修回日期: 2007-08-24
接受日期: 2007-09-01
在线出版日期: 2007-09-08
急性胰腺炎(acute pancreatitis, AP)是一种常见的急重症, 经研究证实多种细胞因子的活化与AP的发生进展关系密切. 而Toll样受体4(Toll-like receptor 4, TLR4)、核因子-κB(nuclear factor kappa B, NF-κB)与这些细胞因子的活化密切相关. 他们在AP发生发展中作为介导炎症反应的枢纽起着重要作用. 以往认为TLR4触发细胞内信号传导通路, 从而激活NF-κB, 调控大量细胞因子释放, 介导炎性反应. 目前对TLR4/NF-κB信号通路具体的传导路径及参与因子又有了新的认识, 本文概述了TLR4/NF-κB信号通路, 且概述了TLR4/NF-κB信号通路相关下调因子、增强因子, 及能激活NF-κB的其他因子.
引文著录: 王昆宁, 徐敏. TLR4, NF-κB与急性胰腺炎. 世界华人消化杂志 2007; 15(25): 2684-2689
Revised: August 24, 2007
Accepted: September 1, 2007
Published online: September 8, 2007
Acute pancreatitis (AP) is a serious commonly-occurring disease. Toll-like receptor 4 (TLR4) and nuclear factor kappa B (NF-κB) are closely related to the activation of many cytokines that have important roles in the occurrence and development of AP. It is already acknowledged that TLR4 and NF-κB have roles in the pathogenesis of acute necrotizing pancreatitis (ANP) because they are essential in the inducing and mediating of inflammation. It is thought that TLR4 induces LPS signaling, which leads to the activation and translocation of NF-κB, and then stimulates the production of proinflammatory cytokines that result in the occurrence of inflammation. Recently, however, new concepts about the specific signaling pathway of TLR4/NF-κB and the factors participating in it have been proposed. This review summarizes the TLR4/NF-κB signaling pathway and outlines the factors that can down- or up-regulate TLR4/NF-κB expression and other factors that activate NF-κB.
- Citation: Wang KN, Xu M. Relationship of toll-like receptor 4, nuclear factor kappa B and acute pancreatitis. Shijie Huaren Xiaohua Zazhi 2007; 15(25): 2684-2689
- URL: https://www.wjgnet.com/1009-3079/full/v15/i25/2684.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i25.2684
急性胰腺炎(acute pancreatitis, AP)是一种常见的急重症, 发病率较高, 尤其是急性坏死性胰腺炎(acute necrotizing pancreatitis, ANP), 病死率可高达30%[1]. 经研究证实多种细胞因子如TNF-α、IL-1β、IL-6等的活化与急性胰腺炎的发生进展关系密切[2]. 而Toll样受体4(Toll-like receptor 4, TLR4)、核因子-κB(nuclear factor kappa B, NF-κB)与这些细胞因子的活化密切相关.
Toll样受体(TLRs)被认为是目前哺乳动物唯一将细胞外抗原识别信息向细胞内传递并引发炎症反应的关键跨膜蛋白[3]. 该受体族作为机体炎性反应链的启动蛋白, 从源头为急性胰腺炎发病机制的研究提供了新的方向. TLR4在该受体家族中最早被发现, 他可以和许多内源性及外源性配体结合[4]. 他被激活后通过一系列的跨膜信号传导激活NF-κB, 后者通过对多种基因表达的调控, 调控众多炎症介质和细胞因子的表达, 发挥防御和免疫调节作用. NF-κB的活化在急性胰腺炎的发生与进展中起着特殊的作用, NF-κB可能调控着ANP的启动. ANP时全身系统性炎症反应综合征(systematic inflammatory response syndrome, SIRS)是引发多器官功能不全综合征(MODS)的一个重要环节, NF-κB在其中起着"信使"作用. 发病初期, 局部炎症可通过NF-κB介导多种炎性细胞因子激活, 这些因子逐渐扩散至全身各个器官而引起SIRS. 因此TLRs/NF-κB通路在AP炎症发生发展中起很重要的作用.
