修回日期: 2009-03-09
接受日期: 2009-03-23
在线出版日期: 2009-04-28
目的: 观察骨髓间充质干细胞(BMSCs)移植通过抑制RhoA-ROCK信号转导通路对大鼠急性肝损伤修复过程的影响.
方法: 贴壁筛选法培养、纯化♂SD大鼠BMSCs. 将健康♀SD大鼠随机分为3组: 正常对照组(N组, n = 10)、CCl4组(C组, n = 10)及CCl4+BMSCs组(T组, n = 10). N组不予任何处理; T组和C组腹腔注射0.1 mL/100 g 600 mL/L CCl4花生油溶液制造急性肝损伤模型后24 h, 分别经鼠尾静脉移植的间充质干细胞和等量PBS. 各组于不同时间点留取标本, 采用HE染色和血清肝功能酶学指标观察受损肝脏恢复过程; RT-PCR方法检测RhoA mRNA的表达; Western blot检测RhoA蛋白的表达.
结果: 与C组相比, T组移植BMSCs后能显著改善CCl4急性损伤大鼠肝功能(1 d, ALT: 89.70±3.09 U/L vs 147.59±6.83 U/L, AST: 263.67±17.05 U/L vs 472.68±19.04 U/L, P<0.01或0.05; 7 d, ALT: 42.38±14.31 U/L vs 92.75±6.70 U/L, AST 173.85±16.80 U/L vs 260.41±25.35 U/L, 均P<0.05), 并迅速修复肝脏结构. N组大鼠肝脏RhoA mRNA和蛋白表达量极低, C组经CCl4损伤后RhoA mRNA和蛋白表达量迅速增加(1.39±0.046 vs 0.57±0.010, 1.23±0.020 vs 0.35±0.036, 均P<0.01), 此后表达量缓慢降低. T组经BMSCs移植后, 与C组比较, RhoA mRNA和蛋白表达水平迅速下降.
结论: Rho-ROCK信号转导通路参与CCl4导致的急性肝损伤发生、发展和修复全过程. BMSCs可能通过抑制RhoA-ROCK信号转导通路加速受损肝脏修复.
引文著录: 梁昌宇, 覃山羽, 姜海行, 王东旭, 苏思标, 梁梓宇. 骨髓间充质干细胞对大鼠急性肝损伤修复的影响. 世界华人消化杂志 2009; 17(12): 1178-1184
Revised: March 9, 2009
Accepted: March 23, 2009
Published online: April 28, 2009
AIM: To observe the effects of bone marrow mesenchymal stem cells (BMSCs) on restoration of acute liver injury induced by carbon tetrachloride (CCl4) in rats via RhoA-ROCK signaling pathway.
METHODS: BMSCs were isolated from male Sprague-Dawley (SD) rats, cultured and purified in a BMSCs culture system in vitro using their adherent characteristics. Rats were divided into 3 groups: normal control (N, n = 10), CCl4 (C group for short hereafter, n = 10) and CCl4 plus BMSCs (T group for short hereafter, n = 10). All the rats were sampled at the appointed time and various target markers were determined as follows: hematoxylin and eosin (HE) staining, as well as the index changes of liver's enzymology, were applied for evaluating the improvement of hepatic histological damage. The expression of RhoA mRNA was determined by reverse transcription-polymerase chain reaction (RT-PCR), and the expression of RhoA protein was assayed by Western blot.
RESULTS: In the T group, liver function of CCl4-induced acute hepatic injury after BMSCs transplantation was improved markedly as compared with the C group (1 d, ALT: 89.70 ± 3.09 vs 147.59 ± 6.83; AST: 263.67 ± 17.05 vs 472.68 ± 19.04, P < 0.01 or 0.05; 7 d, ALT: 42.38 ± 14.31 vs 92.75 ± 6.70; AST: 173.85 ± 16.80 vs 260.41 ± 25.35, all P < 0.05), consistent with the improvement of hepatic histology. Little expression of RhoA was detected in the normal control group. In the C group, both mRNA and protein of RhoA were increased significantly as compared with normal control (1.39 ± 0.046 vs 0.57 ± 0.010, 1.23 ± 0.020 vs 0.35 ± 0.036, both P < 0.01), then was decreased slowly. In the T groups, the expression mRNA and protein of RhoA were obviously decreased, in concomitant with the restoration of liver histology and function, as compared with C group.
