修回日期: 2013-12-30
接受日期: 2014-01-08
在线出版日期: 2014-02-28
目的: 探讨糖基化终末产物(advanced glycation end products, AGEs)对结肠平滑肌细胞内钙离子浓度的影响及可能机制.
方法: 酶解法分离培养SD大鼠结肠平滑肌细胞(smooth muscle cell, SMC), α-actin免疫荧光鉴定; 激光共聚焦显微镜检测(SMC)钙闪烁; 蛋白激酶C(protein kinase C, PKC)活性检测试剂盒检测细胞PKC活性.
结果: 细胞免疫荧光鉴定大鼠结肠SMC. 不同浓度AGEs(50、100 μg/mL)刺激结肠SMC后, 与对照组比较, 钙离子浓度显著降低(56.7%±3.6%、78.6%±5% vs 99.6%±3.1%, P<0.05, P<0.01), 50 μg/mL为体外最大有效浓度. 与对照组相比, AGEs(50、100 μg/mL)升高细胞内PKC活性. PKC抑制剂chelerythrine可阻断AGEs介导的钙离子浓度降低(70.7%±3.7% vs 87.1%±2.5%, P<0.05).
结论: AGEs可激活PKC通路、从而降低胞内钙离子的浓度, 最终抑制大鼠结肠平滑肌收缩.
核心提示: 本研究提示糖基化终末产物(advanced glycation end products, AGEs)可激活结肠平滑肌细胞内的蛋白激酶C(protein kinase C, PKC), PKC磷酸化下游底物丝/苏氨酸残基, 调节结肠平滑肌细胞内钙离子浓度, 参与细胞收缩等功能.
引文著录: 朱滢, 王庆娥, 王云, 巩尧瑶, 孙晓萌, 林琳. 蛋白激酶C在糖基化终末产物介导结肠平滑肌细胞内钙离子浓度中的作用. 世界华人消化杂志 2014; 22(6): 874-879
Revised: December 30, 2013
Accepted: January 8, 2014
Published online: February 28, 2014
AIM: To investigate the effect of advanced glycation end products (AGEs) on intracellular calcium concentration in isolated colonic smooth muscle cells and the possible mechanisms involved.
METHODS: Colonic smooth muscle cells were isolated from normal adult rats, and immumofluorescence staining for α-actin was used to identify smooth muscle cells. The responsiveness of colonic smooth muscle cells to AGEs was measured by confocal laser scanning microscopy. Intracellular Ca2+ concentration ([Ca2+]i) was determined by Fluo3/AM based digital microfluorimetric measurement. Protein kinase C (PKC) activity was detected by PKC activity assay. PKC inhibitor chelerythrine was used to examine the role of PKC in AGEs-mediated inhibition of [Ca2+]i in colonic smooth muscle cells.
RESULTS: Colonic smooth muscle cells were successfully isolated from normal rats and identified by immunofluorescence staining. AGEs inhibited [Ca2+]i in a concentration-dependent manner. AGEs at a concentration of 50 or 100 µg/mL significantly inhibited the mean [Ca2+]i compared with the control group (56.7% ± 3.6%, 78.6% ± 5% vs 99.6% ± 3.1%, P < 0.05, P < 0.01). PKC activity increased in SMCs treated with 50 µg/mL or 100 µg/mL of AGEs compared with the control group. Pretreatment with chelerythrine (1 µmol/L) reduced AGEs-mediated inhibition of [Ca2+]i (70.7% ± 3.7% vs 87.1% ± 2.5%, P < 0.05).
CONCLUSION: AGEs inhibit [Ca2+]i in colonic smooth muscle cells in a PKC-dependent manner.