TLR4是Ⅰ型跨膜蛋白, 进化中高度保守. 结构上包括3部分: 胞外区、跨膜区和胞内区. 研究发现Toll家族成员胞膜外区的变异程度很高, 说明不同成员可能与不同配体结合. 胞内区为Toll同源结构域(Toll-homology domain), 由于与白介素-1受体(interleukin-1 receptor, IL-1R)家族成员胞质区高度同源, 故又称TLR/IL-1R或TIR结构域. 他是TLR4向下游进行信号传导的核心元件, 这一区段的突变或序列缺失将阻断信号的传递. 高度的同源性使TLR和IL-1R激活的胞内信号转导在很大程度上保持一致性.
TLRs可以识别外源性的病原相关的分子模式(pathogen-associated molecular patterns, PAMPs), 即可引起机体炎症反应的微生物结构成分的统称, 是病原微生物表达的一些保守序列, 包括细菌的细胞壁成分(如革兰氏阴性菌的脂多糖、革兰氏阳性菌的肽聚糖和胞壁酸、酵母细胞壁上的甘露糖及结核分枝杆菌细胞壁上的脂质和多糖)、细菌的鞭毛蛋白、细菌DNA和病毒RNA. TLR4的外源性配体主要是脂多糖(lipopolysaccharide, LPS). TLR4也能识别内源性配体如黏多糖(glycosaminoglycans, GAGs)降解产物如硫酸肝素(heparan sulfate, HS)和透明质酸(hyaluronic, HA)、纤维连接蛋白(fibronectin)中的EDA(extra domain A)片段、热休克蛋白(heat shock protein, HSP)等.
NF-κB系由两种Rel家族蛋白构成二聚体, 其内部有一保守的中心区域: Rel同源结构域, 内含DNA结合区、二聚体化区与核定位序列(nuclear localization sequence, NLS). 活性NF-κB以二聚体形式存在, Rel蛋白I/J可形成多种多样的二聚体p50/p50、p65/p65、p50/p65等, 在诱导细胞特异性、亚细胞结构中的位置、与NF-κB抑制蛋白(inhibitor of NF-κB, IκB)相互作用及活性等方面各有差异, 分别对特定启动子和增强子起独特和重要作用. 当细胞因子、LPS、病毒作用于效应细胞, 通过包括TLRs在内的特异通路激活NF-κB诱导激酶(NF-κB inducing kinase, NIK), NIK可以活化IκB激酶(IκB kinase, IKK), IKK使IκB磷酸化而降解, 使NF-κB获自由从胞质移入胞核内, 激活一系列基因表达, 如细胞因子(IL-1、IL-6、IL-8、MIP2、IL-12)、黏附分子(E-选择素、P-选择素、VCAM-1)、补体(C3、C4)、急性期反应蛋白(C反应蛋白、al-酸糖蛋白)以及酶类(NO合成酶、环氧合酶-2)等.