CONCLUSION: Rho-ROCK signaling pathways are involved in the process of acute hepatic injury in rats induced by CCl4. BMSCs transplantation may accelerate the restoration of acute liver injury by inhibition of RhoA-ROCK signaling pathway.
- Citation: Liang CY, Qin SY, Jiang HX, Wang DX, Su SB, Liang ZY. Effects of bone marrow stem cell transplantation on acute hepatic injury induced by carbon tetrachloride in rats. Shijie Huaren Xiaohua Zazhi 2009; 17(12): 1178-1184
- URL: https://www.wjgnet.com/1009-3079/full/v17/i12/1178.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i12.1178
干细胞移植作为治疗终末期肝病的手段, 已经越来越引起临床的关注[1-3]. 骨髓间充质干细胞(bone marrow mesenchymal stem cells, BMSCs)是骨髓内的一种非造血干细胞[4-5], 既有自我复制和高度增殖的能力[6-7], 又有多向分化的潜能[8-10], 可作为干细胞移植的重要细胞来源, 但目前干细胞移植的治疗机制尚不清楚. 小分子 Rho GTP酶家族是相对分子质量为(20-30)×103的Ras超家族成员, 根据其序列和功能可分为3个亚族, 即Rho(包括RhoA、RhoB和RhoC等)、Rac(包括Rac1、Rac2和Rac3等)和Cdc42(包括CDC42、TC10和TCL等)[11]. RhoA是Rho信号通路最重要的信号分子, 激活后的RhoA能调节影响包括细胞骨架的重构、转录因子激活等许多生物学效应. RhoA主要通过下游效应分子Rho相关激酶(Rho-associated kinase, ROCK)发挥作用. 激活状态的RhoA与ROCK结合, 进一步磷酸化ROCK下游底物 Myosin phosphatase target subunit 1(MYPT1), 使肌浆内肌球蛋白轻链(myosin light chain, MLC)磷酸化水平升高, 从而调节细胞的多种行为[11-12]. 近期研究发现, Rho-ROCK信号转导通路与肝脏的慢性炎症和纤维化、缺血再灌注损伤等有密切关系[13-16]. 我们通过移植同种异基因BMSCs, 检测干细胞移植后大鼠肝脏损伤的恢复程度, 以及RhoA mRNA和蛋白的表达, 初步探讨BMSCs通过抑制Rho-ROCK信号转导通路修复肝脏损伤的机制.
SD♂大鼠10只, ♀大鼠30只, 体质量150-180 g(广西医科大学实验动物中心); DMEM-LG培养液(美国Gibco公司); 特级胎牛血清(美国Hyclone公司); 小鼠抗RhoA mAb(美国Santa Cruz公司); TRIzol(美国Invitrogen公司); 逆转录试剂盒(美国MBI公司).
1.2.1 造模及分组: 新鲜配制的浓度为600 mL/L CCl4花生油溶液, 剂量为0.1 mL/100 g, 腹腔注射入♀大鼠体内, 制造急性肝损伤模型, 并随机分为CCl4(C)组和CCl4+BMSCs(T)组, 每组各10只. 另10只健康♀大鼠为正常对照(N)组.
