修回日期: 2006-09-17
接受日期: 2006-09-25
在线出版日期: 2006-12-08
目的: 探讨人肝癌细胞株mtDNA缺失对肿瘤MDR表型的影响.
方法: 采用MTT方法检测细胞药物敏感性, 免疫组化方法检测P-gp, MRP表达. 对比分析SK-Hep1细胞和rhoo SK-Hep1细胞药物敏感性和P-gp, MRP表达的差异.
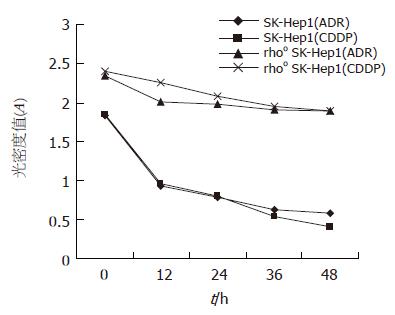
结果: 阿霉素(ADR)、顺铂CDDP对SK-Hep1细胞的12, 24, 36, 48 h抑制率分别为56%, 61%, 72%, 75%和54%, 60%, 77%, 81%, 而对rhoo SK-Hep1细胞的抑制率分别为10%, 18%, 20%, 22%和19%, 20.4%, 21.3%, 22.5%. SK-Hep1与rhoo SK-Hep1细胞比较, 药物敏感性显著降低(P<0.01). 较之于SK-Hep1细胞, rhoo SK-Hep1细胞P-gp, MRP表达明显增加(37% vs 20%, P<0.01; 33% vs 18%, P<0.01).
结论: mtDNA缺失的肿瘤细胞对化疗药物有显著抗性, P-gp, MRP表达增加可能是其MDR表型产生的重要原因.
引文著录: 谈荣玖, 陈宗琼, 江明万, 唐春红. 人肝癌细胞株线粒体DNA缺失对多药耐药表型的影响. 世界华人消化杂志 2006; 14(34): 3311-3313
Revised: September 17, 2006
Accepted: September 25, 2006
Published online: December 8, 2006
AIM: To clarify the influence of mitochondrial DNA depletion on the multidrug resistance (MDR) phenotype of human hepatoma cells.
METHODS: The sensitivities of hepatoma cells to chemotherapy were determined by MTT method, and P-glycoprotein (P-gp) and MDR protein (MRP) expression were detected by immunohistochemistry. The differences of P-gp and MRP expression and sensitivities to chemotherapeutic druds were comparatively analyzed between SK-Hep1 and rhoo SK-Hep1 cells.
RESULTS: Adriamycin inhibited SK-Hep1 cells with a rate of 56%, 61%, 72%, and 75%, respectively, at 12, 24, 36, and 48 h, while cisplatin inhibited them with a rate of 54%, 60%, 77%, and 81%. However, adriamycin inhibited rhoo SK-Hep1 cells with a rate of 10%, 18%, 20%, and 22%, while cisplatin inhibited them with a rate of 19%, 20.4%, 21.3%, and 22.5%, respectively, at 12, 24, 36, and 48 h. There were significant differences between SK-Hep1 and rhoo SK-Hep1 cells (P < 0.01). The expression of P-gp and MRP were markedly higher in rhoo SK-Hep1 cells than those in SK-Hep1 cells (37% vs 20%, P < 0.01; 33% vs 18%, P < 0.01).
CONCLUSION: Cells with mitochondrial DNA depletion have resistance to chemotherapy, and the increased expression of P-gp and MRP may contribute to MDR of tumor cells.