- Citation: Zhu Y, Wang QE, Wang Y, Gong YY, Sun XM, Lin L. Advanced glycation end products inhibit intracellular calcium concentration in colon smooth muscle cells in a protein kinase C-dependent manner. Shijie Huaren Xiaohua Zazhi 2014; 22(6): 874-879
- URL: https://www.wjgnet.com/1009-3079/full/v22/i6/874.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i6.874
糖尿病胃肠动力障碍是糖尿病(diabetes mellitus, DM)的慢性并发症之一, 发生于75%的DM患者[1,2]. 糖基化终末产物(advanced glycation end products, AGEs)在DM患者血清及各种组织中均显著升高, 他与DM肾病、DM血管病变的研究颇多, 与DM胃肠动力障碍的研究很少[3,4]. 细胞外钙内流和内钙释放是平滑肌细胞(smooth muscle cell, SMC)收缩的决定因素; DM结肠SMC内钙离子信号通路异常, 导致钙离子浓度异常[5,6]. 本文探讨AGEs是否影响结肠SMC内钙离子浓度及可能机制, 为DM胃肠道动力障碍的研究提供依据.
清洁级SD大鼠, 体质量150-200 g, 由南京医科大学实验动物中心提供; DMEM培养基、胎牛血清、青链霉素混悬液、大豆胰蛋白酶抑制剂(Gibco, USA); α-actin抗体(Abgent, USA); AGEs(Merck Millipore, Germany); FITC标记山羊抗大鼠IgG二抗(Bioworld, USA); 蛋白激酶C(protein kinase C, PKC)检测试剂盒(Enzo, USA); PKC抑制剂chelerythrine、Ⅱ型胶原酶(Sigma, USA); Fluo-3/AM(Invitrogen, USA).
1.2.1 结肠SMCs的分离及原代培养: SD大鼠断颈处死, 快速自肛门上2 cm取结肠10 cm左右, 用Hepes-Ringer缓冲液反复冲洗, 去除黏膜和浆膜层、剪碎平滑肌组织、加入消化液(0.1%的II型胶原酶和0.01%的大豆胰蛋白酶抑制剂)消化、离心, 含10%胎牛血清及DMEM培养液重悬细胞、过筛; 锥虫蓝染色确认细胞活力>90%, 于37 ℃、95%O2和5%CO2条件下培养, SMCs长至致密单层时, 传代培养, 采用2代SMCs进行实验.
1.2.2 结肠SMCs的鉴定: 取对数生长期的SMCs, 胰蛋白酶消化、制成单细胞悬液、接种到放有载玻片的培养皿中、CO2培养箱培养1-3 d, 待SMCs长至单层时, 吸去培养液, PBS冲洗、冰丙酮固定、PBS冲洗、BSA封闭30 min后吸去、加α-actin一抗(1:100), 阴性对照不加一抗, 4 ℃过夜, PBS冲洗; 加羊抗兔IgG二抗(1:500), 室温避光湿盒中孵育1 h, 冲洗、hoechest33258染核3 min、PBS冲洗、封片、镜下观察特异性荧光.
1.2.3 细胞内钙离子浓度的检测: 用DMSO溶解的Fluo-3/AM为钙荧光探针, SMCs接种于玻底皿中, PBS冲洗后, 加5 μmol/L的Fluo-3/AM, 置于37 ℃、95%O2和5%CO2孵育箱40 min、PBS冲洗; 激光共聚焦显微镜激发光波488 nm, 发射光波为515 nm, 采样间歇为2 s, 记录单个SMC在给予AGEs前后荧光强度变化(荧光强度表示为F/F0, F0为初始荧光强度).
1.2.4 PKC活性检测: 蛋白裂解液提取各组细胞蛋白, 4 ℃、12000 r/min、5 min、取上清, BCA法测定蛋白总量, 加待检样品30 µL于包被的反应孔中, 37 ℃孵育2 h, 洗涤、加酶标抗体30 µL, 室温孵育2 h, 洗涤、加显色液30 µL、显色30-45 min、终止反应, 在450 nm波长读板.
