修回日期: 2011-01-25
接受日期: 2011-02-15
在线出版日期: 2011-04-08
目的: 研究姜辣素辅助昂丹司琼对顺铂所致大鼠急性期和延迟期异嗜行为的抑制作用.
方法: 大鼠随机分成正常对照组、模型组、昂丹司琼组、姜辣素组及姜辣素+昂丹司琼组, 记录顺铂注射后0-24 h和48-72 h各组大鼠摄食量及啃食高岭土量. 72 h后取延髓极后区及近端空肠组织, 免疫组织化学方法测定组织中P物质表达.
结果: 顺铂注射后0-24 h和48-72 h内, 姜辣素组、昂丹司琼+姜辣素组摄食量较模型组显著增加(t = 13.333, 15.000, 13.8333, 15.167, 均P<0.01), 啃食高岭土量显著减少(t = 9.122, 12.986, 8.525, 8.902, 均P<0.01); 姜辣素组和昂丹司琼+姜辣素组摄食量均显著高于昂丹司琼组(t = 8.000, 9.667, 11.500, 12.833, 均P<0.01); 昂丹司琼+姜辣素组啃食高岭土量显著低于昂丹司琼组(t = 2.499, 3.999, 均P<0.01); 姜辣素组啃食高岭土量在0-24 h内高于昂丹司琼组(t = 1.365, P<0.05), 48-72 h内低于昂丹司琼组(t = 3.622, P<0.01). 昂丹司琼+姜辣素组在0-24 h内啃食高岭土量显著低于姜辣素组(t = 3.865, P<0.01), 在48-72 h内啃食高岭土量及摄食量与姜辣素组均无显著差异(t = 0.377, 1.333, 均P>0.05). 姜辣素组及昂丹司琼+姜辣素组的AP区P物质免疫组织化学表达均少于昂丹司琼组(t = 2.217, 2.408, 均P<0.01).
结论: 姜辣素具有可促进大鼠摄食并可抑制顺铂所致的异嗜作用, 与昂丹司琼联用可有效抑制急性期和延迟期异嗜行为, 其机制可能与抑制P物质表达有关.
引文著录: 杨玉玲, 岳旺, 田字彬, 王茂龙, 杨志宏, 张琪, 魏良洲, 赵清喜, 石振艳. 姜辣素辅助昂丹司琼对顺铂所致大鼠异嗜行为的抑制作用. 世界华人消化杂志 2011; 19(10): 1034-1039
Revised: January 25, 2011
Accepted: February 15, 2011
Published online: April 8, 2011
AIM: To investigate whether ondansetron in combination with gingerol has suppressive effect on cisplatin-induced acute and delayed pica behavior in rats.
METHODS: Rats were divided randomly into blank control group, model control group, ondansetron group, gingerol group, and ondansetron plus gingerol group. Kaolin and food ingestion were recorded during 0-24 h and 48-72 h after cisplatin administration. At 72 h, the area postrema and jejunum tissue samples were taken to detect the immunoexpression of substance P by immunohistocheminstry.
RESULTS: During 0-24 h and 48-72 h after cisplatin administration, food ingestion increased significantly (t = 13.333, 15.000, 13.8333, 15.167, all P < 0.01) but kaolin ingestion decreased significantly (t = 9.122, 12.986, 8.525, 8.902, all P < 0.01) in the gingerol group and ondansetron plus gingerol group compared to the model control group. Compared to the ondansetron group, food ingestion was significantly higher in the gingerol group and ondansetron plus gingerol group (t = 8.000, 9.667, 11.500, 12.833, all P < 0.01), and kaolin ingestion was significantly lower in the ondansetron plus gingerol group (t = 2.499, 3.999, all P < 0.01). Kaolin ingestion was higher during 0-24 h (t = 1.365, P < 0.05) but lower during 48-72 h (t = 3.622, P < 0.01) in the gingerol group than in the ondansetron group. Compared to the gingerol group, kaolin ingestion decreased significantly in the ondansetron plus gingerol group during 0-24 h (t = 3.865, P < 0.01). No significant difference was noted in kaolin and food ingestion during 48-72 h between the ondansetron plus gingerol group and the gingerol group (t = 0.377, 1.333, all P > 0.05). The immunoexpression levels of substance P in the AP of gingerol group and ondansetron plus gingerol group were lower than that in the ondansetron group (t = 2.217, 2.408, all P < 0.01).