- Citation: Tan RJ, Chen ZQ, Jiang MW, Tang CH. Influence of mitochondrial DNA depletion on multidrug resistance phenotype of human hepatoma cell line. Shijie Huaren Xiaohua Zazhi 2006; 14(34): 3311-3313
- URL: https://www.wjgnet.com/1009-3079/full/v14/i34/3311.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i34.3311
肿瘤细胞对一种化疗药物耐药的同时, 也可能对其他的化疗药物存在耐药的现象, 称为多药耐药(multidrug resistance, MDR).原发性肝癌是常见的恶性肿瘤之一, 临床所见者以中晚期居多. 化疗是治疗的重要手段之一, 但原发性肝癌原发性耐药占很大的比例, 故化疗疗效常欠理想. 研究发现, 线粒体DNA(mitochondrial DNA, mtDNA)突变可能与肿瘤MDR产生有关[1-3]. 我们以人肝癌细胞SK-Hep1及rhoo SK-Hep1细胞株为研究对象, 研究mtDNA缺失对化疗药物敏感性和多药耐药相关因子表达的影响.
SK-Hep1细胞购自中国科学院上海生命科学研究院细胞与生物化学研究所, rhoo SK-Hep1细胞由第三军医大学凌贤龙博士馈赠. 浓缩型鼠抗人P-糖蛋白(P-gp), 多药耐药相关蛋白(MRP) mAb均为福州迈新公司产品, EnVision法免疫组化试剂盒为丹麦Dako公司产品. 顺铂(CDDP, 济南齐鲁), 阿霉素(ADR, 深圳万乐).
1.2.1 细胞培养: SK-Hep1细胞在DMEM培养基, 含100 mL/L FCS, 50 mL/L CO2, 37 ℃条件下进行培养. rhoo SK-Hep1细胞通过溴化乙锭诱导获得, 培养条件: 高糖DMEM培养基, 含15 mL/L FCS, 100 mg/L丙酮酸、50 mg/L 5'-溴-脱氧尿嘧啶, 50 mL/L CO2, 37 ℃条件下进行培养.
1.2.2 MTT方法检测药物敏感性: 将对数期生长的细胞接种于96孔培养板中, 培养12 h后, 弃去培养液, 分别加入化疗药物CDDP 10 mg/L, ADR 10 mg/L, 继续培养48 h; 加入5 g/L的MTT 20 mL, 4 h后加入DMSO 200 mL, 以490 nm为检测波长, 测定各孔的光密度值, 计算抑制率.
1.2.3 免疫组化: 采用微波EnVision二步法免疫组化技术检测P-gp、MRP在SK-Hep1和rhoo SK-Hep1细胞中的表达. 按试剂盒说明书进行操作, 一抗工作浓度为1:100, DAB显色. P-gp, MRP以细胞质/或细胞膜着棕黄色颗粒为阳性细胞. 随机计数10个高倍视野, 计算阳性细胞百分率.
统计学处理 采用SPSS10.0统计软件进行χ2检验及t检验.
12, 24, 36, 48 h ADR, CDDP对SK-Hep1细胞的抑制率分别为56%, 61%, 72%, 75%和54%, 60%, 77%, 81%, 而对rhoo SK-Hep1细胞的抑制率分别为10%, 18%, 20%, 22%和19%, 20.4%, 21.3%, 22.5%. SK-Hep1与rhoo SK-Hep1细胞比较, 都有显著差异(P<0.01, 图1).
随机计数10个高倍视野阳性细胞数, 计算平均百分比. SK-Hep1细胞P-gp, MRP免疫组化染色阳性细胞百分比为20%, 18%, 而rhoo SK-Hep1细胞为37%和33%, 两者相比有非常显著差异(P<0.01).
线粒体是真核细胞内含有核外遗传物质的细胞器, 是细胞内能量合成的主要场所, 亦在细胞凋亡及细胞内其他生物学行为的信号传递过程中扮演重要角色[4]. 研究显示, mtDNA缺失的肿瘤细胞获得MDR表型. 本研究表明, mtDNA缺失细胞对化疗药物有明显抗性, 较之于SK-Hep1细胞, rhoo SK-Hep1细胞对ADR, CDDP敏感性显著降低, P-gp, MRP表达增加. Pillay et al[3]报道r˚细胞对ADR的敏感性显著降低, 同时对其他非DNA作用药物存在MDR现象, 并发现r˚细胞高表达P-gp; 引起r˚细胞MDR表型还可能与线粒体热休克蛋白60(HSP60)高表达有关[5]. 结直肠癌的研究显示, HSP60在结直肠癌组织高表达[6]. HSP60与细胞凋亡有关, 他能增加胱氨酸蛋白酶的活性; 还可能参与了细胞器特异的应激反应[7]. Calcabrini et al[8]对结肠癌细胞株LoVo的研究显示, 敏感细胞株细胞内ROS产物显著高于药物抵抗细胞株, 化疗药物的细胞毒性主要是通过细胞内存在的ROS来实现的, 应用ALDH和超氧歧化酶后其细胞毒性消失. 透射电镜研究发现, 药物抵抗细胞株有显著的线粒体修饰, 线粒体膜过极化. 给予BASO和spermine后线粒体膜去极化. Harper et al[9]比较了药物敏感和药物抵抗细胞株线粒体差异, 发现多药耐药细胞株: 线粒体膜电位较低; 逆线粒体膜质子梯度的质子转运少, 存在质子"泄漏"现象; 能量供给以脂肪为主, 细胞质内糖酵解率高; 应激状态时, 细胞内ROS水平低; 正常非应激状态, DNA损伤多于应激状态; 对凋亡不易感. 线粒体耦合蛋白2(UCP2)可能与上述差异的发生有关.
消化系统肿瘤是我国常见的恶性肿瘤, 原发性耐药占很大的比例, 故化疗疗效不理想. 因此, 研究肿瘤多药耐药(MDR)的发生机制有重要理论和应用价值. 研究显示, mtDNA 缺失的肿瘤细胞获得MDR表型, 但其机制尚不明确.
肿瘤细胞耐药给临床化疗带来困难. 本文探讨人肝癌细胞株mtDNA缺失对肿瘤MDR表型的影响, 为肝癌化疗提供了参考依据.
本文探讨人肝癌细胞株mtDNA缺失对肿瘤MDR表型的影响, 结果发现SK-Hep1与rhoo SK-Hep1细胞比较, 药物敏感性显著降低. 较之于SK-Hep1细胞, rhoo SK-Hep1细胞Pgp, MRP表达明显增加, 为化疗提供重要的参考依据, 论文方法科学, 结果可靠, 对临床治疗有参考意义.
电编: 张敏 编辑:王晓瑜
| 1. | Salvioli S, Storci G, Pinti M, Quaglino D, Moretti L, Merlo-Pich M, Lenaz G, Filosa S, Fico A, Bonafe M. Apoptosis-resistant phenotype in HL-60-derived cells HCW-2 is related to changes in expression of stress-induced proteins that impact on redox status and mitochondrial metabolism. Cell Death Differ. 2003;10:163-174. [PubMed] [DOI] |
| 2. | Grandjean F, Bremaud L, Robert J, Ratinaud MH. Alterations in the expression of cytochrome c oxidase subunits in doxorubicin-resistant leukemia K562 cells. Biochem Pharmacol. 2002;63:823-831. [PubMed] [DOI] |
| 3. | Pillay V, Martinus RD, Hill JS, Phillips DR. Upregulation of P-glycoprotein in rat hepatoma rho(o) cells: implications for drug-DNA interactions. J Cell Biochem. 1998;69:463-469. [PubMed] [DOI] |
| 5. | Shen DW, Akiyama S, Schoenlein P, Pastan I, Gottesman MM. Characterisation of high-level cisplatin-resistant cell lines established from a human hepatoma cell line and human KB adenocarcinoma cells: cross-resistance and protein changes. Br J Cancer. 1995;71:676-683. [PubMed] [DOI] |
| 6. | Cappello F, Bellafiore M, Palma A, David S, Marciano V, Bartolotta T, Sciume C, Modica G, Farina F, Zummo G. 60KDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur J Histochem. 2003;47:105-110. [PubMed] [DOI] |
| 7. | Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, Hoogenraad NJ. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240:98-103. [PubMed] [DOI] |
| 8. | Calcabrini A, Arancia G, Marra M, