDG PET/CT诊断胰腺癌的文献, 并手工检索国内外相关医学杂志. 严格按照纳入排除标准筛选文献, 采用QUADAS量表评价文献质量, 利用Meta-Disc 1.4统计软件进行异质性分析和定量合成, 计算相应验后合并灵敏度和特异度, 并绘制SROC曲线评估诊断效能, 同时运用Stata12.0软件检测纳入研究是否存在发表偏倚.
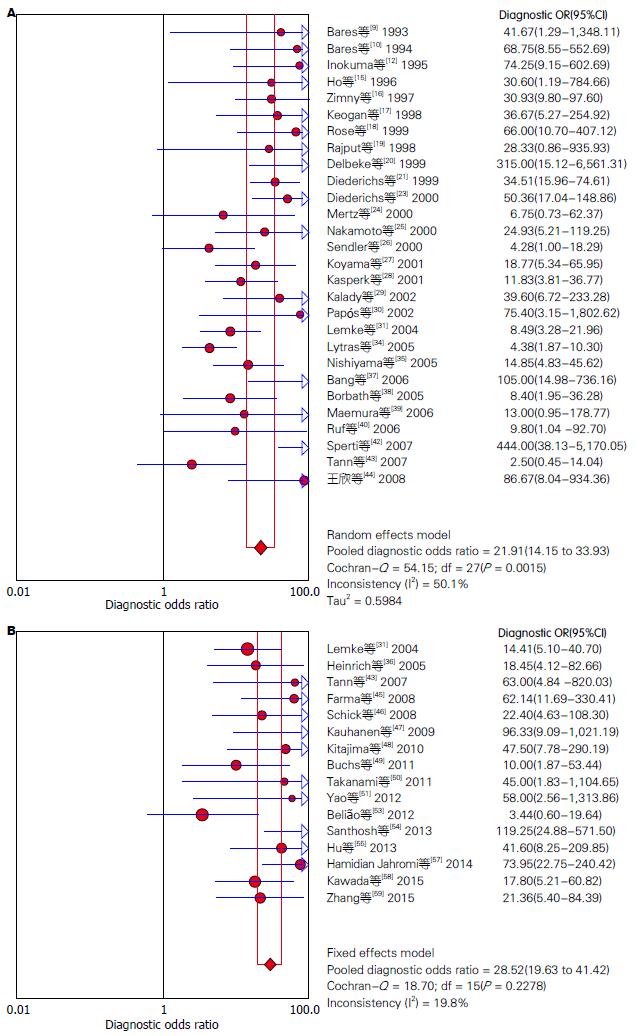
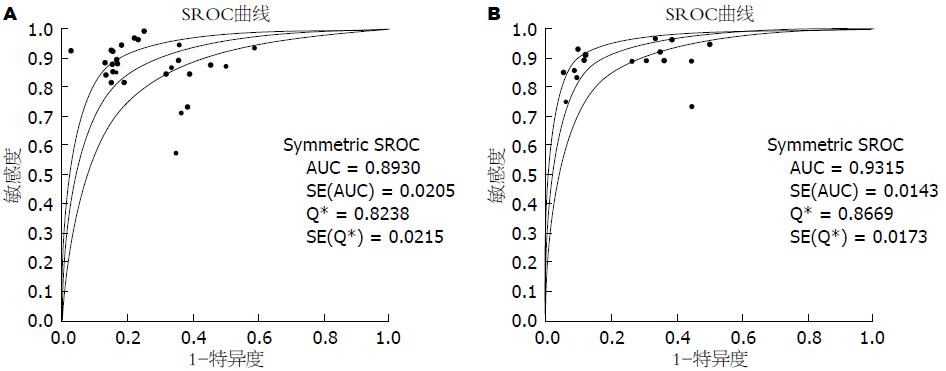
结果: 共纳入51篇英文文献, Meta分析表明: 18F-FDG PET诊断胰腺癌的合并灵敏度(pooled sensitivity)为87%(95%CI: 85%-89%)、合并特异度(pooled specificity)为78%(95%CI: 74%-81%)、诊断比值比(diagnosis odds ratio, DOR)为21.91(95%CI: 14.15-33.93)、阳性似然比(positive likelihood ratio, +LR)为3.38(95%CI: 2.64-4.33)、阴性似然比(negative likelihood ratio, -LR)为0.18(95%CI: 0.14-0.23)、SROC曲线下面积为0.8930; 18F-FDG PET/CT诊断胰腺癌的合并的灵敏度为91%(95%CI: 88%-93%)、合并特异度为77%(95%CI: 72%-82%)、DOR为28.52(95%CI: 19.63-41.42)、+LR为3.57(95%CI: 2.96-4.31)、-LR为0.14(95%CI: 0.11-0.18)、SROC曲线下面积为0.9315.
结论: 18F-FDG PET/CT及18F-FDG PET诊断胰腺癌的诊断效能均显著高于CT, 且18F-FDG PET/CT诊断灵敏度较18F-FDG PET高, 漏诊率低, 具有更高的诊断价值, 两者均可作为传统检查不能发现的胰腺癌的辅助诊断工具.
核心提示: 本文采用循证医学方法探讨18F-脱氧葡萄糖(18F-fluorodeoxyglucose, 18F-FDG)正电子发射计算机断层显像(positron emission tomography, PET)与18F-FDG PET/计算机断层扫描(computed tomography)诊断胰腺癌的价值, 结果表明在常规影像学检查阴性的疑似患者中具有较高价值, 可作为早期识别胰腺癌的良好辅助检查之一.
引文著录: 蒋晓涵, 胡乃中, 魏明通. 18F-FDG PET与18F-FDG PET/CT在胰腺癌中诊断价值的Meta分析. 世界华人消化杂志 2016; 24(1): 136-146
Revised: November 12, 2015
Accepted: November 23, 2015
Published online: January 8, 2016
AIM: To evaluate the value of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) and 18F-FDG PET/computed tomography (CT) in the diagnosis of pancreatic cancer.
METHODS: Medline, EMBASE, Science Direct, Springer link, CBM, Cnki, Wan fang and VIP databases were searched by computer before April 1, 2015 to retrieve articles on the study of 18F-FDG PET and 18F-FDG PET/CT in diagnosing pancreatic cancer. Studies were selected according to the inclusion and exclusion criteria, and quality assessment was made using the QUADAS scale. Meta-Disc 1.4 software was used to analyze the heterogeneity of the included articles, and the SROC curve was plotted to calculate the pooled sensitivity and specificity. The publication bias was assessed with Stata 12.0 software.
RESULTS: A total of 51 English-language articles were included. The summary sensitivity and specificity of 18F-FDG PET in diagnosing pancreatic cancer were 87% (95%CI: 85%-89%) and 78% (95%CI: 74%-81%), respectively. The positive and negative likelihood ratios were 3.38 (95%CI: 2.64-4.33) and 0.18 (95%CI: 0.14-0.23), respectively. The diagnostic odds ratio (DOR) was 21.91 (95%CI: 14.15-33.93), and the area under the SROC curve was 0.8930. The summary sensitivity and specificity of 18F-FDG PET/CT in diagnosing pancreatic cancer were 91% (95%CI: 88%-93%) and 77% (95%CI: 72%-82%), respectively. The positive and negative likelihood ratios were 3.57 (95%CI: 2.96-4.31) and 0.14 (95%CI: 0.11-0.18), respectively. The DOR was 28.52 (95%CI: 19.63-41.42), and the area under the SROC curve was 0.9315.
CONCLUSION: 18F-FDG PET/CT and 18F-FDG PET have higher diagnostic value than CT in diagnosing pancreatic cancer. 18F-FDG PET/CT is superior to 18F-FDG PET in terms of sensitivity and both of them can be used as diagnostic tools for pancreatic cancer with negative traditional examinations.
- Citation: Jiang XH, Hu NZ, Wei MT. Value of 18F-fluorodeoxyglucose positron emission tomography and 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis of pancreatic cancer: A systemic review and meta-analysis. Shijie Huaren Xiaohua Zazhi 2016; 24(1): 136-146
- URL: https://www.wjgnet.com/1009-3079/full/v24/i1/136.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v24.i1.136
胰腺癌(pancreatic cancer)是临床常见消化系恶性肿瘤之一, 发病例数逐年上升[1], 该病起病隐匿, 诊断时多为中晚期或已发生远处转移, 手术率低[2-5], 病死率高. 研究[6]表明胰腺癌若能早期诊断和治疗, 5年生存率可显著提高. 目前, 胰腺癌的诊断方法主要包括血清学检测及影像学检查. 血清学检测简单便捷, 最常见的为血清肿瘤标志物糖类抗原19-9(carbohydrate antigen 19-9, CA19-9)检测, 但其在许多胰腺良性疾病或胆道疾病中假阳性率高, 临床应用尚有限. 影像学检查包括: 计算机体层成像(computed tomography, CT)、核磁共振成像(magnetic resonance imaging, MRI)、超声内镜(B ultrasound, Bus)及内镜下逆行胰胆管造影(endoscopic retrograde cholangiopancreatography, ERCP)等, 其中, CT是胰腺显像最常用方法, 但是在鉴别胰腺良恶性疾病中仍存在不足. 理想的胰腺癌诊断方法应具有较高灵敏度和特异度且能评估预后和监测复发. 正电子发射计算机断层显像(positron emission tomography, PET)是近年来受到极大关注的新型影像学方法, 他利用进入人体并参与体内生物活动的各种示踪剂发射的射线而通过计算机显像的, 其中, 18F-脱氧葡萄糖(18F-fluorodeoxyglucose, 18F-FDG)是临床最常用的显像剂, 包括18F-FDG PET及将PET与CT融为一体的18F-FDG PET/CT. 胰腺癌细胞的生长代谢活跃, 对葡萄糖需求量高, 可较正常胰腺细胞摄取更多的18F-FDG而在PET图像上形成高代谢的功能图像, 因此18F-FDG PET及PET/CT可对胰腺癌作出诊断[7]. 目前国内外对18F-FDG PET及PET/CT在诊断胰腺癌的诊断价值的研究较多, 但相互之间仍有争议, 且国内尚无文献对其在胰腺癌的诊断价值进行系统性评价. 因此, 本文拟采用循证医学方法对18F-FDG PET及PET/CT在诊断胰腺癌的诊断价值进行系统评价.
计算机检索Medline、EMBASE、Science Direct、Springer link、CBM、中国知网、万方以及维普等常用数据库自建库至2015-04-01公开发表的有关18F-FDG PET及18F-FDG PET/CT诊断胰腺癌价值的文献. 检索策略: ("positron emission tomography OR PET")、("pancreatic cancer OR pancreatic tumor OR pancreatic carcinoma OR pancreatic neoplasms"). 尽可能对以上检索词采用不同策略检索, 并进一步追查已纳入文献或者综述文献的参考文献以全面收集资料. 纳入标准: (1)研究目的为影像学18F-FDG PET或18F-FDG PET/CT诊断胰腺癌价值的诊断试验; (2)纳入研究对象: 胰腺癌、良性胰腺疾病. 其中胰腺癌或良性胰腺疾病的诊断由病理学、组织学或临床随访最终确定, 各组均需有足够的样本量且具有代表性; (3)纳入研究的仪器参数及显像剂的量应注明, 且PET或PET/CT诊断胰腺癌的诊断标准应明确; (4)文献中应给出或可计算出四格表相关数据的值. 包括: 真阳性(true positive, TP)、假阳性(false positive, FP)、假阴性(false negative, FN)、真阴性(true negative, TN). 排除标准: (1)非诊断性试验研究, 如病例对照研究等应当排除; (2)人口统计学资料不全或无法从文献中获得灵敏度及特异度的研究; (3)重复性试验中, 发表较早、样本量较小或文章质量较低的文献予以排除; (4)试验设计缺乏严格对照的文献、评述类文献、文摘、讲座等非原始研究和动物实验等基础研究及学位论文、会议论文等予以排除.