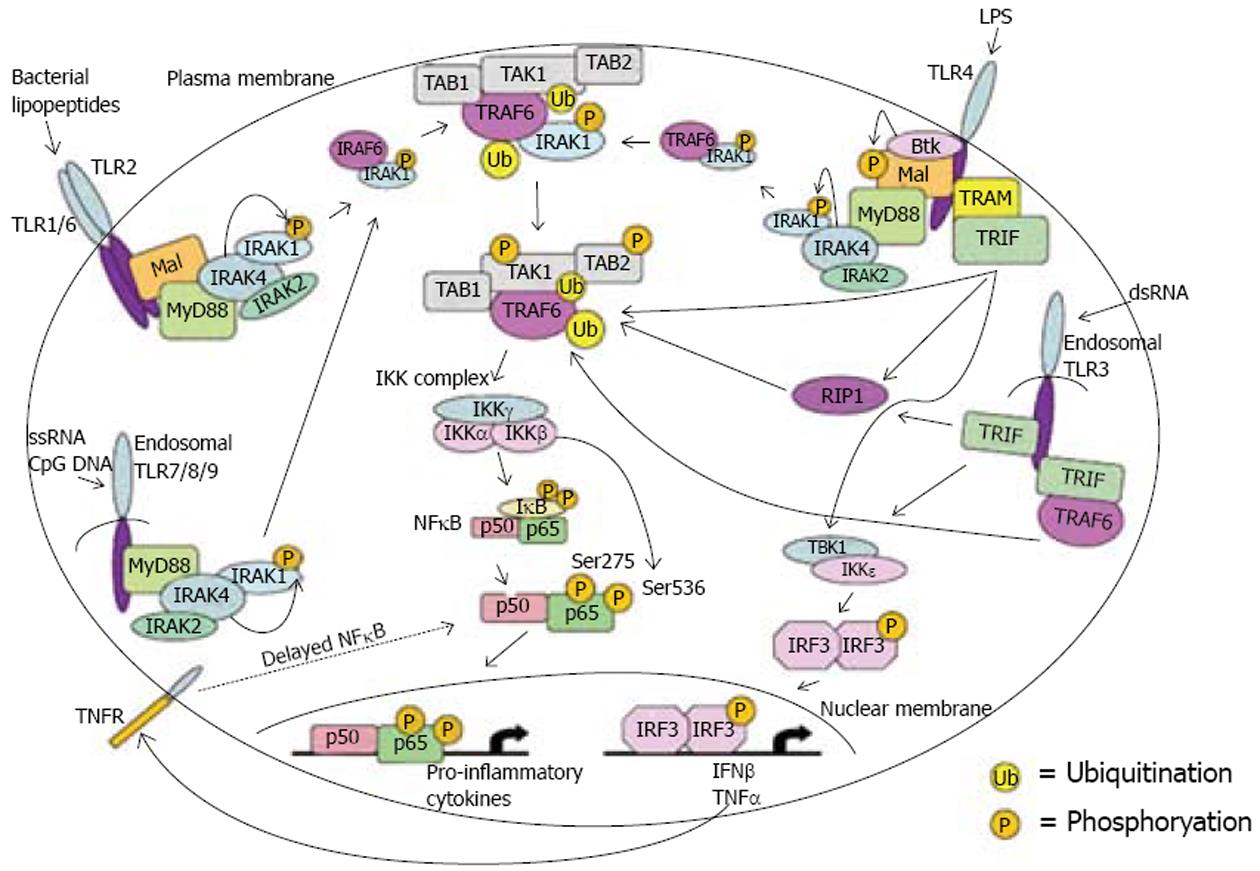
目前的研究发现, TLR4的活化需要四种接头蛋白(adaptor protein)的调节, 即髓样分化蛋白88(myeloid differentiation factor 88, MyD88)、MyD88接头蛋白(MyD88-adaptor-like, MAL)、Toll样受体相关的干扰素活化子(TRIF)和TRIF相关的接头分子(TRIF-related adaptor molecule, TRAM). 四种接头蛋白都可以将信号传递给TIR结构域, 进而激活NF-κB. TLR4/NF-κB信号通路主要有2条胞内信号传导途径[5](图1).
MyD88依赖型途径, 主要是介导NF-κB活化和细胞因子产生. MyD88是一种接头分子, TLR4上的TIR结构域与MyD88蛋白羧基端相互作用使其活化. 活化的MyD88可诱导IL-1受体相关激酶(interleukin-1 receptor-associated kinase, IRAK)磷酸化, 进而激活胞质内的肿瘤坏死因子受体相关因子6(TNF receptor-associated factor 6, TRAF6). 活化后的TRAF6通过转化生长因子TGF-β活化激酶(TGF-β activated kinase, TAK1)与IKK信号级联, 使IκB磷酸化而降解, 从而使NF-κB游离并移位到细胞核中, 结合到靶向的DNA, 促使TNF-α及IL-1、6、8、12等多种炎症基因的转录[6-10].
3.2.1 MyD88接头蛋白(MAL, 也可称为TIR domain-containing adaptor protein, TIRAP, 包含TIR结构域的接头蛋白)负责的MyD88非依赖型途径: TLR4上的TIR结构域与MAL通过一系列相互作用使NF-κB p65亚单位上的S536磷酸化, 从而NF-κB游离并移位到细胞核中, 结合到靶向的DNA, 促使促炎症细胞因子的表达[11-13].
3.2.2 Toll样受体相关的干扰素活化子(TRIF接头蛋白, 也称为TIR-containing adaptor molecule-1, TICAM-1, 包含TIR结构域的接头分子)负责的MyD88非依赖型途径: TLR4的TIR结构域先结合TRIF相关的接头分子(TRAM), TRAM和TIRAP高度同源, 是TLR4和TRIF连接的桥梁, 继而结合TRIF[14-15]. TRIF可以直接结合TRAF6, 使TAK1以一种不依赖于IRAKs的方式活化, 然后导致IKK复合体的活化[16-17]; TRIF也可先结合到受体相互作用蛋白1(receptor interacting protein 1, RIP1), 然后结合TRAF6导致IKK复合体的活化[18-20]; TRIF还可以和TANK结合激酶1(TANK binding kinase 1, TBK1)/IκB激酶ε(IKKε)相互作用使干扰素调节因子3(IFN regulatory factor3, IRF3)活化, 并转移到细胞核, 结合到他的靶向基因.
已有研究证实IL-4可下调TLRs的表达[27]. Nomura et al[28]研究认为, LPS刺激可引起细胞表面跨膜受体TLR4-MD2(MD2是TLR4识别LPS反应中的一个重要的膜蛋白)表达减少, 而IL-1β通过白细胞介素1受体(interleukin-1 receptor, IL-1R)发挥作用, 不会影响TLR4-MD2的表达. Zhang et al[29]研究证实Toll样蛋白相互作用蛋白(Toll-interacting protein, Tollip)对TLR介导的信号传导通路起负调节作用, 在静止期细胞Tollip与IRAK形成复合物; 当细胞受刺激时, Tollip可抑制IRAK的活性, 从而阻断TLR介导的信号转导. 还有研究认为RIP1可以介导TRIF-诱导的NF-κB活化, 而RIP3能在TRIF-RIP1-诱导的NF-κB通路中起负性调节作用[18-20]. 另有研究证实单免疫球蛋白白介素1受体相关分子(single immunoglobulin interleukin-1 receptor-related molecule, SIGIRR)在IL-1和LPS/TLR4信号通路中起负性调节作用[30]. 还有研究发现甲状腺受体互动蛋白6(Thyroid receptor-interacting protein 6, TRIP6)的过度表达可以减轻受体相互作用蛋白2(receptor interacting protein 2, RIP2)介导的NF-κB活化, 而TRIP6的显性失活突变体或受RNA干扰的TRIP6可以抑制TNF、IL-1、TLR2或Nod1活化NF-κB[31-35]. Wullaert et al证实结合锌指蛋白A20(锌指蛋白A20是一种多刺激因子诱导的NF-κB活化的细胞抑制物, 并在阻断NF-κB应答中起重要作用)的NF-κB活化抑制剂-3(A20-binding inhibitor of NF-kappaB activation, ABIN-3)在LPS/TLR4通路中起作用[36-39]. ABIN-3过度表达可以抑制LPS/TLR4通路中NF-κB依赖的TRAF6下游水平和IκB激酶β(IκB kinase β, IKKβ)上游水平之间的基因表达, 从而可能对TLR4的表达产生影响.
还有研究发现了具有特征性的TLR4增强子, 可增强TLR4的表达[40]. Siren et al[41], Ostelund et al[42]研究认为干扰素-α(IFN-α)可以增强TLR信号通路中MyD88及其他调节分子和激酶的表达. IFN-α通过上调TLR3、TLR4和TLR7的表达增强巨噬细胞TLR的应答.
