修回日期: 2007-12-28
接受日期: 2008-01-10
在线出版日期: 2008-01-18
目的: 探讨骨髓形成肝细胞的分子机制.
方法: 采用移植♂骨髓的♀小鼠模型,♀Balb/c小鼠随机分为模型组和正常对照组, 选用小鼠基因表达谱寡核苷酸芯片, 利用反转录酶合成掺有荧光标记的cDNA探针, 将探针与基因表达谱芯片杂交观察小鼠肝组织基因表达谱的变化, 筛选样本之间杂交信号比值有差异表达的基因. 采用生物信息学方法分析模型组中小鼠肝组织再生相关基因的信号通路变化规律.
结果: ♀小鼠在移植♂骨髓6 mo后肝组织的基因表达谱显著不同, 对照组相对于正常组的差异表达基因有865条, 已知功能基因有447条, 其中上调基因92条, 下调基因355条. 与肝再生相关基因信号通路涉及HGF, TGF-β, Focal Adhesion, JAK-Stat, VEGF等信号通路, 他们相关基因的上调表达促进肝细胞的增殖分化. TGF-β信号通路中相关基因下调, 抑制信号通路的激活, 减弱肝再生的负性作用, 利于肝细胞的增殖分化.
结论: ♀小鼠移植♂骨髓后的肝组织基因表达谱显著变化, 有可能通过其肝再生相关基因的信号通路激活或抑制调节肝再生.
引文著录: 李晶津, 李瀚旻, 高翔, 晏雪生. 移植雄性骨髓的雌性小鼠肝组织肝再生相关基因的信号通路. 世界华人消化杂志 2008; 16(2): 150-155
Revised: December 28, 2007
Accepted: January 10, 2008
Published online: January 18, 2008
AIM: To probe into the mechanism underlying the transformation of bone marrow into hepatic cells.
METHODS: Inter-sexual bone marrow transplantation models were induced by transplanting bone marrow from male mice into the liver cells from female mice. Female Balb/C mice were randomly divided model group and normal control group. The oligo-necleotide acid chip with mouse gene expression spectrum was selected, and reverse transcription enzyme was used to synthesize the fluorescence-labeled cDNA probe. The probe and gene expression microchip were hybridized to observe changes of gene expression in the liver issues, and genes with a different hybrid signal ratio were selected and the changes of liver regeneration-related gene pathways in female mice transplanted bone marrow from male mice were analyzed.
RESULTS: A significance difference in the expression of genes was found in liver tissue from the female mice transplanted bone marrow from male mice after 6 mo between the model and normal control groups. Eight hundred and sixty-five genes had different expressions, including 447 recognized functional genes, of which 92 were up-regulated genes and 355 down-regulated genes. The up-regulated genes involving the signal pathways of HGF, TGF-β, focal adhesion, JAK-Stat and VEGF could promote the proliferation and differentiation of hepatic cells. The down-regulated genes could inhibit the activation of TGF-β signaling pathways and the negative effect of liver regeneration, thus contributing to the proliferation and differentiation of liver cells.
CONCLUSION: The gene expression spectrum is remarkably changed in liver tissue from female mice transplanted bone marrow from male mice by activating gene signal pathways and inhibiting liver regeneration.
- Citation: Li JJ, Li HM, Gao X, Yan XS. Liver regeneration-related gene signaling pathways in female mice transplanted bone marrow from male mice. Shijie Huaren Xiaohua Zazhi 2008; 16(2): 150-155
- URL: https://www.wjgnet.com/1009-3079/full/v16/i2/150.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i2.150
鉴于有关人类胚胎干细胞的研究受到伦理道德和法律的限制, 成体干细胞的研究越来越受到重视. 最近的许多研究证明, 骨髓中的干细胞在特定环境下可分化成为多种组织细胞[1], 骨髓干细胞向肝细胞横向分化的潜能已得到肯定[2-8]. 但骨髓形成肝细胞的分子机制, 特别是有关这一过程的相关基因的信号转导通路尚缺乏系统深入的研究. 我们利用移植♂骨髓的♀小鼠模型(骨髓干细胞转化肝细胞的经典动物模型), 采用小鼠基因表达谱寡核苷酸芯片技术, 在探讨肝再生过程中基因表达谱变化规律的基础上, 对♀小鼠移植♂骨髓后肝组织相关基因的信号通路进行分析.
Balb/c小鼠, 4周龄, SPF级, 湖北省预防医学科学院实验动物中心提供, 动管证号: SCXK(鄂)2003-0005. ♀小鼠随机分为两组, A组为对照组(正常♀小鼠), B组为移植♂骨髓的♀小鼠, 每组40只, 骨髓移植术前1 wk起饮水中加入红霉素(250 mg/L)和庆大霉素(320 mg/L)以清洁消化道. Cy5 dCTP、Cy3 dCTP (Amersham); RNA inhibitor(TaKaRa); QIAquick Nucleotide Removal Kit(Qiagen); 小鼠16K v1.0基因表达谱寡核苷酸芯片由上海生物芯片公司提供. UVP杂交炉; Agilent扫描仪; Agilent 2100 Bioanalyzer.
♂小鼠颈椎脱臼处死, 750 mL/L乙醇浸泡10 min, 无菌条件下取股骨、剪开干骺端, 用5 mL注射器吸取RPMI-1640液0.5 mL冲出骨髓, 过6号针头3次、4号针头两次制成单个细胞悬液, 记数并调整细胞密度为1×1010/L. ♀小鼠接受60Co源γ射线全身照射(总剂量9 Gy), 然后经尾静脉输入制备好的♂小鼠骨髓细胞悬液0.2 mL(约2×106个细胞). 移植♂小鼠骨髓的♀小鼠饲养于SPF级动物房, 饮水中加入红霉素(250 mg/L)和庆大霉素(320 mg/L), 每天更换垫料和饮水, 垫料、饲料和饮水均经高温消毒. 骨髓移植6 mo后, 颈椎脱臼处死小鼠, 无菌条件下切取肝组织用液氮速冻后保存于-80 ℃待测.
肝组织标本抽提并纯化总RNA后完成如下操作. 在cDNA第1链合成过程中, 通过反转录酶将CyDye标记核苷酸直接掺入到cDNA链中制备探针荧光探针. 使用QIAquick Nucleotide Removal Kit纯化荧光探针. 将纯化好的探针转入酶标板, 分别测定A260、A550、A650以定量, Cy3 probe(pmol) = A550×洗脱体积/0.15, Cy5 probe (pmol) = A650×洗脱体积/0.25. 将探针吸回PCR管, 真空加热抽干, 避光保存于-20 ℃, 待杂交. 移植♂小鼠骨髓的♀小鼠(Cy3标记)与正常♀小鼠(Cy5标记)合做芯片. 洗涤盖玻片, 用50 mL离心管分别配制ddH2O、950 mL/L乙醇、ddH2O并置于沸水中加热, 将盖玻片依次放入ddH2O、950 mL/L乙醇、ddH2O, 各3 min, 最后放入50 mL离心管中1000 r/min离心3 min, 去除残留的水渍, 放置备用. 用纯水配制PBS溶液, 杂交前加入适量到杂交盒内以保持杂交体系100%的湿度. 平衡杂交炉, 用水平仪校准杂交炉, 保持水平, 确保杂交芯片的均一性. 使用Agilent Scanner获取图像, 利用Split-tiff软件和图像分析软件Imagene, 确定杂交点的范围, 过滤背景噪音, 提取得到基因表达的荧光信号强度值. 将所得数据导入分析软件Genespring进行数据标准化处理, 计算得到Ratio值(即两种荧光Cy3与Cy5的比值), Ratio值≤0.5为基因表达水平下调, Ratio≥2为基因表达水平上调. 甄选出肝再生相关的差异表达的基因, 登陆http://www.genome.jp/京都基因和基因组百科全书(KEGG)的网页, 搜取相关基因的资料, 并得出相关信号通路的图示, 供分析肝再生相关信号通路和其中差异表达的基因.
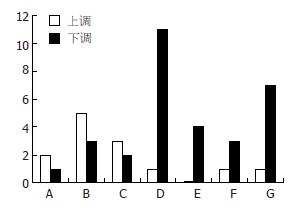
在所检测的基因中, 对照组相对于正常♀小鼠的差异表达基因有865条, 已知功能基因有447条, 其中上调基因92条、下调基因355条(图1-2).
涉及肝再生相关的信号通路主要有细胞凋亡信号通路、Focal Adhesion通路、MAPK信号通路、WNT信号转导通路、Toll样受体信号通路、JAK-Stat信号通路、VEGF信号通路、TGF-β通路的基因. 主要有45条差异表达基因, 其中含不同信号通路中相同的基因(图3, 表1).
| 通路名称 | 信号比值 | Gene-symbol | Gene-description |
| Apoptosis | 0.367 | NFKB2 | Nuclear factor of kappa light polypeptidegene enhancer in B-cells 2, p49/p100 |
| 2.951 | MAP3K14 | Mitogen-activated protein kinase kinase kinase 14 | |
| 2.733 | PIK3RL | Phosphatidylinositol 3-kinase, regulatory subunit, | |
| polypeptide 1(p85 alpha) | |||
| Cytokine-cytokine receptor | 0.481 | BMP2 | Bone morphogenetic protein 2 |
| interaction | 0.25 | TGFβ2 | Transforming growth factor, beta 2 |
| 2.136 | HGF | Hepatocyte growth factor | |
| 2.001 | VEGFC | Vascular endothelial growth factor C | |
| 0.231 | IL12Rβ2 | nterleukin 12 receptor, beta 2 | |
| 2.001 | CSF3R | Colony stimulating factor 3 receptor (granulocyte) | |
| 2.026 | IL6Rα | Interleukin 6 receptor, alpha | |
| 0.493 | IL13 | Interleukin 13 | |
| 2.05 | NGFR | Nerve growth factor receptor | |
| Jak-Stat signaling pathway | 2.733 | PIK3RL | Phosphatidylinositol 3-kinase, regulatory subunit, polypeptide 1(p85 alpha) |
| 2.001 | CSF3R | Colony stimulating factor 3 receptor(granulocyte) | |
| 0.231 | IL12Rβ2 | Interleukin 12 receptor, beta 2 | |
| 2.026 | IL6Rα | Interleukin 6 receptor, alpha | |
| 0.493 | IL13 | Interleukin 13 | |
| MAPK signaling pathway | 0.475 | PPM1B | Protein phosphatase 1B, Magnesium dependent, beta isoform |
| 0.298 | PLA2GIB | Phospholipase A2, group IB, pancreas | |
| 0.367 | NFKB2 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2, p49/p100 | |
| 2.951 | MAP3K14 | Mitogen-activated protein kinase kinasekinase 14 | |
| 0.463 | PRKCβ | Protein kinase C, beta | |
| 0.442 | MAP3K6 | Mitogen-activated protein kinase kinase kinase 6 | |
| 0.25 | TGFβ2 | Transforming growth factor, beta 2 | |
| 0.376 | MAPK8IP2 | Mitogen-activated protein kinase 8 interacting protein 2 | |
| 0.238 | HSPA5 | Heat shock 70 kDa protein 5(glucose-regulated protein, 78 kDa) | |
| 0.338 | HSPA8 | Heat shock 70 kDa protein 8 | |
| 0.35 | HSPA1l | Heat shock 70 kDa protein 1-like | |
| 0.263 | FOS | FBJ osteosarcoma oncogene | |
| TGF-beta signaling pathway | 0.25 | TGFβ2 | Transforming growth factor, beta 2 |
| 0.429 | DCN | Decorin | |
| 0.481 | BMP2 | Bone morphogenetic protein 2 | |
| 0.429 | BMP4 | Bone morphogenetic protein 4 | |
| Toll-like receptor signaling | 0.367 | NFKB2 | Nuclear factor of kappa light polypeptidegene enhancer in |
| pathway | B-cells 2, p49/p100 | ||
| 0.263 | FOS | FBJ osteosarcoma oncogene | |
| 0.395 | TIRAP | Toll-interleukin 1 receptor(TIR)domain-containing adaptor protein | |
| 2.733 | PIK3RL | Phosphatidylinositol 3-kinase, regulatory subunit, polypeptide 1(p85 alpha) | |
| WNT signaling pathway | 0.463 | PRKCβ | Protein kinase C, beta |
| 0.479 | WNT4 | Wingless-related MMTV integration site 4 | |
| 0.332 | FOSL1 | Fos-like antigen 1 | |
| 2.357 | CSNK1α1 | Casein kinase 1, alpha 1 | |
| 0.436 | 2410091N08RIK | RIKEN cDNA 2410091N08 gene | |
| 0.334 | FRATL | Frequently rearranged in advanced T-cell lymphomas | |
| 0.263 | CSNKLE | Casein kinase 1, epsilon | |
| 0.262 | LRP5 | Low density lipoprotein receptor-related protein 5 |
在♀小鼠移植♂骨髓后, 其体内骨髓形成肝细胞这一过程可以分为骨髓细胞迁移至肝脏和骨髓细胞在肝脏分化为肝细胞两个阶段, 从骨髓干细胞的相关研究可以发现能够引起骨髓干细胞动员和归巢的信号主要有间质细胞源性因子1(stromal cell derived factor-1, SDF-1)及其受体CXCR4、干细胞因子(stem cell factor, SCF)及其受体c-kit、集落刺激因子(colony stimulating factor, CSF)、血管内皮生长因子(vascular endothelial growth factor, VEGF)、整合素等[9]. 脏器损伤(本实验为致死性放射性损伤, 包括肝损伤)可以引起一系列信号释放, 刺激骨髓动员多种干细胞迁移至病变处, 进而分化为相应的组织细胞进行自然的代偿性修复, 而通过各种途径引入体内的外源性骨髓干细胞也可接受上述信号的刺激, 定向归巢至损伤处, 发挥增殖修复作用. 移植♂骨髓的♀小鼠模型的肝组织中G-CSF3, TNF-α, IL-6α, NGF等细胞因子/生长因子的受体表达上调. CSF是刺激骨髓干细胞动员和归巢的信号之一, 主要包括G-CSF、粒细胞巨噬细胞集落刺激因子(granulocyte macrophage colony stimulating factor, GM-CSF), G-CSF广泛用于干细胞的动员和归巢, 但其中机制尚不明确; GM-CSF可引导人CD34+造血祖细胞骨髓归巢, 还能增加骨髓内皮前体细胞和肌源性前体细胞的动员. TNF-α主要由活化的单核巨噬细胞产生, 抗原刺激的T淋巴细胞、活化的NK细胞和肥大细胞等也能分泌, 其生物学活性取决于浓度的高低, 低浓度的TNF-α作用于局部的白细胞和血管内皮细胞中, 诱发炎症反应; 而高浓度的TNF-α则可进入血流引起全身反应. IL-6主要由单核细胞、淋巴细胞、成纤维细胞产生, 是B淋巴细胞终末分化因子、造血前体细胞克隆刺激因子, 对早期的造血干细胞的增殖分化起重要作用, 能刺激粒系和巨噬系造血祖细胞集落的形成, 能促进巨核细胞的增殖分化而加速血小板的再生[10].
♀小鼠移植♂骨髓后, 其肝组织与肝再生相关基因涉及多条信号通路. 其中HGF表达上调, TGF-β表达下调, MAPK和WNT与肝再生相关的经典信号通路的相关基因表达下调. HGF是一种多功能因子, 参与组织再生、肿瘤入侵和多种细胞修复过程[11-16]. HGF的受体c-Met具有酪氨酸激酶活性, 能介导各种HGF信号的传递. 蛋白激酶C/A的激活、cAMP浓度的升高、细胞因子和炎症因子都可促进HGF的的表达. HGF与其受体结合并激活受体上的PTK后, 受体Y1349VHV及Y1356VNV序列中的Tyr首先自身磷酸化, 该磷酸化序列被含SH2或SH3区蛋白质, 如PI23K所识别, 使二者短暂结合, 受体PTK进而使之磷酸化. 这些靶蛋白中的磷酸化Tyr序列再被其他含SH2或SH3区的蛋白质所识别. 通过这种识别和结合机制, 几条信号通路同时被启动, 经瀑布式的磷酸化反应, 将信号逐级放大, 最终传到细胞核内的转录机构, 导致细胞的增殖分化[17-19]. ♀小鼠移植♂骨髓后, 其肝组织HGF表达上调, 提示通过激活其信号通路, 有利于肝组织细胞的增殖分化.
TGF-β通路是正常肝再生的负性调控通路, 肝再生失调时TGF-β通过Smads通路激活HSC, 促进胶原蛋白基因表达, 使ECM合成、沉积而促进肝纤维化[20-28]. TGF-β与受体TGFβR结合后使Smad2、Smad3磷酸化, 随即与Smad4形成异源寡聚体复合物, 转入胞核与相应转录因子作用, 调节靶基因的转录, 促进HSC增殖, 产生胶原, 同时抑制ECM降解, 加速肝纤维化的发展. 在信号传递的过程中, Smad7发挥负反馈的调节作用, 他与Smad2/3竞争性地与TGFβR1结合, 抑制Smad2/3的磷酸化及其下游的信号转导[29]. ♀小鼠移植♂骨髓后, 其肝组织TGF-β呈下调表达, 提示可通过抑制TGF-β信号通路激活的复杂作用而有利于肝再生(包括骨髓干细胞转化为肝细胞的机制). MAPK和WNT与肝再生相关的经典信号通路的相关基因表达下调, 其可能的作用机制和意义尚需进一步研究.
本文围绕肝再生这一重大而关键的科学问题, 在前期研究的基础上, 研究骨髓移植后放射性肝损伤的肝再生相关基因信号通路的变化规律.
潘兴华, 副主任医师, 成都军区昆明总医院病理实验科
本文利用移植♂骨髓的♀小鼠模型, 采用基因芯片技术, 在探讨肝再生过程中基因表达谱变化规律的基础上, 对♀小鼠移植♂骨髓后肝组织肝再生相关基因的信号通路进行分析, 为研究骨髓移植治疗肝损伤提供科学依据.
本文研究骨髓干细胞转化肝细胞的分子机制, 为揭示肝再生的调控机制, 为临床应用骨髓干细胞移植治疗肝损伤奠定坚实的实验基础.
本文研究MSC移植治疗肝损伤及其机制有重要意义, 对同行有一定参考价值.
编辑: 程剑侠 电编:郭海丽
| 2. | Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433-445. [PubMed] |
| 3. | Omori N, Omori M, Evarts RP, Teramoto T, Miller MJ, Hoang TN, Thorgeirsson SS. Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver regeneration in the adult rat. Hepatology. 1997;26:720-727. [PubMed] |
| 4. | Omori M, Omori N, Evarts RP, Teramoto T, Thorgeirsson SS. Coexpression of flt-3 ligand/flt-3 and SCF/c-kit signal transduction system in bile-duct-ligated SI and W mice. Am J Pathol. 1997;150:1179-1187. [PubMed] |
| 5. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [PubMed] |
| 6. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [PubMed] |
| 7. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [PubMed] |
| 10. | Patchen ML, MacVittie TJ, Williams JL, Schwartz GN, Souza LM. Administration of interleukin-6 stimulates multilineage hematopoiesis and accelerates recovery from radiation-induced hematopoietic depression. Blood. 1991;77:472-480. [PubMed] |
| 11. | Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160-169. [PubMed] |
| 12. | Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500-504. [PubMed] |
| 13. | Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156-164. [PubMed] |
| 14. | Miyazaki M, Akiyama I, Sakaguchi M, Nakashima E, Okada M, Kataoka K, Huh NH. Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem Biophys Res Commun. 2002;298:24-30. [PubMed] |
| 15. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [PubMed] |
| 16. | 唐 力军, 高 毅, 张 志, 李 浩, 单 毓强. HGF+FGF-体外定向4诱导人骨髓来源的多能成体祖细胞向肝样细胞分化的特征性表型鉴定. 解放军医学杂志. 2004;29:973-976. |
| 17. | Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, Barbacid M, Sabe H, Hanafusa H, Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777-2785. [PubMed] |
| 18. | Mayer BJ, Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993;3:8-13. [PubMed] |
| 19. | Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261-271. [PubMed] |
| 20. | Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548-557. [PubMed] |
| 21. | Shek FW, Benyon RC. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur J Gastroenterol Hepatol. 2004;16:123-126. [PubMed] |
| 22. | Nakamura T, Ueno T, Sakamoto M, Sakata R, Torimura T, Hashimoto O, Ueno H, Sata M. Suppression of transforming growth factor-beta results in upregulation of transcription of regeneration factors after chronic liver injury. J Hepatol. 2004;41:974-982. [PubMed] |
| 23. | Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34:89-100. [PubMed] |
| 24. | Zhang LH, Pan JP, Yao HP, Sun WJ, Xia DJ, Wang QQ, He L, Wang J, Cao X. Intrasplenic transplantation of IL-18 gene-modified hepatocytes: an effective approach to reverse hepatic fibrosis in schistosomiasis through induction of dominant Th1 response. Gene Ther. 2001;8:1333-1342. [PubMed] |
| 25. | Zhang L, Mi J, Yu Y, Yao H, Chen H, Li M, Cao X. IFN-gamma gene therapy by intrasplenic hepatocyte transplantation: a novel strategy for reversing hepatic fibrosis in Schistosoma japonicum-infected mice. Parasite Immunol. 2001;23:11-17. [PubMed] |
| 26. | Mignon A, Guidotti JE, Mitchell C, Fabre M, Wernet A, De La Coste A, Soubrane O, Gilgenkrantz H, Kahn A. Selective repopulation of normal mouse liver by Fas/CD95-resistant hepatocytes. Nat Med. 1998;4:1185-1188. [PubMed] |
| 27. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-807. [PubMed] |
| 28. | Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol. 2002;71:731-740. [PubMed] |