1.2.1 文献筛选以及资料提取: 两位研究者遵循盲法原则, 独立阅读、筛选文献并进行数据提取. 对有分歧的文献或数据通过讨论或由第三个研究者决定其是否纳入. 提取信息包括: 作者、发表时间、样本量及诊断试验的各项参数信息(敏感度、特异度、TP、FP、FN、TN等). 文章中不详细的数据结果可通过联系作者或检索同一研究发表的相关文献予以补充.
1.2.2 文献质量评估: 根据Whiting等[8]制订的QUADAS质量标准的14个条目, 由2名评价者独立对纳入文献进行质量评价, 包括变异、偏倚和报告质量三个方面, 对每条标准划分为"是"、"否"、"不明确"3个结果. "是"表示入选文献符合该项条目, 计2分; "否"表示不满足, 计0分; 无法提取足够的信息判定为"不清楚", 计1分. 总分0-15分表示文献质量较低, 16-28分表示文献质量较高.
统计学处理 异质性分析: 通过SROC曲线及Spearman相关分析判断有无阈值效应导致的异质性, 非阈值效应采用Cochran Q统计量检验, 用I2评估异质性大小. I2<25%提示有轻度异质性, I2在25%和75%之间提示中度异质性, I2>75%以上则提示明显异质性, 显著性水平设定为P = 0.1. 对于异质性较大的研究(P<0.1且I2>75%), 采用随机效应模型进行合并数据分析, 无明显异质性(P>0.1且I2<75%)的采用固定效应模型进行数据分析. 合并统计量: 采用Meta-Disc 1.4软件进行Meta分析, 汇总18F-FDG PET及18F-FDG PET/CT诊断胰腺癌的诊断效能, 包括: 合并的灵敏度(pooled sensitivity, Sen)和合并特异度(pooled specificity, Spe), 阳性似然比(positive likelihood ratio, +LR), 阴性似然比(negative likelihood ratio, -LR), SROC曲线下面积(area under curve, AUC)等, 所有的结果均用95%CI可信区间表示, 并运用约登指数(Youden index) = Sen+Spe-1评估试验的准确精度. 发表偏倚检测: 运用Stata12.0软件, 通过观察Deek's图识别纳入文献是否存在发表偏倚.
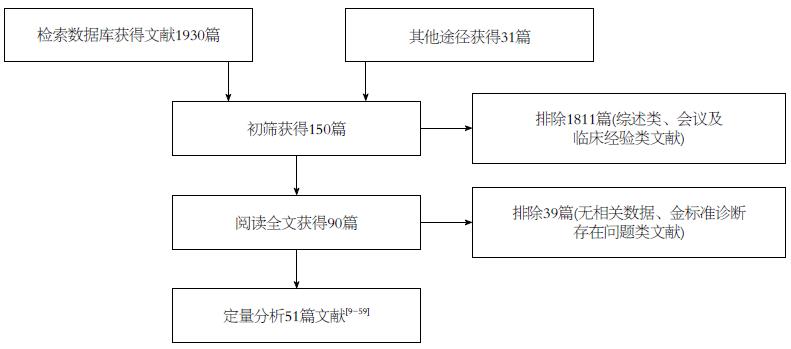
经过两位研究者(蒋晓涵与魏明通)独立进行筛选文献后, 共检索相关文献1961篇, 首先经阅读摘要和全文, 排除非胰腺癌病例或者综述类文献1871篇, 最后剩余90篇. 严格按照文献的纳入与排除标准对所纳入全文阅读文献进行筛选和剔除, 最终纳入英文文献51篇[9-59]. 文献筛选的PRISMA流程如图1. 其中, 18F-FDG PET诊断胰腺癌的诊断价值研究共纳入29个研究总计2011例研究对象, 其中胰腺癌组患者1422例, 非胰腺癌组589例; 18F-FDG PET在鉴别诊断胰腺癌与慢性胰腺炎的价值研究中纳入8个研究, 共427例患者, 其中胰腺癌组患者232例, 慢性胰腺炎患者195例; 而18F-FDG PET/CT 诊断胰腺癌的诊断价值研究共纳入16个研究, 总计993例患者, 其中胰腺癌组患者654例, 非胰腺癌组患者339例; 同时我们也对给出了CT诊断胰腺癌诊断价值的22个研究进行汇总, 共1227例患者, 其中胰腺癌患者868例, 非胰腺癌患者359例.
我们严格按照QUADAS量表对所纳入的51篇文献进行质量评价, 评价结果表明: 纳入文献的质量评分为15-27分, 平均24.1分, 其中16-28分的文献34篇, <16分的文献17篇, 文献的整体质量较高. 其中低分文献主要是样本量较少, 试验结果差异较大, 胰腺癌诊断中病理学诊断比例偏少等原因所导致的混合偏倚.