Magder et al[43]研究证明TLR4的细胞内信号通路和一个已被证实的NF-κB的激活因子IL-1的细胞内信号通路很相似, TLR4和TNF-α调控的细胞内信号通路都可以激活NF-κB. 有研究证实TLR、TNF、IL-1和胞质蛋白Nod 1能诱导NF-κB活化, 这是甲状腺受体互动蛋白6(TRIP6)和多个信号蛋白如肿瘤坏死因子受体相关因子2(TNF receptor-associated factor 2, TRAF2)、肿瘤坏死因子受体相关因子6(TNF receptor-associated factor 6, TRAF6)、MyD88及白细胞介素1受体相关激酶1(IRAK1)相互作用完成的[31-35].
Magder et al[43]还发现有一种LPS的自分泌作用可以直接诱导TNFα表达. Berclaz et al发现TLR4信号通路成员中CD14、RP105(也称CD180, 是B细胞所特有的TLR同源体, 可以像TLR4一样介导LPS信号通路[48-49])、IRAK-M的mRNA和蛋白表达是粒-巨噬细胞集落刺激因子(GM-CSF)依赖性的[44-47]. 但是其他TLR-4通路成员如MD-2、TLR4、IRAK1、IRAK-2、TIRAP/MAL、MyD88、IRAK-4、TRAF-6、NF-κB及IKKβ的表达既不是GM-CSF依赖性的, 也不是转录因子PU.1依赖性的.
这些研究说明TLR4表达的多少受IL-4、TLR4增强子、IL-1β等细胞因子的影响, 而影响TLR4表达量的细胞因子如IL-4、IL-1β又和NF-κB有关, 同时这些研究说明能激活NF-κB的除了TLR4调控的细胞内信号通路外还有TNF-α、IL-1调控的细胞内信号通路, 所以在ANP的发生、发展中, TLR4和NF-κB可能不是简单的上下游关系, 他们在一些细胞因子的共同参与下可能构成复杂的循环网络, 相互调节. 这打破了以往"TLR4和NF-κB是上下游关系"的认识, 为更进一步深入研究ANP提供了新的方向. 认识到TLR4和NF-κB之间可能是一个循环网络, 搞清楚这个循环网络中谁是主角、谁是配角, 从而找出治疗ANP准确的干预靶点对重症急性胰腺炎(severe acute pancreatitis, SAP)的临床治疗有很大意义.
急性胰腺炎是一种常见的急重症, 尤其是急性坏死性胰腺炎. 多种细胞因子如TNF-α、IL-1β、IL-6等的活化与急性胰腺炎的发生进展关系密切, 而Toll样受体4、核因子-κB与这些细胞因子的活化密切相关, TLR4/NF-κB通路在AP炎症发生发展中起很重要的作用. 因此对TLR4/NF-κB信号通路的研究倍受关注.
研究认为TLR4/NF-κB信号通路具体有几条传导途径? 以往认为TLR4/NF-κB信号通路主要有2条胞内信号传导途径, 分别由接头蛋白--髓样分化蛋白88和MyD88接头蛋白调节. 目前研究发现, TLR4/NF-κB信号通路有另外2条传导途径: 即通过另外2种接头蛋白--Toll样受体相关的干扰素活化子和TRIF相关的接头分子调节TLR4的活化, 进而激活NF-κB, 现阶段的研究热点、重点及亟待研究的问题是对TLR4/NF-κB信号通路中新的调节因子的研究.
Toll样受体被认为是目前哺乳动物唯一将细胞外抗原识别信息向细胞内传递并引发炎症反应的关键跨膜蛋白, 该受体族作为机体炎性反应链的启动蛋白, 从源头为急性胰腺炎发病机制的研究提供了新的方向. TLR4在该受体家族中最早被发现, 他可以和许多内源性及外源性配体结合.