1.2.2 BMSCs的分离与培养: 按文献方法[17]在无菌条件下分离SD♂大鼠股骨骨髓细胞, 于37℃、饱和湿度、50 mL/L CO2培养箱中培养, 利用BMSCs与其他贴壁细胞的贴壁差异性严格控制传代时胰酶的量和消化时间, 传代纯化BMSCs, 倒置相差显微镜下观察细胞形态. 取第4代细胞(Passege4, P4), 2.5 g/L胰酶消化, PBS重悬细胞, 调整细胞浓度为1012个/L.
1.2.3 BMSCs移植: 造模后24 h内T组经鼠尾静脉移植109个(1 mL)BMSCs; C组经鼠尾静脉移植等量PBS; N组不予任何处理. 各组分别于术后第1、3、7、14和30天等眼球取血行肝功能检测(ALT和AST). 颈椎脱臼法处死大鼠, 留取肝脏组织分别行HE染色等检查.
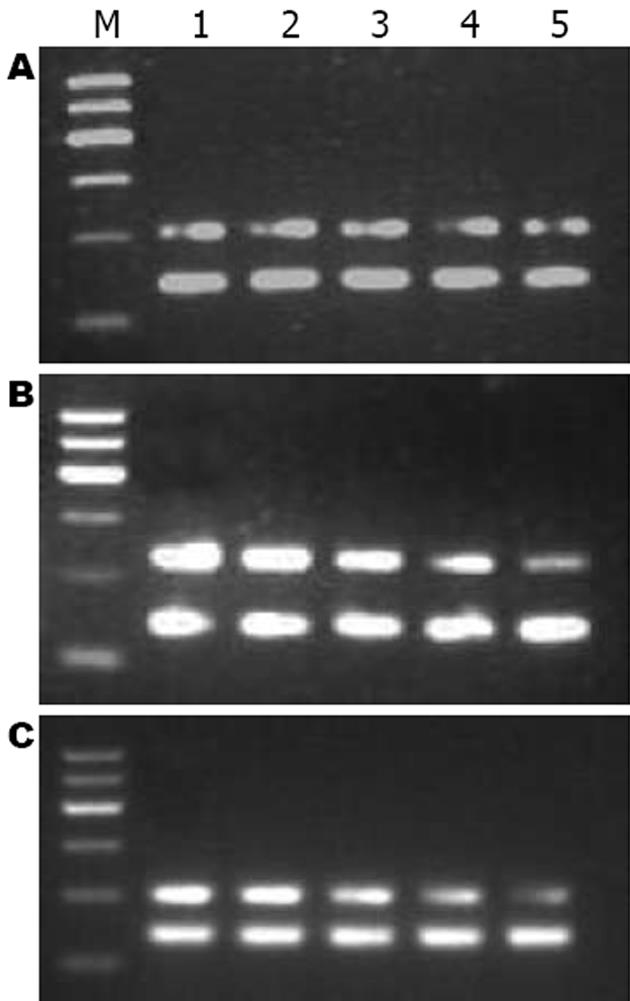
1.2.4 大鼠肝组织总RNA提取和逆转录酶-聚合酶链反应(RT-PCR): 冰上称取-80℃冻存的肝组织0.1 g, TRIzol一步抽提法提取总RNA. 按逆转录试剂盒说明书进行逆转录, 并根据以下条件进行目的基因的扩增: 95℃预变性5 min进入循环, 95℃变性45 s, 55℃退火45 s, 72℃ 1 min, 共35个循环后, 72℃延伸5 min, 以GAPDH为内参照. 扩增引物由上海生工生物工程公司合成. RhoA的上游引物(205 bp)5'-TGGTGATGGAGCTTGTGGTAAG-3', 下游引物5'-AACATCAGTGTCTGGGTAGGAG-3'. GAPDH上游引物(140 bp): 5'-GCCAGTAGACTCCACGACAT-3', 下游引物5'-GCAAGTTCAACGGCACAG-3'. 取6 μL PCR产物及6 μL DNA Marker进行17 g/L琼脂糖凝胶电泳, 采用凝胶图像分析仪进行吸光度扫描, 观察条带的灰度强弱, 以RhoA/GAPDH的灰度比值表示相对RhoA mRNA水平.