CONCLUSION: Gingerol is able to improve food ingestion and inhibit cisplatin-induced pica behavior in rats probably by restraining the immunoexpression of substance P. Ondansetron in combination gingerol is able to effectively inhibit cisplatin-induced acute and delayed pica in rats.
- Citation: Yang YL, Yue W, Tian ZB, Wang ML, Yang ZH, Zhang Q, Wei LZ, Zhao QX, Shi ZY. Ondansetron in combination with gingerol suppresses cisplatin-induced pica in rats. Shijie Huaren Xiaohua Zazhi 2011; 19(10): 1034-1039
- URL: https://www.wjgnet.com/1009-3079/full/v19/i10/1034.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v19.i10.1034
恶心呕吐是肿瘤化疗最常见不良反应之一, 按发生时间可分为预期性、急性和迟发性3大类[1]. 目前临床常用止吐药物5-HT3受体阻滞剂控制急性化疗性呕吐(chemotherapy-induced nausea and vomiting, CINV)效果显著, 但对于延迟性呕吐效果较差. 姜辣素(gingerol)是生姜的提取物, 其主要成分有[2]-、[4]-、[6]-姜酚和姜烯酚[2,3]等, 研究显示姜辣素可作用于多个呕吐相关受体, 具有良好的止吐效果[4]. 顺铂是强致吐性化疗药, 是呕吐模型的常用催吐剂. 大鼠无呕吐反射, 但顺铂注射后可出现啃食高岭土的异嗜行为, 其延髓背面极后区(the area postrema, AP)内具有与人类呕吐化学感受器触发区(chemoreceptor trigger zone, CTZ)相似的解剖结构, 可以产生条件味觉厌恶(conditioned taste aversion, CTA)反应, 故大鼠异嗜高岭土行为可以间接反映呕吐的程度[5]. 本研究利用顺铂所致大鼠异嗜模型, 探讨姜辣素及其与昂丹司琼联用对顺铂所致大鼠急性期和延迟期异嗜高岭土行为的抑制作用.
姜辣素(宝鸡宏远生物制药, 2005071223)溶于1%西黄芪胶; 顺铂(齐鲁制药, 903005CE)溶于50 ℃温生理盐水; 盐酸昂丹斯琼注射液(奥赛康制药, 090102); 兔抗P物质试剂盒(北京博奥森生物, bs-0064R).
1.2.1 动物实验: 健康♂成年wistar大鼠[华中科技大学实验动物中心, SCXK(鄂)2004-0007]30只, 质量200-250 g, 室温(22 ℃±2 ℃)单笼饲养, 12 h明暗周期(18:00-6:00为暗周期). 随机分成5组: 正常对照组(n = 6), 模型组(n = 6), 昂丹司琼组(n = 6), 姜辣素组(n = 6), 姜辣素+昂丹司琼组(n = 6). 除正常对照组外, 其余组大鼠用顺铂(3 mg/kg)腹腔注射(ip)造模, 并于顺铂注射前1 h及注射后24、48 h各组分别给予以下处理: 生理盐水(10 mg/kg, ip), 西黄芪胶(10 mg/kg, ip), 昂丹司琼(4 mg/kg, ip), 姜辣素(400 mg/kg, ip)[4], 姜辣素(400 mg/kg, ip)+昂丹司琼(4 mg/kg, ip); 观察顺铂注射后0-24 h、48-72 h各组大鼠摄食量和啃食高岭土量. 72 h后处死大鼠, 取AP区及近端空肠(距幽门约20 cm)组织于4%甲醛4 ℃固定.