统计学处理 采用SPSS17.0软件包分析, 非参数检验之K-S检验进行数据正态性分析, 各组数据均呈正态分布, 以mean±SD表示, 各组间比较采用单因素方差分析和成组t检验, P<0.05为差异有统计学意义.
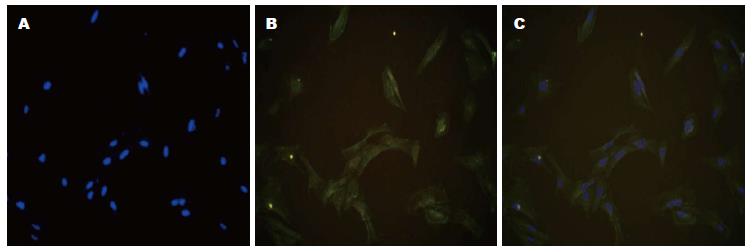
细胞核Hoechst染色呈蓝色, 大部分细胞α-actin荧光反应阳性, 细胞呈梭形(胞质见红色荧光)(图1).
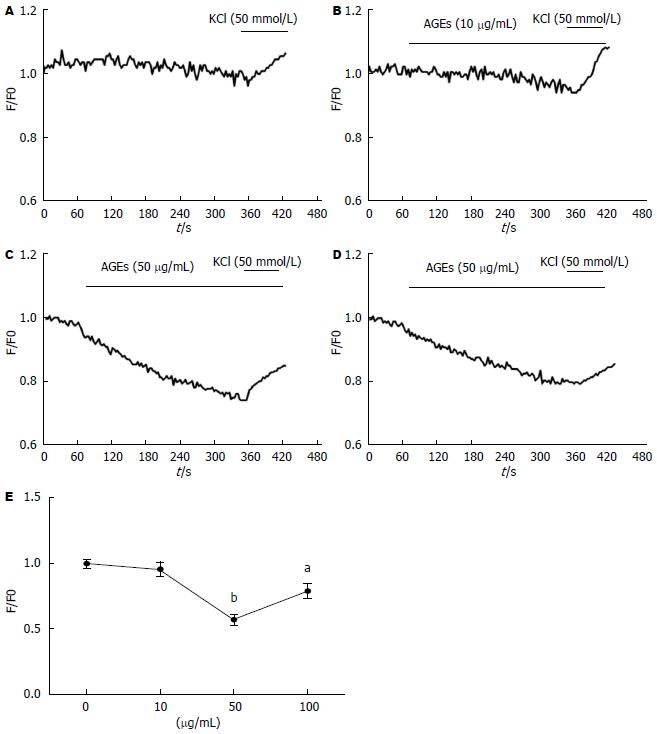
与对照组相比, AGEs在50和100 µg/mL时, 降低Fluo-3/AM负载的钙离子荧光强度(56.7%±3.6%、78.6%±5% vs 99.6%±3.1%, P<0.05, P<0.01), 说明AGEs可降低SMCs内钙离子浓度; AGEs在10 µg/mL时, 与对照组的差异无统计学意义; 实验结束后加入50 mmol/L氯化钾, 钙离子显著升高表示细胞活性良好(图2).
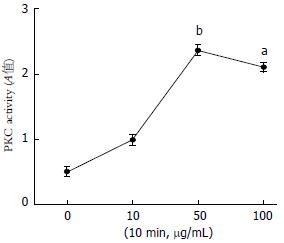
与对照组相比, AGEs在50 µg/mL和100 µg /mL时, 升高细胞内PKC活性(2.3600±0.0723、2.1060±0.0625 vs 0.5123±0.0614, P<0.05, P<0.01), 而AGEs在10 mg/mL时, 与对照组差异无统计学意义(图3).