2.3.1 异质性检验: 对阈值效应导致的异质性检验, 计算敏感度与(1-特异度)对数的Spearman相关系数, 纳入研究中18F-FDG PET及18F-FDG PET/CT诊断胰腺癌的诊断试验相关Spearman系数依次为: -0.119, P = 0.545; 0.382, P = 0.144, 不具有统计学意义, 且观察SROC曲线不存在"肩臂状"分布, 因此不存在阈值效应. 同时检验了纳入文献中报告了CT诊断胰腺癌价值的试验的Spearman系数为0.085, P = 0.707, 也不存在阈值效应.
对非阈值效应导致的异质性检验, 在文献筛选和质量评价后, 我们分别提取51篇纳入研究的四格表数据(TP、FP、FN、TN), 并采用Meta-Disc 1.4软件进行Meta分析, 18F-FDG PET及18F-FDG PET/CT诊断胰腺癌的异质性分析分别如图2. 异质性分析表明: 18F-FDG PET诊断胰腺癌的合并的DOR为21.91(95%CI: 14.15-33.93), 各项纳入研究之间存在中等程度异质性(P = 0.0015, I2 = 50.1%), 采用随机效应模型; 18F-FDG PET/CT诊断胰腺癌的合并DOR为28.52(95%CI: 19.63-41.42), 各项研究间无明显异质性(P = 0.2278, I2 = 19.8%), 故采用固定效应模型. 同时我们根据纳入文献中报告的CT诊断胰腺癌的诊断价值进行系统评价, 结果表明, CT诊断胰腺癌的合并DOR为7.63(95%CI: 5.21-11.16), 存在中度异质性(P = 0.1084, I2 = 28.2%).
2.3.2 18F-FDG PET及18F-FDG PET/CT诊断胰腺癌的效能及价值: Meta-disc 1.4软件合并分析18F-FDG PET及18F-FDG PET/CT、CT诊断胰腺癌的诊断价值, 详如表1, 并绘制其合并的SROC曲线以及曲线下面积AUC如图3. 同时我们对给出了CT诊断胰腺癌及18F-FDG PET鉴别诊断胰腺癌及慢性胰腺炎诊断价值的研究进行汇总, 结果如表1. 由以上数据可以得出: 18F-FDG PET/CT诊断胰腺癌的灵敏度较18F-FDG PET高, 漏诊率低, 特异度基本一致, 两者诊断效能均显著高于CT. 其中18F-FDG PET在特异性鉴别诊断胰腺癌与慢性胰腺炎时准确度高.
| 项目 | 合并灵敏度(%) | 合并特异度(%) | 阳性似然比 | 阴性似然比 | 约登指数 |
| PET | 87(95%CI: 85-89) | 78(95%CI: 74-81) | 3.38(95%CI: 2.64-4.33) | 0.18(95%CI: 0.14-0.23) | 0.65 |
| PET/CT | 91(95%CI: 88-93) | 77(95%CI: 72-82) | 3.57(95%CI: 2.96-4.31) | 0.14(95%CI: 0.11-0.18) | 0.68 |
| CT | 80(95%CI: 77-83) | 64(95%CI: 59-69) | 1.95(95%CI: 1.59-2.39) | 0.32(95%CI: 0.24-0.43) | 0.44 |
| PET21 | 92(95%CI: 88-95) | 86(95%CI: 80-90) | 5.95(95%CI: 4.20-8.43) | 0.10(95%CI: 0.06-0.16) | 0.78 |
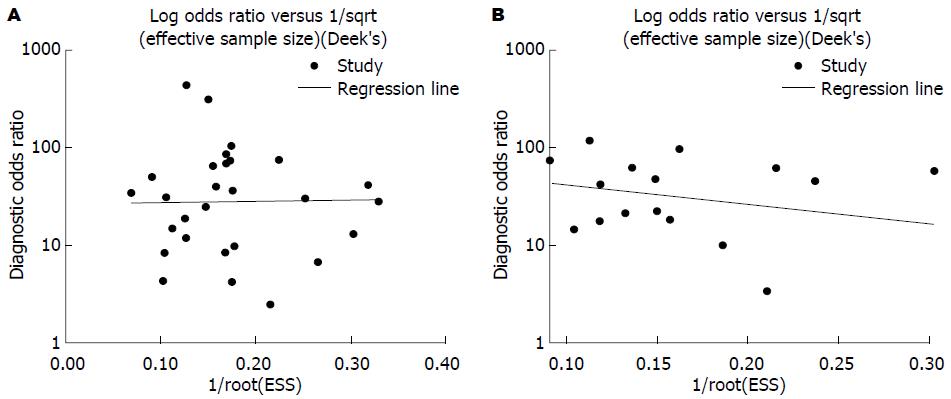
2.3.3 发表偏倚检验结果 Stata12.0软件对18F-FDG PET及18F-FDG PET/CT诊断胰腺癌价值的研究进行发表偏倚检测, 结果显示: 纳入的29个有关18F-FDG PET诊断胰腺癌价值的诊断研究Deek's图对称, P = 0.963(>0.05), 不具有统计学意义; 纳入的16个有关18F-FDG PET/CT诊断胰腺癌价值的诊断研究Deek's图对称, P = 0.420(>0.05), 不具有统计学意义(图4). 结果表明均不存在发表偏倚.