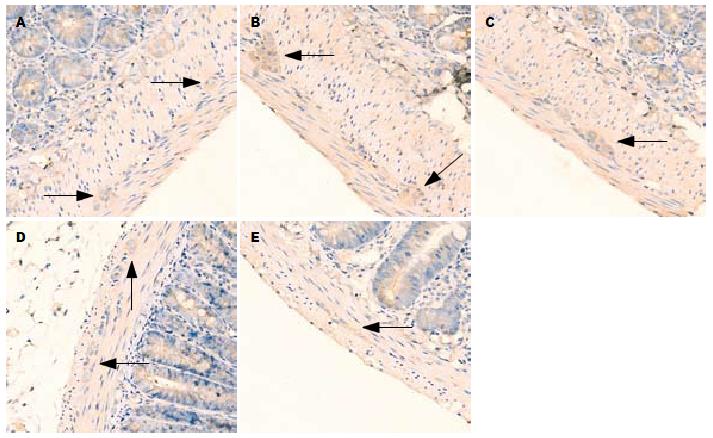
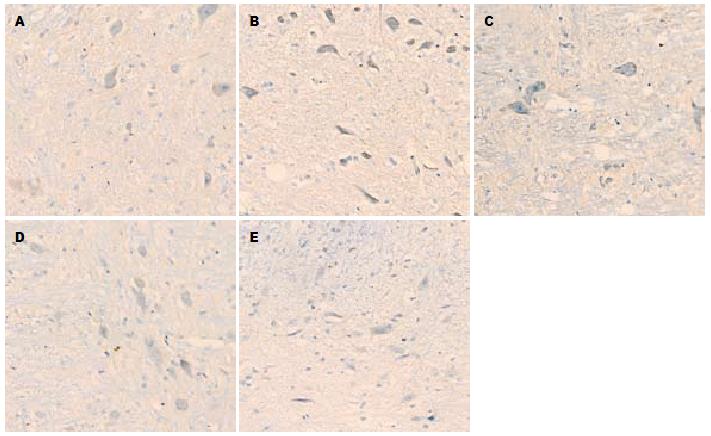
1.2.2 免疫组织化学和图像分析: 组织先后在20%蔗糖和30%蔗糖中, 4 ℃冰箱内脱水, 液氮中速冻1 min后切片(4 μm厚). 严格按照抗P物质(bs-0064R)免疫组织化学试剂盒说明操作. 将切片用pH7.4的PBS液室温下漂洗, 10%正常山羊血清液封闭, 加1:200稀释的兔多克隆P物质抗体, PBS液漂洗后滴加生物素化二抗, DAB显色剂显色, 苏木素复染, 无水酒精脱水, 二甲苯透明, 中性树胶封片. 200倍光镜下随机选取5个视野并照相, 观察外周空肠及AP区组织阳性产物的分布、染色情况. 黄光源下为浅黄色背景, 标记阳性颗粒在细胞质表达, 呈棕黄色或棕褐色. 对每例切片, 按Ferrier所采取半定量积分法[6]判断结果: A(按切片中阳性细胞占切片中总细胞数的比例评分): 未见阳性细胞为0分, 阳性细胞占整个视野细胞数的1%-25%为1分, 26%-50%为2分, 51%-75%为3分, 76%-100%为4分; B(按切片中阳性细胞显色有无及深浅评分): 无显色为0分, 淡黄色为1分, 棕黄色为2分, 棕褐色为3分. 每例积分 = A×B, 两者积分相乘结果作为阳性表达强度.
统计学处理 计量资料用mean±SD表示, 采用SPSS13.0统计分析软件, 应用单因素方差分析和LSD-t检验分析, 以P<0.05为差异有统计学意义.
模型组较正常对照组啃食高岭土量显著增多, 摄食量显著减少(均P<0.01). 昂丹司琼组、姜辣素组和昂丹司琼+姜辣素组啃食高岭土量较模型组均显著减少(均P<0.01). 姜辣素组啃食高岭土量多于昂丹司琼组(P<0.05); 昂丹司琼+姜辣素组啃食高岭土量明显低于昂丹司琼组、姜辣素组(均P<0.01). 昂丹司琼组摄食量多于模型组(P<0.05); 姜辣素组、昂丹司琼+姜辣素组摄食量明显多于模型组、昂丹司琼组(均P<0.01).姜辣素组摄食量与昂丹司琼+姜辣素组摄食量比较无显著性差异(P>0.05, 表1).
| 分组 | 0-24 h | 48-72 h | ||
| 高岭土量(g) | 摄食量(g) | 高岭土(g) | 摄食量(g) | |
| 正常对照组 | 0.352±0.267bd | 30.000±3.899bd | 0.319±0.178bd | 30.833±4.916bd |
| 模型组 | 14.270±1.677d | 14.167±3.971c | 9.706±1.775d | 16.333±3.386 |
| 昂丹司琼组 | 3.783±0.817b | 19.500±3.182a | 4.803±1.223b | 18.667±2.944 |
| 姜辣素组 | 5.148±1.184bc | 27.500±4.506bd | 1.182±0.368bd | 30.167±2.927bd |
| 昂丹司琼+姜辣素组 | 1.283±0.804bdf | 29.167±5.636bd | 0.804±0.442bd | 31.500±3.082bd |
模型组啃食高岭土量显著多于正常对照组(P<0.01)、摄食量显著低于正常对照组(P<0.01).昂丹司琼组、姜辣素组、昂丹司琼+姜辣素组啃食高岭土量较模型组均显著减少(均P<0.01).姜辣素组、昂丹司琼+姜辣素组啃食高岭土量显著低于昂丹司琼组(均P<0.01). 昂丹司琼组摄食量与模型组比较无显著性差异(P>0.05). 姜辣素组、昂丹司琼+姜辣素组摄食量明显多于模型组、昂丹司琼组(均P<0.01). 昂丹司琼+姜辣素组啃食高岭土量、摄食量与姜辣素组比较均无显著性差异(P>0.05, 表1).
P物质阳性颗粒在近端空肠组织表达于肌间神经节(图1), 在AP区表达于神经元(图2). 模型组空肠组织和AP区P物质免疫组织化学阳性积分均显著大于正常对照组(均P<0.01). 昂丹司琼组、姜辣素组和昂丹司琼+姜辣素组空肠组织和AP区的P物质免疫组织化学阳性积分均显著小于模型组(均P<0.01). 在空肠组织中, 昂丹司琼+姜辣素组的积分值小于昂丹司琼组(P<0.01)和姜辣素组(P<0.05), 而姜辣素组与昂丹司琼组相比无显著性差异(P>0.05). 在AP区组织中, 姜辣素组和昂丹司琼+姜辣素组的P物质免疫组织化学阳性积分均显著小于昂丹司琼组(均P<0.01), 姜辣素组和昂丹司琼+姜辣素组之间无显著性差异(P>0.05, 表2).
化疗药物(顺铂等)通过作用于多个位点诱发恶心呕吐反射, 化疗药物注射后, 产生多种自由基, 促使肠嗜铬细胞释放5-HT, 5-HT作用于位于肠壁迷走神经传入纤维末梢的5-HT3受体, 迷走神经传入纤维将产生的神经冲动上传至脑干背侧的孤束核和AP, 即迷走神经复合体背侧. 迷走神经复合体背侧多种神经递质受体均参与呕吐反射的发生, 如神经激肽-1(neurokinin-1, NK1)受体、5-HT3受体和多巴胺-2(dopamine-2, D2)受体[7]等; 迷走神经传出纤维自迷走神经复合体背侧出发将冲动传至呕吐中枢, 呕吐中枢支配效应器从而引发呕吐反射[8]. AP是脑室周围器官, 位于第4脑室两侧闩上方, 缺乏有效的血脑屏障, 可以感受血液和脑脊液的呕吐刺激信号, 因此称为呕吐化学感受器触发区(chemoreceptor trigger zone, CTZ)[9]. CINV按发生时间不同可分为预期性、急性和迟发性三大类[1]. 急性恶心呕吐主要发生于化疗开始后24 h内, 24 h后的恶心呕吐称为延迟性恶心呕吐[10](顺铂所致的恶心呕吐主要发生在48-72 h, 且对常用的5-HT3受体阻滞剂反应不敏感[11]). 急性CINV的发生主要与肠道嗜铬细胞释放的5-HT有关[12], 昂丹司琼即竞争性结合5-HT3受体抑制5-HT的作用而发挥抗呕吐作用. 20世纪90年代发现神经激肽P物质及其受体NK1在延迟性CINV中具有重要作用[11,13], NK1受体阻滞剂治疗延迟性CINV效果良好[14,15].