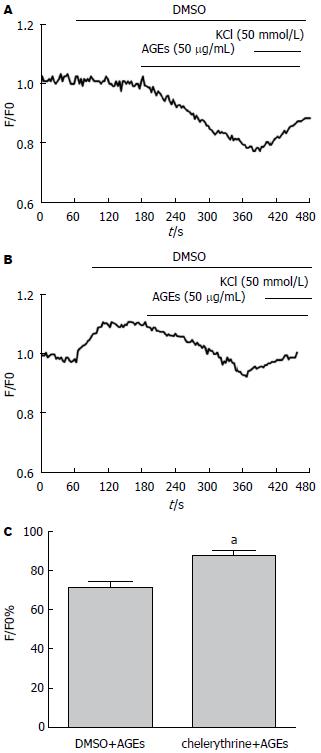
预先给予PKC抑制剂chelerythrine(1 µmol/L)可显著升高Fluo-3/AM负载的钙离子荧光强度, 说明细胞内钙离子浓度明显增加; 再加入AGEs后荧光强度下降, 但下降较溶剂对照组(DMSO, 1 µL)明显减少. 提示PKC抑制剂chelerythrine阻断AGEs介导的钙离子浓度降低(70.7%±3.7% vs 87.1%±2.5%, P<0.05)(图4).
本实验证实: AGEs可以影响细胞内钙离子浓度, 其通过活化PKC, 来降低细胞内钙离子浓度; 而PKC抑制剂chelerythrine则可阻断AGEs降低细胞内钙离子浓度的作用. 提示PKC参与了AGEs抑制大鼠结肠SMCs钙信号的作用, 减少钙离子释放[7,8].
细胞外钙内流和内钙释放是SMCs收缩的决定因素, 经典的钙致敏平滑肌收缩机制是: 钙离子与钙调蛋白结合, 再结合并激活肌球蛋白轻链激酶(myosin light chain kinase, MLCK), 激活的MLCK磷酸化肌球蛋白轻链(myosin light chain, MLC), 促进肌动蛋白和肌球蛋白之间的横桥周期、导致平滑肌收缩. 而肌球蛋白轻链磷酸酶(myosin light chain phosphase, MLCP)可使肌球蛋白轻链去磷酸化、导致平滑肌松弛[9,10].
研究发现PKC有多种亚型, 其中α、β、γ等亚型主要分布于胃肠SMC上, PKC可被DAG、Ca2+或磷脂激活, 参与调节细胞收缩、细胞增殖、代谢和凋亡等多种过程[11-13]. 生理条件下, 磷脂酶C(phospholipase C, PLC)激活, 水解4, 5-二磷酸磷脂酰肌醇(4, 5-phosphatidylinositol bisphosphate, PIP2)产生三磷酸肌醇(inositol triphosphate, InsP3)和甘油二酯(diaglycerides, DAG), InsP3与内质网上InsP3R结合、引起内质网钙离子释放, 细胞内钙离子浓度升高; 同时PKC活化负反馈调节钙离子的释放[14-16], PKC这种保护性负反馈机制一方面源于细胞内钙过度释放、导致内质网自身应急信号的激活, 另一方面可防止细胞内钙过度释放引起能量浪费和细胞损伤[17,18]; 该生理过程决定着多种生物学效应, 如钙依赖性酶和通道的激活, 细胞收缩等[19-21]. 另有文献报道, 其他一些蛋白激酶如PKG、PKA也能负反馈调节细胞内钙离子浓度, 发挥相应的生物学功能[22-24].
文献报道AGEs在DM患者血清及组织中比正常人明显升高, 他可通过氧化应激、糖基化修饰某些蛋白在DM并发症中起重要作用[25,26]. 本实验的新颖点: 从DM结肠SMCs病变入手, 探讨AGEs是否通过SMCs内钙离子途径、最终影响SMCs收缩的. 遗憾的是: AGEs作用受体及信号通路尚不明确(尤其是胃肠平滑肌AGEs的作用受体不明), 因此没有特异性受体拮抗剂[27-29], 不能直接证明AGEs降低SMCs内钙离子浓度的信号通路. 另外, 调节SMCs内钙离子的机制很多, AGEs是否影响细胞外钙离子内流? 是作用于胞膜上T型钙通道或者L型钙通道还是抑制内质网上InsP3R3或ryanodine受体释放钙离子[30]? 仍有待研究证实. 最后, SMCs内钙离子浓度的检测还可以结合流式细胞术及膜片钳技术多重证实.