胰腺癌是一种恶性度极高的消化系肿瘤[60], 发病率呈增长趋势, 病死率居恶性肿瘤第4位[61]. 早期诊断胰腺癌可通过手术切除延长生存期, 但多数胰腺癌诊断时已中晚期或发生淋巴转移, 无法行手术切除, 预后极差. 因此, 胰腺癌的早期诊断是改善预后的关键. 早期诊断包括临床表现, 血清学检测及影像学检查三方面. 胰腺癌起病隐匿, 早期缺乏典型临床表现, 诊断困难. 血清肿瘤标志物如CA19-9、CEA等敏感度较高, 但缺乏特异性, 诊断价值局限. 影像学诊断方法中, Bus应用广泛但易受肠道内气体干扰而导致病变显示欠佳, ERCP特异性高但有创伤性且并发症多而应用较少, CT、MRI常用且对胰腺形态、结构病变的诊断有重要意义, 但鉴别胰腺病变的良恶性存在不足. 18F-FDG PET及PET/CT是新型无创性功能代谢显像, 他主要是通过18F-FDG显像剂示踪发射射线传递到计算机而显像. 18F-FDG是葡萄糖类似物, 能在体内参与葡萄糖代谢, 胰腺癌组织细胞在缺血缺氧环境下, 细胞膜葡萄糖转运蛋白-1高度表达, 胞内己糖激酶-Ⅱ活性增高, 这将促使胰腺组织细胞摄取18F-FDG增高[62-64]. 18F-FDG高摄取水平可通过视觉观察及标准摄取值(SUV)判定. 胰腺组织呈高摄取状态时, 其放射性浓聚程度高于肝右叶下段并且SUV值将高于阈值. 18F-FDG PET是分子水平的功能显像, 18F-FDG PET/CT则是集解剖和功能于一身的综合显像, 两者均为全身显像, 对胰腺癌淋巴结及远处转移的诊断准确率较高, 有利于指导胰腺癌术前分期和临床策略的制定, 因此较传统解剖显像的CT、MRI有独特优势. 已有研究[9-59]对18F-FDG PET及PET/CT的诊断价值进行预测. 本文即采用系统评价的方法, 根据已发表的关于18F-FDG PET及PET/CT诊断胰腺癌诊断价值的文献, 对18F-FDG PET及PET/CT诊断胰腺癌的诊断价值进行系统评价.
本研究共纳入英文文献51篇, 均为18F-FDG PET、18F-FDG PET/CT诊断胰腺癌的诊断试验. 定量合成相关统计量结果显示: 异质性检验表明各纳入研究间不存在阈值效应, 18F-FDG PET/CT及18F-FDG PET诊断胰腺癌的灵敏度、特异度、阳性似然比, 约登指数、SROC下面积均较CT高, 诊断效能高, 且18F-FDG PET/CT灵敏度优于18F-FDG PET, 诊断价值更高. 其中18F-FDG PET在鉴别诊断胰腺癌及慢性胰腺炎时灵敏度、特异度较好, 误诊、漏诊率低, 可见, 18F-FDG PET及18F-FDG PET/CT作为胰腺癌早期诊断的影像学指标均有较高诊断价值. 但是, 这两种影像学方法在临床工作者及消化科医生中关注度不高, 因此我们建议: 对临床上有相关临床表现怀疑胰腺癌需进一步确诊时, 可优先使用传统诊断方法, 如CT、MRI等, 他们检出率较高, 且有确诊意义, 但对部分传统检查方法检测为阴性但又高度怀疑胰腺癌的患者, 在情况允许下可行18F-FDG PET或18F-FDG PET/CT检查, 诊断价值较高, 漏诊率低, 且已有指南表明, 18F-FDG PET或18F-FDG PET/CT在早期发现胰腺癌转移及进行胰腺癌分期中具有独特优势, 对疾病的评估更加直观, 价值更高. 其较血清学指标受干扰因素少, 但与影像学医师的阅片水平有直接关系, 且由于价格昂贵性价比不高而限制了应用, 因此, 根据患者个体化, 正确选择18F-FDG PET或18F-FDG PET/CT等影像学方法辅助诊断胰腺癌是一位合格的医务工作者应有的能力.
系统评价作为一种观察性研究, 具有一定的局限性, 本文主要存在以下局限: (1)语言偏倚: 检索文献的语种限于英文, 肯定会造成漏检其他语种的相关研究而影响试验结果; (2)文献质量偏倚: 我们对纳入文献进行严格的质量评估, 尽可能保证高质量文献的纳入, 但不同研究间质量仍存在差异, 这难免会影响结果的可靠性, 导致偏倚.
本研究通过汇总关于18F-FDG PET及18F-FDG PET/CT诊断胰腺癌的相关研究文献对其诊断胰腺癌的效能进行评价. 增大样本量可有效规避不具有代表性的研究对结果造成的偏倚, 使结果更具有可信性, 但是由于纳入研究间人口统计学资料及试验的实施过程中的诸多差异, 均可使试验结果产生异质性, 本文存在中等程度异质性, 产生原因可能有: (1)各研究的纳入对象地理区域不同, 胰腺癌的发病率和发现时间有所不同; (2)不同研究间金标准存在差异, 部分研究金标准仅为病理学结果, 但有部分研究还包含了临床随访诊断为胰腺癌的病例, 可产生异质性; (3)使用18F-FDG PET及18F-FDG PET/CT诊断胰腺癌最简单常用的是视觉观察法, 但影像学医师的阅片水平差异会对结果产生直接影响, 同时还有部分研究包含了SUV值测定诊断胰腺癌, 而有的研究则没有, 这可使结果产生异质性.
本文采用系统评价的方法对18F-FDG PET及18F-FDG PET/CT诊断胰腺癌的价值进行评价, 但由于18F-FDG PET或18F-FDG PET/CT的价值局限于当前的检测水平, 因此, 随着科技水平及影像学设备的发展, 今后仍需大样本, 高质量研究来进一步验证18F-FDG PET或18F-FDG PET/CT早期诊断胰腺癌和评估胰腺癌的预后的价值.
胰腺癌是常见消化系恶性肿瘤之一, 早期诊断率低, 病死率高, 18氟-脱氧葡萄糖正电子发射计算机断层显像(18F-fluorodeoxyglucose positron emission tomography, 18F-FDG PET)与18F-FDG PET/计算机体层成像(computed tomography, CT)为新型无创功能显像方法, 在胰腺癌细胞中可呈高代谢功能图像, 已有研究对其诊断胰腺癌的价值进行预测, 但国内尚无循证医学证据对其价值进行系统评价.
李兆申, 教授, 主任医师, 博士生导师, 上海长海医院消化科
胰腺癌早期诊断困难, 病死率高, 胰腺癌细胞可较正常胰腺细胞摄取更多的18F-FDG而在PET图像上形成高代谢功能图像, 因此18F-FDG PET及PET/CT可对胰腺癌做出诊断, 但目前尚无文献对其在胰腺癌的诊断价值进行系统性评价. 因此, 本文采用循证医学方法对18F-FDG PET及PET/CT在诊断胰腺癌的诊断价值进行汇总, 为临床提供证据.
本文结合近年最新的诊断试验研究, 运用循证医学方法进一步明确和证实了18F-FDG PET及18F-FDG PET/CT在胰腺癌诊断中的价值, 为临床选择提供证据.
相比较胰腺癌传统解剖显像的诊断方法, 18F-FDG PET及18F-PET/CT的敏感度、特异度及准确性更高, 可作为传统检查阴性而高度怀疑胰腺癌时的辅助诊断工具, 且已有指南表明, 18F-FDG PET或18F-FDG PET/CT在早期发现胰腺癌转移及进行胰腺癌分期中具有独特优势, 临床医生应合理选择应用.
本文应用系统评价的方法对18F-FDG PET及18F-FDG PET/CT诊断胰腺癌价值进行汇总, 结果可信, 具备一定的科学性、创新性, 对临床工作有指导意义.
编辑: 郭鹏 电编: 都珍珍
| 1. | Bednar F, Simeone DM. Pancreatic cancer stem cell biology and its therapeutic implications. J Gastroenterol. 2011;46:1345-1352. [PubMed] [DOI] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] |
| 3. | Witkowski ER, Smith JK, Tseng JF. Outcomes following resection of pancreatic cancer. J Surg Oncol. 2013;107:97-103. [PubMed] [DOI] |
| 4. | Jelski W, Kutylowska E, Laniewska-Dunaj M, Szmitkowski M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) as candidates for tumor markers in patients with pancreatic cancer. J Gastrointestin Liver Dis. 2011;20:255-259. [PubMed] |
| 5. | Gui JC, Yan WL, Liu XD. CA19-9 and CA242 as tumor markers for the diagnosis of pancreatic cancer: a meta-analysis. Clin Exp Med. 2014;14:225-233. [PubMed] [DOI] |
| 6. | Tsuchiya R, Noda T, Harada N, Miyamoto T, Tomioka T, Yamamoto K, Yamaguchi T, Izawa K, Tsunoda T, Yoshino R. Collective review of small carcinomas of the pancreas. Ann Surg. 1986;203:77-81. [PubMed] [DOI] |
| 7. | Jadvar H, Fischman AJ. Evaluation of pancreatic carcinoma with FDG PET. Abdom Imaging. 2001;26:254-259. [PubMed] [DOI] |
| 8. | Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. [PubMed] |
| 9. | Bares R, Klever P, Hellwig D, Hauptmann S, Fass J, Hambuechen U, Zopp L, Mueller B, Buell U, Schumpelick V. Pancreatic cancer detected by positron emission tomography with 18F-labelled deoxyglucose: method and first results. Nucl Med Commun. 1993;14:596-601. [PubMed] [DOI] |
| 10. | Bares R, Klever P, Hauptmann S, Hellwig D, Fass J, Cremerius U, Schumpelick V, Mittermayer C, Büll U. F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology. 1994;192:79-86. [PubMed] [DOI] |
| 11. | Friess H, Langhans J, Ebert M, Beger HG, Stollfuss J, Reske SN, Büchler MW. Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Gut. 1995;36:771-777. [PubMed] [DOI] |
| 12. | Inokuma T, Tamaki N, Torizuka T, Magata Y, Fujii M, Yonekura Y, Kajiyama T, Ohshio G, Imamura M, Konishi J. Evaluation of pancreatic tumors with positron emission tomography and F-18 fluorodeoxyglucose: comparison with CT and US. Radiology. 1995;195:345-352. [PubMed] |
| 13. | Kato T, Fukatsu H, Ito K, Tadokoro M, Ota T, Ikeda M, Isomura T, Ito S, Nishino M, Ishigaki T. Fluorodeoxyglucose positron emission tomography in pancreatic cancer: an unsolved problem. Eur J Nucl Med. 1995;22:32-39. [PubMed] [DOI] |
| 14. | Stollfuss JC, Glatting G, Friess H, Kocher F, Berger HG, Reske SN. 2-(fluorine-18)-fluoro-2-deoxy-D-glucose PET in detection of pancreatic cancer: value of quantitative image interpretation. Radiology. 1995;195:339-344. [PubMed] [DOI] |
| 15. | Ho CL, Dehdashti F, Griffeth LK, Buse PE, Balfe DM, Siegel BA. FDG-PET evaluation of indeterminate pancreatic masses. J Comput Assist Tomogr. 1996;20:363-369. [PubMed] |
| 16. | Zimny M, Bares R, Fass J, Adam G, Cremerius U, Dohmen B, Klever P, Sabri O, Schumpelick V, Buell U. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: a report of 106 cases. Eur J Nucl Med. 1997;24:678-682. [PubMed] [DOI] |
| 17. | Keogan MT, Tyler D, Clark L, Branch MS, McDermott VG, DeLong DM, Coleman RE. Diagnosis of pancreatic carcinoma: role of FDG PET. AJR Am J Roentgenol. 1998;171:1565-1570. [PubMed] [DOI] |
| 18. | Rose DM, Delbeke D, Beauchamp RD, Chapman WC, Sandler MP, Sharp KW, Richards WO, Wright JK, Frexes ME, Pinson CW. 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann Surg. 1999;229:729-737; discussion 737-738. [PubMed] [DOI] |
| 19. | Rajput A, Stellato TA, Faulhaber PF, Vesselle HJ, Miraldi F. The role of fluorodeoxyglucose and positron emission tomography in the evaluation of pancreatic disease. Surgery. 1998;124:793-797; discussion 797-798. [PubMed] [DOI] |
| 20. | Delbeke D, Rose DM, Chapman WC, Pinson CW, Wright JK, Beauchamp RD, Shyr Y, Leach SD. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med. 1999;40:1784-1791. [PubMed] |
| 21. | Diederichs CG, Staib L, Glasbrenner B, Guhlmann A, Glatting G, Pauls S, Beger HG, Reske SN. F-18 fluorodeoxyglucose (FDG) and C-reactive protein (CRP). Clin Positron Imaging. 1999;2:131-136. [PubMed] [DOI] |