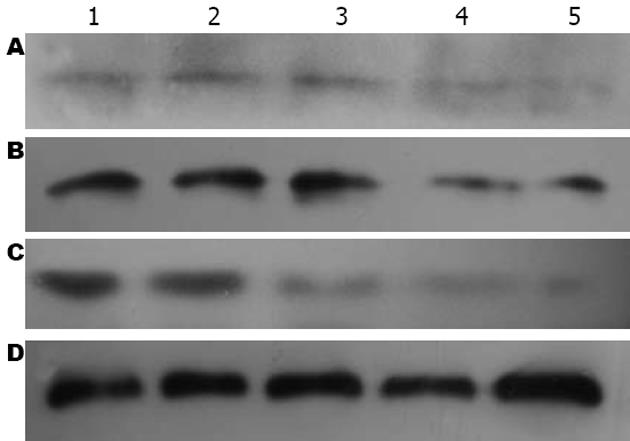
1.2.5 Western blot检测: 用细胞裂解液提取各组肝组织总蛋白, 考马斯亮蓝比色法测定蛋白含量, 上样量为100 μg, 蛋白进行120 g/L SDS-PAGE凝胶电泳, NC转膜, 非特异性封闭; 加入一抗小鼠抗RhoA mAb(1∶500稀释)、4℃过夜, 加入辣根过氧化物酶标记的二抗进行杂交. ECL发光剂1 min, 曝光、显影、定影. 数码成像分析系统软件对结果进行分析, 以RhoA/GAPDH的灰度比值表示相对RhoA蛋白水平.
统计学处理 数据资料以mean±SD表示并应用统计软件SPSS11.5进行分析, 以P<0.05为有统计学差异, P<0.01为有显著性差异.
接种后24 h后首次换液,可见少量类圆形细胞贴壁生长. 原代培养3 d后,镜下可见单个或少量呈集落生长的贴壁细胞, 形态大多呈短梭形; 7-10 d后, 细胞集落不断扩大并形成融合单层, 细胞形态大多呈长梭形或多角形; 传至P4, BMSCs逐渐纯化, 类似成纤维细胞, 呈旋涡状生长.
大鼠在造模后均反应迟钝, 饮食及活动减少, 毛发蓬乱, 尿色加深. C组3 d后逐渐好转, 活动增多, 饮食良好, 体质量逐渐增加, 14 d后与正常大鼠无异. T组在移植BMSCs后24 h即逐渐好转, 活动增多, 正常饮食饮水, 体质量回升.
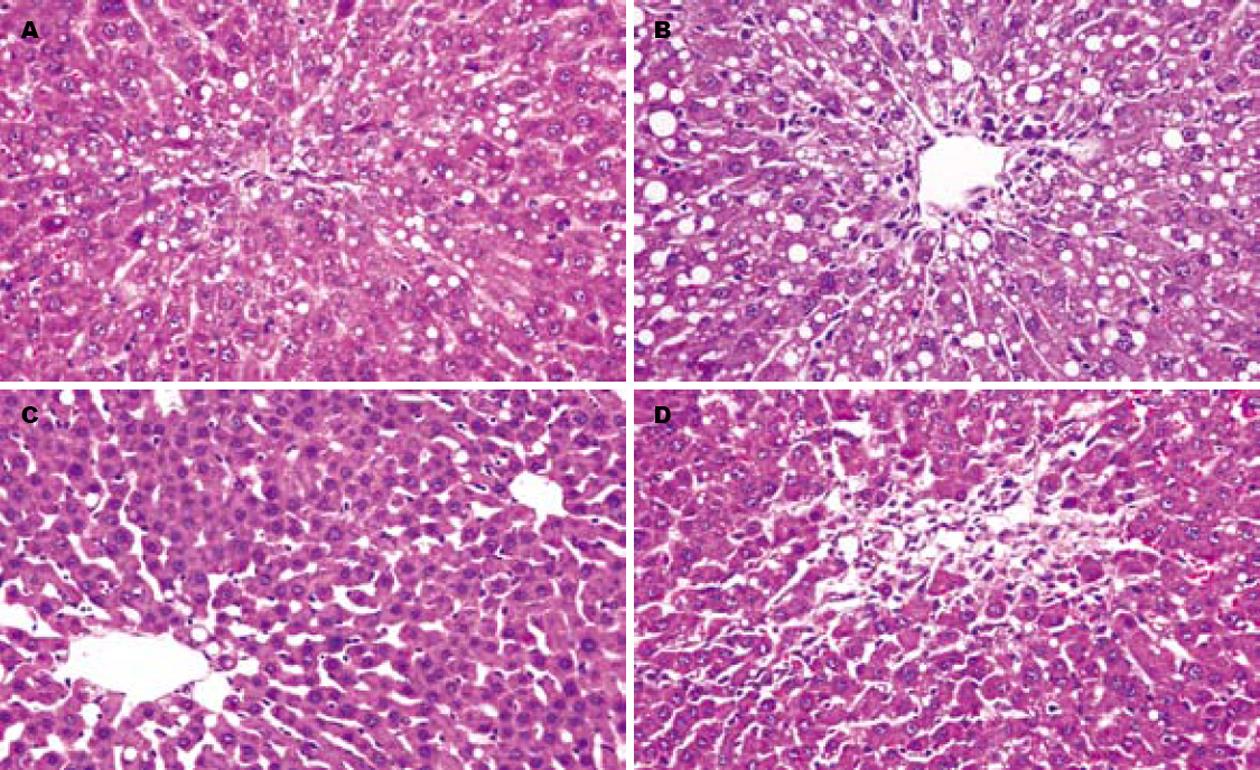