总之, AGEs可以降低结肠SMC内钙离子浓度从而抑制收缩. AGEs激活PKC降低细胞内钙离子浓度, 是细胞内钙离子降低的重要因素. DM患者结肠动力障碍与患者血清及组织中AGEs增多相关, AGEs对结肠平滑肌是否有直接抑制作用, 以及AGEs降低结肠SMCs内钙离子的其他机制, 仍待研究.
糖尿病(diabetes mellitus, DM)胃肠动力障碍与胃肠自主神经、Cajal间质细胞及平滑肌细胞病变相关, 平滑肌病变在DM胃肠动力障碍中的研究已成为重点, 其中DM结肠平滑肌细胞内钙离子信号通路异常, 导致钙离子浓度异常, 是本病的病理基础之一.
刘长征, 副教授, 中国医学科学院基础医学研究所
糖基化终末产物(advanced glycation end products, AGEs)在DM患者血清及各种组织中均显著升高, 他与DM肾病、DM血管病变的研究颇多. 但AGEs与 DM结肠平滑肌病变相关迄今为止尚未报道.
国内外研究报道DM胃肠动力障碍存在胃肠道平滑肌病变, 且DM结肠平滑肌细胞内存在钙离子信号通路异常.
文本首次采用激光共聚焦的方法观测AGEs对结肠平滑肌细胞内钙离子浓度的影响及可能机制, 为DM胃肠道动力障碍研究提供依据.
本研究提示AGEs抑制结肠平滑肌细胞内钙离子浓度, 可能是DM胃肠动力障碍的相关机制, 为临床治疗DM胃肠动力障碍提供治疗靶点.
本文有一定的学术价值, 研究论点新颖, 为探索DM胃肠动力障碍、平滑肌病变提供了新思路.
编辑: 郭鹏 电编:鲁亚静
| 1. | Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131-18, e26. [PubMed] [DOI] |
| 2. | Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322. [PubMed] [DOI] |
| 3. | Neves D. Advanced glycation end-products: a common pathway in diabetes and age-related erectile dysfunction. Free Radic Res. 2013;47 Suppl 1:49-69. [PubMed] [DOI] |
| 4. | Zhao Z, Liu J, Shi B, He S, Yao X, Willcox MD. Advanced glycation end product (AGE) modified proteins in tears of diabetic patients. Mol Vis. 2010;16:1576-1584. [PubMed] |
| 5. | Touw K, Chakraborty S, Zhang W, Obukhov AG, Tune JD, Gunst SJ, Herring BP. Altered calcium signaling in colonic smooth muscle of type 1 diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G66-G76. [PubMed] [DOI] |
| 6. | Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230-44237. [PubMed] [DOI] |
| 7. | Yue C, Ku CY, Liu M, Simon MI, Sanborn BM. Molecular mechanism of the inhibition of phospholipase C beta 3 by protein kinase C. J Biol Chem. 2000;275:30220-30225. [PubMed] [DOI] |
| 8. | Codazzi F, Teruel MN, Meyer T. Control of astrocyte Ca(2+) oscillations and waves by oscillating translocation and activation of protein kinase C. Curr Biol. 2001;11:1089-1097. [PubMed] [DOI] |
| 9. | Gao N, Huang J, He W, Zhu M, Kamm KE, Stull JT. Signaling through myosin light chain kinase in smooth muscles. J Biol Chem. 2013;288:7596-7605. [PubMed] [DOI] |
| 10. | Ihara E, Chappellaz M, Turner SR, MacDonald JA. The contribution of protein kinase C and CPI-17 signaling pathways to hypercontractility in murine experimental colitis. Neurogastroenterol Motil. 2012;24:e15-e26. [PubMed] [DOI] |
| 11. | Young SH, Wu SV, Rozengurt E. Ca2+-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor require negative feedback by protein kinase C. J Biol Chem. 2002;277:46871-46876. [PubMed] [DOI] |
| 12. | Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C22-C33. [PubMed] |
| 13. | Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11-21. [PubMed] [DOI] |
| 14. | Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1, 4, 5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527-1530. [PubMed] |
| 15. | Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047-5061. [PubMed] [DOI] |
| 16. | Lo KJ, Luk HN, Chin TY, Chueh SH. Store depletion-induced calcium influx in rat cerebellar astrocytes. Br J Pharmacol. 2002;135:1383-1392. [PubMed] [DOI] |
| 17. | Montero M, Lobatón CD, Gutierrez-Fernández S, Moreno A, Alvarez J. Modulation of histamine-induced Ca2+ release by protein kinase C. Effects on cytosolic and mitochondrial [Ca2+] peaks. J Biol Chem. 2003;278:49972-49979. [PubMed] [DOI] |
| 18. | Hajnóczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun. 2003;304:445-454. [PubMed] [DOI] |
| 19. | Zhu J, Chen L, Xia H, Luo HS. Mechanisms mediating CCK-8S-induced contraction of proximal colon in guinea pigs. World J Gastroenterol. 2010;16:1076-1085. [PubMed] [DOI] |
| 20. | Gong YY, Si XM, Lin L, Lu J. Mechanisms of cholecystokinin-induced calcium mobilization in gastric antral interstitial cells of Cajal. World J Gastroenterol. 2012;18:7184-7193. [PubMed] [DOI] |
| 21. | Schöfl C, Börger J, Mader T, Waring M, von zur Mühlen A, Brabant G. Tolbutamide and diazoxide modulate phospholipase C-linked Ca(2+) signaling and insulin secretion in beta-cells. Am J Physiol Endocrinol Metab. 2000;278:E639-E647. [PubMed] |
| 22. | Murthy KS. cAMP inhibits IP(3)-dependent Ca(2+) release by preferential activation of cGMP-primed PKG. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1238-G1245. [PubMed] |
| 23. | Soulsby MD, Wojcikiewicz RJ. The type III inositol 1, 4, 5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites. Biochem J. 2005;392:493-497. [PubMed] [DOI] |
| 24. | Straub SV, Giovannucci DR, Bruce JI, Yule DI. A role for phosphorylation of inositol 1, 4, 5-trisphosphate receptors in defining calcium signals induced by Peptide agonists in pancreatic acinar cells. J Biol Chem. 2002;277:31949-31956. [PubMed] [DOI] |
| 25. | Fujimoto E, Kobayashi T, Fujimoto N, Akiyama M, Tajima S, Nagai R. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414. [PubMed] [DOI] |
| 26. | Wei Q, Ren X, Jiang Y, Jin H, Liu N, Li J. Advanced glycation end products accelerate rat vascular calcification through RAGE/oxidative stress. BMC Cardiovasc Disord. 2013;13:13. [PubMed] [DOI] |
| 27. | Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol. 2011;7:526-539. [PubMed] [DOI] |
| 28. | Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med (Berl). 2009;87:235-247. [PubMed] [DOI] |
| 29. | Fujita T. AGE/RAGE axis in the development of abdominal aortic aneurysm. Ann Surg. 2010;252:203-25; author reply 205. [PubMed] [DOI] |
| 30. | Dabertrand F, Nelson MT, Brayden JE. Ryanodine receptors, calcium signaling, and regulation of vascular tone in the cerebral parenchymal microcirculation. Microcirculation. 2013;20:307-316. [PubMed] [DOI] |