| 22. | Imdahl A, Nitzsche E, Krautmann F, Högerle S, Boos S, Einert A, Sontheimer J, Farthmann EH. Evaluation of positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose for the differentiation of chronic pancreatitis and pancreatic cancer. Br J Surg. 1999;86:194-199. [PubMed] [DOI] |
| 23. | Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, Beger HG, Reske SN. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109-116. [PubMed] [DOI] |
| 24. | Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc. 2000;52:367-371. [PubMed] [DOI] |
| 25. | Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, Hosotani R, Imamura M, Konishi J. Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer. 2000;89:2547-2554. [PubMed] |
| 26. | Sendler A, Avril N, Helmberger H, Stollfuss J, Weber W, Bengel F, Schwaiger M, Roder JD, Siewert JR. Preoperative evaluation of pancreatic masses with positron emission tomography using 18F-fluorodeoxyglucose: diagnostic limitations. World J Surg. 2000;24:1121-1129. [PubMed] [DOI] |
| 27. | Koyama K, Okamura T, Kawabe J, Nakata B, Chung KH, Ochi H, Yamada R. Diagnostic usefulness of FDG PET for pancreatic mass lesions. Ann Nucl Med. 2001;15:217-224. [PubMed] [DOI] |
| 28. | Kasperk RK, Riesener KP, Wilms K, Schumpelick V. Limited value of positron emission tomography in treatment of pancreatic cancer: surgeon's view. World J Surg. 2001;25:1134-1139. [PubMed] [DOI] |
| 29. | Kalady MF, Clary BM, Clark LA, Gottfried M, Rohren EM, Coleman RE, Pappas TN, Tyler DS. Clinical utility of positron emission tomography in the diagnosis and management of periampullary neoplasms. Ann Surg Oncol. 2002;9:799-806. [PubMed] [DOI] |
| 30. | Papós M, Takács T, Trón L, Farkas G, Ambrus E, Szakáll S, Lonovics J, Csernay L, Pávics L. The possible role of F-18 FDG positron emission tomography in the differential diagnosis of focal pancreatic lesions. Clin Nucl Med. 2002;27:197-201. [PubMed] |
| 31. | Lemke AJ, Niehues SM, Hosten N, Amthauer H, Boehmig M, Stroszczynski C, Rohlfing T, Rosewicz S, Felix R. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: clinical value in pancreatic lesions--a prospective study with 104 patients. J Nucl Med. 2004;45:1279-1286. [PubMed] |
| 32. | Rasmussen I, Sörensen J, Långström B, Haglund U. Is positron emission tomography using 18F-fluorodeoxyglucose and 11C-acetate valuable in diagnosing indeterminate pancreatic masses? Scand J Surg. 2004;93:191-197. [PubMed] |
| 33. | van Kouwen MC, Jansen JB, van Goor H, de Castro S, Oyen WJ, Drenth JP. FDG-PET is able to detect pancreatic carcinoma in chronic pancreatitis. Eur J Nucl Med Mol Imaging. 2005;32:399-404. [PubMed] [DOI] |
| 34. | Lytras D, Connor S, Bosonnet L, Jayan R, Evans J, Hughes M, Garvey CJ, Ghaneh P, Sutton R, Vinjamuri S. Positron emission tomography does not add to computed tomography for the diagnosis and staging of pancreatic cancer. Dig Surg. 2005;22:55-61; discussion 62. [PubMed] [DOI] |
| 35. | Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Tsutsui K, Wakabayashi H, Ohkawa M. Evaluation of delayed additional FDG PET imaging in patients with pancreatic tumour. Nucl Med Commun. 2005;26:895-901. [PubMed] [DOI] |
| 36. | Heinrich S, Goerres GW, Schäfer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, Hany TF, von Schulthess GK, Clavien PA. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242:235-243. [PubMed] [DOI] |
| 37. | Bang S, Chung HW, Park SW, Chung JB, Yun M, Lee JD, Song SY. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol. 2006;40:923-929. [PubMed] [DOI] |
| 38. | Borbath I, Van Beers BE, Lonneux M, Schoonbroodt D, Geubel A, Gigot JF, Deprez PH. Preoperative assessment of pancreatic tumors using magnetic resonance imaging, endoscopic ultrasonography, positron emission tomography and laparoscopy. Pancreatology. 2005;5:553-561. [PubMed] |
| 39. | Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, Jinnouchi S, Aikou T. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435-441. [PubMed] [DOI] |
| 40. | Ruf J, Lopez Hänninen E, Böhmig M, Koch I, Denecke T, Plotkin M, Langrehr J, Wiedenmann B, Felix R, Amthauer H. Impact of FDG-PET/MRI image fusion on the detection of pancreatic cancer. Pancreatology. 2006;6:512-519. [PubMed] [DOI] |
| 41. | Singer E, Gschwantler M, Plattner D, Kriwanek S, Armbruster C, Schueller J, Feichtinger H, Roka R, Moeschl P, Weiss W. Differential diagnosis of benign and malign pancreatic masses with 18F-fluordeoxyglucose-positron emission tomography recorded with a dual-head coincidence gamma camera. Eur J Gastroenterol Hepatol. 2007;19:471-478. [PubMed] |
| 42. | Sperti C, Bissoli S, Pasquali C, Frison L, Liessi G, Chierichetti F, Pedrazzoli S. 18-fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2007;246:932-937; discussion 937-939. [PubMed] [DOI] |
| 43. | Tann M, Sandrasegaran K, Jennings SG, Skandarajah A, McHenry L, Schmidt CM. Positron-emission tomography and computed tomography of cystic pancreatic masses. Clin Radiol. 2007;62:745-751. [PubMed] [DOI] |
| 45. | Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, Eikman EA, Malafa M. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465-2471. [PubMed] [DOI] |
| 46. | Schick V, Franzius C, Beyna T, Oei ML, Schnekenburger J, Weckesser M, Domschke W, Schober O, Heindel W, Pohle T. Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangio-pancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging. 2008;35:1775-1785. [PubMed] [DOI] |
| 47. | Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, Rinta-Kiikka I, Alanen K, Borra RJ, Puolakkainen PA. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957-963. [PubMed] [DOI] |
| 48. | Kitajima K, Murakami K, Yamasaki E, Kaji Y, Shimoda M, Kubota K, Suganuma N, Sugimura K. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent pancreatic cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Mol Imaging Biol. 2010;12:452-459. [PubMed] |
| 49. | Buchs NC, Bühler L, Bucher P, Willi JP, Frossard JL, Roth AD, Addeo P, Rosset A, Terraz S, Becker CD. Value of contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography in detection and presurgical assessment of pancreatic cancer: a prospective study. J Gastroenterol Hepatol. 2011;26:657-662. [PubMed] [DOI] |
| 50. | Takanami K, Hiraide T, Tsuda M, Nakamura Y, Kaneta T, Takase K, Fukuda H, Takahashi S. Additional value of FDG PET/CT to contrast-enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Ann Nucl Med. 2011;25:501-510. [PubMed] [DOI] |
| 51. | Yao J, Gan G, Farlow D, Laurence JM, Hollands M, Richardson A, Pleass HC, Lam VW. Impact of F18-fluorodeoxyglycose positron emission tomography/computed tomography on the management of resectable pancreatic tumours. ANZ J Surg. 2012;82:140-144. [PubMed] [DOI] |
| 52. | Herrmann K, Erkan M, Dobritz M, Schuster T, Siveke JT, Beer AJ, Wester HJ, Schmid RM, Friess H, Schwaiger M. Comparison of 3'-deoxy-3'-[18F]fluorothymidine positron emission tomography (FLT PET) and FDG PET/CT for the detection and characterization of pancreatic tumours. Eur J Nucl Med Mol Imaging. 2012;39:846-851. [PubMed] [DOI] |
| 53. | Belião S, Ferreira A, Vierasu I, Blocklet D, Goldman S, Metens T, Matos C. MR imaging versus PET/CT for evaluation of pancreatic lesions. Eur J Radiol. 2012;81:2527-2532. [PubMed] [DOI] |
| 54. | Santhosh S, Mittal BR, Bhasin D, Srinivasan R, Rana S, Das A, Nada R, Bhattacharya A, Gupta R, Kapoor R. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in the characterization of pancreatic masses: experience from tropics. J Gastroenterol Hepatol. 2013;28:255-261. [PubMed] [DOI] |
| 55. | Hu SL, Yang ZY, Zhou ZR, Yu XJ, Ping B, Zhang YJ. Role of SUV(max) obtained by 18F-FDG PET/CT in patients with a solitary pancreatic lesion: predicting malignant potential and proliferation. Nucl Med Commun. 2013;34:533-539. [PubMed] [DOI] |
| 56. | Matsumoto I, Shirakawa S, Shinzeki M, Asari S, Goto T, Ajiki T, Fukumoto T, Kitajima K, Ku Y. 18-Fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:712-718. [PubMed] [DOI] |
| 57. | Hamidian Jahromi A, Fallahzadeh MK, Takalkar A, Sheng J, Zibari G, Shokouh Amiri H. Impact of Plasma Glucose Level at the Time of Fluorodeoxyglucose Administration on the Accuracy of FDG-PET/CT in the Diagnosis of Pancreatic Lesions. Int J Endocrinol Metab. 2014;12:e16429. [PubMed] [DOI] |
| 58. | Kawada N, Uehara H, Hosoki T, Takami M, Shiroeda H, Arisawa T, Tomita Y. Usefulness of dual-phase 18F-FDG PET/CT for diagnosing small pancreatic tumors. Pancreas. 2015;44:655-659. [PubMed] [DOI] |
| 59. | Zhang J, Zuo CJ, Jia NY, Wang JH, Hu SP, Yu ZF, Zheng Y, Zhang AY, Feng XY. Cross-modality PET/CT and contrast-enhanced CT imaging for pancreatic cancer. World J Gastroenterol. 2015;21:2988-2996. [PubMed] [DOI] |
| 60. | Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8-29. [PubMed] |
| 61. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed] [DOI] |
| 62. | Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, Beger HG. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med. 1997;38:1344-1348. [PubMed] |
| 63. | Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, Doi R, Hosotani R, Imamura M, Konishi J. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002;43:173-180. [PubMed] |
| 64. | Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625-1632. [PubMed] |