生姜作为传统中药已有一千多年的历史, 有"止呕圣药"之美誉, 动物实验[16,17]和临床研究[18,19]显示, 生姜可有效改善顺铂引起的恶心呕吐. 生姜的有效止呕成分为姜辣素[3], 其抗呕吐机制最初认为为阻断5-HT3受体[20,21], 后证实其并非与5-HT3受体竞争性结合, 而是作用于一个调节性位点, 此位点除可抑制5-HT3受体活性外, 还可调节P物质受体和胆碱能受体活性[22]; 另有研究证实姜辣素可作用于辣椒素受体发挥抗呕吐作用[23]. 因此, 姜辣素可能通过作用于多种受体发挥抗呕吐作用. 因大鼠异嗜高岭土行为可以间接反映呕吐的程度, 本研究利用顺铂所致大鼠异嗜模型, 探讨姜辣素及其与昂丹司琼联用对顺铂所致大鼠急性期和延迟期异嗜高岭土行为的抑制作用, 结果显示, 姜辣素在0-24 h内对大鼠异嗜行为虽有抑制作用, 但其作用弱于昂丹司琼, 而48-72 h内的作用强于昂丹司琼; 昂丹司琼与姜辣素联用后在0-24 h内抑制异嗜行为效果强于两者单一用药, 在48-72 h内强于单用昂丹司琼, 而与单用姜辣素无明显差异, 说明姜辣素可能对大鼠急性(相当于本实验的0-24 h)和延迟性(相当于本实验的48-72 h)异嗜行为均有抑制作用, 抑制延迟性异嗜行为效果优于昂丹司琼. 推测姜辣素及与昂丹司琼联用可能有效缓解顺铂所致急性和延迟性呕吐. 免疫组织化学染色显示, 姜辣素组和昂丹司琼+姜辣素组空肠和AP组织中P物质表达较昂丹司琼组减少, 说明姜辣素可能通过抑制P物质表达发挥抗异嗜行为的作用. 本研究还显示, 姜辣素组大鼠的摄食量均多于模型组和昂丹司琼组, 而昂丹司琼+姜辣素组和姜辣素组比较摄食量无明显差异, 说明姜辣素除具有抗异嗜行为外, 还具有促进大鼠摄食的作用, 而昂丹司琼则无促进大鼠摄食作用. 推测姜辣素的抗呕吐及促摄食作用可能与其改善胃排空障碍、增加胃动力及促进食欲有关[24-27].
恶心、呕吐严重影响肿瘤患者化疗效果和生存质量, 目前临床常用止吐药物作用受体单一、价格昂贵, 故亟待开发一种高效、廉价的抗呕吐药物. 生姜是传统中药中的"止呕圣药", 已有一千多年的历史, 《伤寒杂病论》中止呕方剂中一半以上含有生姜, 姜辣素是其主要止呕成分.
张俊, 副教授, 上海交通大学医学院附属瑞金医院外科
Ryan等通过对600多例肿瘤化疗患者的随机对照双盲试验, 提出生姜止吐效果良好; Abdil-Aziz等研究证明姜辣素的止呕机制不仅限作用于5-HT3受体; 钱秋海等证实姜辣素可作用于5-HT3受体、NK1受体及D2受体.
将昂丹司琼和姜辣素联用, 在增强抑制急性期CINV效果的同时, 可弥补昂丹司琼对延迟期CINV无效的缺点, 并可促进摄食, 保证机体营养摄入.
姜辣素可作为辅助用药与昂丹司琼联用, 增强急性期止吐效果, 并对抗延迟期呕吐, 同时增加摄食, 以保证营养供应. 生姜价格低廉, 姜辣素提取工艺简单, 无不良反应, 可作为常用药物开发应用.
本文思路清晰, 方法准确, 对指导临床化疗相关呕吐的治疗有一定的意义.
编辑: 李薇 电编:李薇
| 1. | Licitra L, Spinazzé S, Roila F. Antiemetic therapy. Crit Rev Oncol Hematol. 2002;43:93-101. [PubMed] [DOI] |
| 2. | Kawai T, Kinoshita K, Koyama K, Takahashi K. Anti-emetic principles of Magnolia obovata bark and Zingiber officinale rhizome. Planta Med. 1994;60:17-20. [PubMed] [DOI] |
| 3. | Yang Y, Kinoshita K, Koyama K, Takahashi K, Kondo S, Watanabe K. Structure-antiemetic-activity of some diarylheptanoids and their analogues. Phytomedicine. 2002;9:146-152. [PubMed] [DOI] |
| 4. | Qian QH, Yue W, Wang YX, Yang ZH, Liu ZT, Chen WH. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch Pharm Res. 2009;32:565-573. [PubMed] [DOI] |
| 5. | Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45:817-821. [PubMed] [DOI] |
| 6. | Ferrier CM, de Witte HH, Straatman H, van Tienoven DH, van Geloof WL, Rietveld FJ, Sweep CG, Ruiter DJ, van Muijen GN. Comparison of immunohistochemistry with immunoassay (ELISA) for the detection of components of the plasminogen activation system in human tumour tissue. Br J Cancer. 1999;79:1534-1541. [PubMed] [DOI] |
| 8. | Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482-2494. [PubMed] [DOI] |
| 9. | McCarthy LE, Borison HL. Cisplatin-induced vomiting eliminated by ablation of the area postrema in cats. Cancer Treat Rep. 1984;68:401-404. [PubMed] |
| 10. | Antonarakis ES, Hain RD. Nausea and vomiting associated with cancer chemotherapy: drug management in theory and in practice. Arch Dis Child. 2004;89:877-880. [PubMed] [DOI] |
| 11. | Kris MG, Roila F, De Mulder PH, Marty M. Delayed emesis following anticancer chemotherapy. Support Care Cancer. 1998;6:228-232. [PubMed] [DOI] |