正常对照组大鼠肝脏表面光滑, 质地柔软, 呈褐色, 光泽明显. HE染色后镜下观察: 腺泡结构清楚, 汇管区清晰, 肝细胞排列呈条索状, 围绕中央静脉呈放射状排列. CCl4腹腔注射后24 h常规病理HE染色显示为弥漫性肝小叶中心带肝细胞气球样变或脂肪变性, 中央静脉周围肝细胞内出现大量的脂肪滴, 可见炎性细胞浸润. 随着观察时间的推移, 上述组织学变化均好转, T组较C组更早更快的恢复, 7 d时肝脏结构完全恢复正常; C组7 d时仍可见脂滴或气球样变, 14 d才恢复正常肝脏组织结构(图1).
造模后大鼠血清的ALT和AST均明显升高, T组和C组相比, 在3 d和7 d时间点ALT和AST明显好转, 2组间比较均有统计学意义; 14 d后, 两组比较没有统计学意义(表1-2).
CCl4造成肝损伤后24 h, C组RhoA mRNA表达(1.39±0.046)较N组(0.57±0.010)显著上调(P<0.01), 此后缓慢下降, 至14 d时才恢复正常水平. T组RhoA mRNA在1、3和7 d时间点较C组显著下降(P<0.01, 表3, 图2), 7 d时已降至正常.
急性肝损伤以短期内大量肝细胞变性、坏死和炎性细胞浸润等为特征, 临床常见有病毒性、药物性、免疫损伤性及酒精性肝损伤. CCl4药物性肝损伤因操作简单、重复率高、费用低廉等特点, 成为应用最广泛的经典模型[18-19]. 6-24 h是CCl4引发肝脏损伤阶段, 而48 h-2 wk则是肝脏损伤后进行自我修复的阶段[20]. 故本研究将移植时间定为损伤后24 h内, 观察时间长度定为30 d, 以便更好地将移植细胞的修复作用与肝脏的自身修复进行比较.