TLR4表达的多少受IL-4、TLR4增强子、IL-1β等细胞因子的影响, 而影响TLR4表达量的细胞因子如IL-4、IL-1β又和NF-κB有关, 同时这些研究说明能激活NF-κB的除了TLR4调控的细胞内信号通路外还有TNF-α、IL-1调控的细胞内信号通路, 所以在ANP的发生、发展中, TLR4和NF-κB可能不是简单的上下游关系, 他们在一些细胞因子的共同参与下可能构成复杂的循环网络, 相互调节. 这打破了以往"TLR4和NF-κB是上下游关系"的认识, 为更进一步深入研究ANP提供了新的方向.
认识到TLR4和NF-κB之间可能是一个循环网络, 搞清楚这个循环网络中谁是主角、谁是配角, 从而找出治疗ANP准确的干预靶点对重症急性胰腺炎(severe acute pancreatitis, SAP)的临床治疗有很大意义.
本文较全面的概述了TLR4-NF-κB信号通路及该通路相关的下调因子和增强因子, 思路明确, 重点突出, 有新意, 对急性胰腺炎引起的全身性炎症反应的发病机制研究有一定的指导意义.
编辑: 何燕 电编:何基才
| 1. | Algul H, Tando Y, Schneider G, Weidenbach H, Adler G, Schmid RM. Acute experimental pancreatitis and NF-kappaB/Rel activation. Pancreatology. 2002;2:s 503-509. [PubMed] [DOI] |
| 2. | Liu HS, Pan CE, Liu QG, Yang W, Liu XM. Effect of NF-kappaB and p38 MAPK in activated monocytes/macrophages on pro-inflammatory cytokines of rats with acute pancreatitis. World J Gastroenterol. 2003;9:2513-2518. [PubMed] |
| 3. | Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927-930. [PubMed] [DOI] |
| 4. | Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172:20-24. [PubMed] [DOI] |
| 5. | Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102-1113. [PubMed] [DOI] |
| 6. | Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK {beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279:55633-55643. [PubMed] [DOI] |
| 7. | Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346-351. [PubMed] [DOI] |
| 8. | Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289-301. [PubMed] [DOI] |
| 9. | Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X. IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFkappa B. J Biol Chem. 2001;276:41661-41667. [PubMed] [DOI] |
| 10. | Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22:7158-7167. [PubMed] [DOI] |
| 11. | Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Wirth T, O'Neill LA. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258-26264. [PubMed] [DOI] |
| 12. | Doyle SL, Jefferies CA, O'Neill LA. Bruton's tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. J Biol Chem. 2005;280:23496-23501. [PubMed] [DOI] |
| 13. | Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489-10495. [PubMed] [DOI] |
| 14. | Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043-1055. [PubMed] [DOI] |
| 15. | Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640-643. [PubMed] [DOI] |
| 16. | Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem. 2003;278:16713-16719. [PubMed] [DOI] |
| 17. | Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A. 2004;101:3533-3538. [PubMed] [DOI] |
| 18. | Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503-507. [PubMed] [DOI] |
| 19. | Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339-3346. [PubMed] [DOI] |
| 20. | Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3-and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280:36560-36566. [PubMed] [DOI] |
| 21. | Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854-1857. [PubMed] [DOI] |
| 22. | Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857-1861. [PubMed] [DOI] |
| 23. | Li X, Massa PE, Hanidu A, Peet GW, Aro P, Savitt A, Mische S, Li J, Marcu KB. IKKalpha, IKKbeta, and NEMO/IKKgamma are each required for the NF-kappa B-mediated inflammatory response program. J Biol Chem. 2002;277:45129-45140. [PubMed] [DOI] |
| 24. | Krappmann D, Wegener E, Sunami Y, Esen M, Thiel A, Mordmuller B, Scheidereit C. The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol Cell Biol. 2004;24:6488-6500. [PubMed] [DOI] |
| 25. | Carayol N, Chen J, Yang F, Jin T, Jin L, States D, Wang CY. A dominant function of IKK/NF-kappaB signaling in global lipopolysaccharide-induced gene expression. J Biol Chem. 2006;281:31142-31151. [PubMed] [DOI] |