| 12. | Endo T, Minami M, Hirafuji M, Ogawa T, Akita K, Nemoto M, Saito H, Yoshioka M, Parvez SH. Neurochemistry and neuropharmacology of emesis - the role of serotonin. Toxicology. 2000;153:189-201. [PubMed] [DOI] |
| 13. | Girish C, Manikandan S. Aprepitant: a substance P antagonist for chemotherapy induced nausea and vomiting. Indian J Cancer. 2007;44:25-30. [PubMed] [DOI] |
| 14. | Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090-3098. [PubMed] [DOI] |
| 15. | Fang ZW, Zhai SD. [A meta-analysis of aprepitant for prevention of chemotherapy-induced nausea and vomiting]. Beijing Daxue Xuebao. 2010;42:756-763. [PubMed] |
| 16. | Yamahara J, Rong HQ, Naitoh Y, Kitani T, Fujimura H. Inhibition of cytotoxic drug-induced vomiting in suncus by a ginger constituent. J Ethnopharmacol. 1989;27:353-355. [PubMed] [DOI] |
| 17. | Haksar A, Sharma A, Chawla R, Kumar R, Arora R, Singh S, Prasad J, Gupta M, Tripathi RP, Arora MP. Zingiber officinale exhibits behavioral radioprotection against radiation-induced CTA in a gender-specific manner. Pharmacol Biochem Behav. 2006;84:179-188. [PubMed] [DOI] |
| 18. | Hickok JT, Roscoe JA, Morrow GR, Ryan JL. A Phase II/III Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Ginger (Zingiber officinale) for Nausea Caused by Chemotherapy for Cancer: A Currently Accruing URCC CCOP Cancer Control Study. Support Cancer Ther. 2007;4:247-250. [PubMed] [DOI] |
| 19. | Zick SM, Ruffin MT, Lee J, Normolle DP, Siden R, Alrawi S, Brenner DE. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer. 2009;17:563-572. [PubMed] [DOI] |
| 20. | Sharma SS, Kochupillai V, Gupta SK, Seth SD, Gupta YK. Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J Ethnopharmacol. 1997;57:93-96. [PubMed] [DOI] |
| 21. | Abdel-Aziz H, Nahrstedt A, Petereit F, Windeck T, Ploch M, Verspohl EJ. 5-HT3 receptor blocking activity of arylalkanes isolated from the rhizome of Zingiber officinale. Planta Med. 2005;71:609-616. [PubMed] [DOI] |
| 22. | Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006;530:136-143. [PubMed] [DOI] |
| 23. | Borrelli F, Capasso R, Pinto A, Izzo AA. Inhibitory effect of ginger (Zingiber officinale) on rat ileal motility in vitro. Life Sci. 2004;74:2889-2896. [PubMed] [DOI] |
| 24. | Sharma SS, Gupta YK. Reversal of cisplatin-induced delay in gastric emptying in rats by ginger (Zingiber officinale). J Ethnopharmacol. 1998;62:49-55. [PubMed] [DOI] |
| 25. | Wu KL, Rayner CK, Chuah SK, Changchien CS, Lu SN, Chiu YC, Chiu KW, Lee CM. Effects of ginger on gastric emptying and motility in healthy humans. Eur J Gastroenterol Hepatol. 2008;20:436-440. [PubMed] [DOI] |
| 26. | Wadikar DD, Nanjappa C, Premavalli KS, Bawa AS. Development of ginger based ready-to-eat appetizers by response surface methodology. Appetite. 2010;55:76-83. [PubMed] [DOI] |
| 27. | Stewart JJ, Wood MJ, Wood CD, Mims ME. Effects of ginger on motion sickness susceptibility and gastric function. Pharmacology. 1991;42:111-120. [PubMed] [DOI] |