国内外的实验均证实, 干细胞移植后在体内微环境[21-23,25]、细胞生长因子[21,23-24,27]、细胞外基质[26-27]等多种因素的共同作用下, 不仅可以分化为肝细胞和胆管上皮细胞, 还有对受损肝细胞的修复功能, 这为以后临床上治疗终末期肝病提供了一个新的选择. 本实验中, BMSCs经鼠尾静脉移植后可循血流方向进入受损肝脏, 并向肝索、肝窦内迁移和定植, 伴随着肝功能的恢复以及肝脏结构的重建与好转, 与以往报道结果一致[17,28]. BMSCs移植后肝功能较早出现了好转, 肝组织结构在短期内并未见到明显的改善, 但最终7 d内完全修复了肝脏的正常结构.
近年研究显示, Rho-ROCK信号通路在肝损伤过程中发挥重要作用. Shiotani et al[15-16]报道Rho-ROCK通路调节肝细胞内的NADPH氧化酶(NADPH oxidase)和核因子-κB(nuclear factor kappa B, NF-κB)等信号转导途径, 介导活性氧(reactive oxygen species, ROS)、TNF-α和IL-1β等炎症因子生成, 加重肝脏移植的超急性排斥反应. Thorlacius et al[29]报道Rho-ROCK信号通路介导内毒素性肝损伤的白细胞浸润, 促使内皮细胞、吞噬细胞和枯否细胞(Kupffer cells)合成及释放TNF-α和CXC趋化因子等炎症介质, 并且诱导肝细胞凋亡. Murata et al[13]报道抑制Rho-ROCK信号通路活化可以阻断肝星状细胞(hepatic stellate cell, HSC)增殖、收缩和迁移, 抑制胶原合成, 促进HSC凋亡等使肝纤维化得以改善或逆转, 同时也可以减轻肝细胞损伤. Ikeda et al[30]通过体外实验证明Rho-ROCK信号通路被Rho-ROCK特异性抑制剂阻断后, 可活化磷脂酰肌醇-3-激酶(phosphatidylinositol 3-kinase, PI3K)/Akt信号通路, 下调Caspase-3活性, 增强Bcl-2表达, 抑制肝脏实质细胞损伤和/或凋亡. 以上证据均表明, Rho-ROCK信号转导通路在炎症介质和细胞因子所介导的细胞损伤中发挥重要作用.
我们的研究表明, 正常大鼠的肝脏组织RhoA mRNA表达微弱, 蛋白表达大多为阴性. CCl4造成肝脏损伤后, RhoA mRNA和蛋白质表达水平明显升高, RhoA-ROCK信号通路被激活, 可能引发ROS、TNF-α、IL-1β和/或细胞凋亡等介导细胞炎性损伤, 从而使肝脏组织细胞损伤和血清ALT和AST水平升高. 而BMSCs移植组可见RhoA mRNA和蛋白质表达减少, 与CCl4损伤组相比, 肝组织结构恢复及肝功能恢复明显加快, 说明RhoA-ROCK信号通路受抑制, 其机制之一可能是BMSCs抑制肝脏损伤时RhoA的激活, 减轻促炎症因子生成、减少血管活性物质活化和氧化应激作用, 抑制肝细胞凋亡, 加速肝脏的恢复过程, 因而BMSCs具有类似Rho-ROCK信号通路抑制剂的作用. 此外, 抑制RhoA活性能调节肌动蛋白聚合与延伸, 促使移植细胞向受损脏器迁移与分化[31-32], 可能对加速肝脏组织结构与功能恢复也起到一定作用. 然而Rho-ROCK信号通路调节以及与其他信号通路的交叉反应极为复杂, 进一步研究该通路, 对于阐明干细胞修复组织损伤机制和建立新的治疗策略具有深远意义.
干细胞移植在治疗终末期肝病方面具有良好的应用前景, BMSCs因其不可替代的多种优势而被认为是最具治疗潜力的供体细胞, 但其治疗机制尚不明确.