| 26. | Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659-663. [PubMed] [DOI] |
| 27. | Staege H, Schaffner A, Schneemann M. Human toll-like receptors 2 and 4 are targets for deactivation of mononuclear phagocytes by interleukin-4. Immunol Lett. 2000;71:1-3. [PubMed] [DOI] |
| 28. | Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476-3479. [PubMed] [DOI] |
| 29. | Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002;277:7059-7065. [PubMed] [DOI] |
| 30. | Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. J Biol Chem. 2005;280:25233-25241. [PubMed] [DOI] |
| 31. | Li L, Bin LH, Li F, Liu Y, Chen D, Zhai Z, Shu HB. TRIP6 is a RIP2-associated common signaling component of multiple NF-kappaB activation pathways. J Cell Sci. 2005;118:555-563. [PubMed] [DOI] |
| 32. | Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190-194. [PubMed] [DOI] |
| 33. | Lu C, Wang A, Dorsch M, Tian J, Nagashima K, Coyle AJ, Jaffee B, Ocain TD, Xu Y. Participation of Rip2 in lipopolysaccharide signaling is independent of its kinase activity. J Biol Chem. 2005;280:16278-16283. [PubMed] [DOI] |
| 34. | Ruefli-Brasse AA, Lee WP, Hurst S, Dixit VM. Rip2 participates in Bcl10 signaling and T-cell receptor-mediated NF-kappaB activation. J Biol Chem. 2004;279:1570-1574. [PubMed] [DOI] |
| 35. | Sarkar A, Duncan M, Hart J, Hertlein E, Guttridge DC, Wewers MD. ASC directs NF-kappaB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol. 2006;176:4979-4986. [PubMed] [DOI] |
| 36. | Wullaert A, Verstrepen L, Van Huffel S, Adib-Conquy M, Cornelis S, Kreike M, Haegman M, El Bakkouri K, Sanders M, Verhelst K. LIND/ABIN-3 is a novel lipopolysaccharide-inducible inhibitor of NF-kappaB activation. J Biol Chem. 2007;282:81-90. [PubMed] [DOI] |
| 37. | Heyninck K, De Valck D, Vanden Berghe W, Van Criekinge W, Contreras R, Fiers W, Haegeman G, Beyaert R. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J Cell Biol. 1999;145:1471-1482. [PubMed] [DOI] |
| 38. | Van Huffel S, Delaei F, Heyninck K, De Valck D, Beyaert R. Identification of a novel A20-binding inhibitor of nuclear factor-kappa B activation termed ABIN-2. J Biol Chem. 2001;276:30216-30223. [PubMed] [DOI] |
| 39. | Gon Y, Asai Y, Hashimoto S, Mizumura K, Jibiki I, Machino T, Ra C, Horie T. A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:330-336. [PubMed] [DOI] |
| 40. | Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275:9773-9781. [PubMed] [DOI] |
| 41. | Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174:1932-1937. [PubMed] [DOI] |
| 42. | Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608-9617. [PubMed] [DOI] |
| 43. | Magder S, Neculcea J, Neculcea V, Sladek R. Lipopolysaccharide and TNF-alpha produce very similar changes in gene expression in human endothelial cells. J Vasc Res. 2006;43:447-461. [PubMed] [DOI] |
| 44. | Berclaz PY, Carey B, Fillipi MD, Wernke-Dollries K, Geraci N, Cush S, Richardson T, Kitzmiller J, O'connor M, Hermoyian C. GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS. Am J Respir Cell Mol Biol. 2007;36:114-121. [PubMed] [DOI] |
| 45. | Berclaz PY, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma -mediated molecular connection between innate and adaptive immunity in the lung. Blood. 2002;100:4193-4200. [PubMed] [DOI] |
| 46. | Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem. 2002;277:42808-42814. [PubMed] [DOI] |
| 47. | Lendemans S, Rani M, Selbach C, Kreuzfelder E, Schade FU, Flohe S. GM-CSF priming of human monocytes is dependent on ERK1/2 activation. J Endotoxin Res. 2006;12:10-20. [PubMed] [DOI] |