张国梁, 主任医师, 天津市第一中心医院消化内科
近年来, BMSCs在肝脏疾病中的应用研究日益增多, 但大多致力于治疗效果的观察和评价, 对其作用机制的研究较少.
Ikeda et al报道Rho-ROCK信号通路被特异性抑制剂阻断后, 可抑制肝脏实质细胞损伤和/或凋亡, Rho-ROCK通路特异性抑制剂对CCl4诱导的急性肝损伤有保护作用.
本文首次研究BMSCs抑制RhoA-ROCK信号转导通路激活加速肝脏修复, 这可能是BMSCs参与肝损伤修复的作用机制之一.
本实验研究RhoA在CCl4诱导的急性肝损伤及BMSCs移植后肝脏修复过程中表达水平的变化, 说明BMSCs抑制RhoA-ROCK信号转导通路激活加速肝脏修复, 为干细胞移植治疗肝脏疾病提供新的理论依据.
本研究设计严谨、合理, 结果可靠, 很好地反映了我国和国际此方面研究的先进水平.
编辑: 李军亮 电编:何基才
| 1. | Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, Rashed LA, Sabry D, Hassouna AA, Hasan NM. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007;40:893-899. [PubMed] [DOI] |
| 2. | Miyazaki M, Hardjo M, Masaka T, Tomiyama K, Mahmut N, Medina RJ, Niida A, Sonegawa H, Du G, Yong R. Isolation of a bone marrow-derived stem cell line with high proliferation potential and its application for preventing acute fatal liver failure. Stem Cells. 2007;25:2855-2863. [PubMed] [DOI] |
| 3. | Khurana S, Mukhopadhyay A. Characterization of the potential subpopulation of bone marrow cells involved in the repair of injured liver tissue. Stem Cells. 2007;25:1439-1447. [PubMed] [DOI] |
| 4. | Koide Y, Morikawa S, Mabuchi Y, Muguruma Y, Hiratsu E, Hasegawa K, Kobayashi M, Ando K, Kinjo K, Okano H. Two distinct stem cell lineages in murine bone marrow. Stem Cells. 2007;25:1213-1221. [PubMed] [DOI] |
| 5. | Miura Y, Gao Z, Miura M, Seo BM, Sonoyama W, Chen W, Gronthos S, Zhang L, Shi S. Mesenchymal stem cell-organized bone marrow elements: an alternative hematopoietic progenitor resource. Stem Cells. 2006;24:2428-2436. [PubMed] [DOI] |
| 6. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [PubMed] [DOI] |
| 7. | Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;249-282. [PubMed] |
| 8. | Wang X, Hisha H, Taketani S, Adachi Y, Li Q, Cui W, Cui Y, Wang J, Song C, Mizokami T. Characterization of mesenchymal stem cells isolated from mouse fetal bone marrow. Stem Cells. 2006;24:482-493. [PubMed] [DOI] |
| 9. | Lysy PA, Campard D, Smets F, Malaise J, Mourad M, Najimi M, Sokal EM. Persistence of a chimerical phenotype after hepatocyte differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 2008;41:36-58. [PubMed] |
| 10. | Li W, Liu SN, Luo DD, Zhao L, Zeng LL, Zhang SL, Li SL. Differentiation of hepatocytoid cell induced from whole-bone-marrow method isolated rat myeloid mesenchymal stem cells. World J Gastroenterol. 2006;12:4866-4869. [PubMed] |
| 12. | Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118-124. [PubMed] [DOI] |
| 13. | Murata T, Arii S, Mori A, Imamura M. Therapeutic significance of Y-27632, a Rho-kinase inhibitor, on the established liver fibrosis. J Surg Res. 2003;114:64-71. [PubMed] [DOI] |
| 14. | Kitamura K, Tada S, Nakamoto N, Toda K, Horikawa H, Kurita S, Tsunematsu S, Kumagai N, Ishii H, Saito H. Rho/Rho kinase is a key enzyme system involved in the angiotensin II signaling pathway of liver fibrosis and steatosis. J Gastroenterol Hepatol. 2007;22:2022-2033. [PubMed] [DOI] |
| 15. | Shiotani S, Shimada M, Suehiro T, Soejima Y, Yosizumi T, Shimokawa H, Maehara Y. Involvement of Rho-kinase in cold ischemia-reperfusion injury after liver transplantation in rats. Transplantation. 2004;78:375-382. [PubMed] [DOI] |
| 16. | Shiotani S, Shimada M, Taketomi A, Soejima Y, Yoshizumi T, Hashimoto K, Shimokawa H, Maehara Y. Rho-kinase as a novel gene therapeutic target in treatment of cold ischemia/reperfusion-induced acute lethal liver injury: effect on hepatocellular NADPH oxidase system. Gene Ther. 2007;14:1425-1433. [PubMed] [DOI] |
| 18. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [PubMed] [DOI] |
| 19. | Sipes IG, el Sisi AE, Sim WW, Mobley SA, Earnest DL. Reactive oxygen species in the progression of CCl4-induced liver injury. Adv Exp Med Biol. 1991;283:489-497. [PubMed] |
| 20. | Chung H, Hong DP, Jung JY, Kim HJ, Jang KS, Sheen YY, Ahn JI, Lee YS, Kong G. Comprehensive analysis of differential gene expression profiles on carbon tetrachloride-induced rat liver injury and regeneration. Toxicol Appl Pharmacol. 2005;206:27-42. [PubMed] [DOI] |
| 21. | Xu J, Deng X, Demetriou AA, Farkas DL, Hui T, Wang C. Factors released from cholestatic rat livers possibly involved in inducing bone marrow hepatic stem cell priming. Stem Cells Dev. 2008;17:143-155. [PubMed] [DOI] |
| 22. | Lemoli RM, Catani L, Talarico S, Loggi E, Gramenzi A, Baccarani U, Fogli M, Grazi GL, Aluigi M, Marzocchi G. Mobilization of bone marrow-derived hematopoietic and endothelial stem cells after orthotopic liver transplantation and liver resection. Stem Cells. 2006;24:2817-2825. [PubMed] [DOI] |
| 23. | Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892-1903. [PubMed] [DOI] |
| 24. | Neuss S, Becher E, Wöltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405-414. [PubMed] [DOI] |
| 25. | Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J Gastroenterol. 2005;11:4497-4504. [PubMed] |
| 26. | Chen SS, Fitzgerald W, Zimmerberg J, Kleinman HK, Margolis L. Cell-cell and cell-extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553-561. [PubMed] [DOI] |
| 27. | Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254-1264. [PubMed] [DOI] |
| 29. | Thorlacius K, Slotta JE, Laschke MW, Wang Y, Menger MD, Jeppsson B, Thorlacius H. Protective effect of fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte recruitment, and hepatocellular apoptosis in septic liver injury. J Leukoc Biol. 2006;79:923-931. [PubMed] [DOI] |
| 30. | Ikeda H, Kume Y, Tejima K, Tomiya T, Nishikawa T, Watanabe N, Ohtomo N, Arai M, Arai C, Omata M. Rho-kinase inhibitor prevents hepatocyte damage in acute liver injury induced by carbon tetrachloride in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G911-G917. [PubMed] [DOI] |
| 31. | Ghiaur G, Lee A, Bailey J, Cancelas JA, Zheng Y, Williams DA. Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment. Blood. 2006;108:2087-2094. [PubMed] [DOI] |
| 32. | Jaganathan BG, Ruester B, Dressel L, Stein S, Grez M, Seifried E, Henschler R. Rho inhibition induces migration of mesenchymal stromal cells. Stem Cells. 2007;25:1966-1974. [PubMed] [DOI] |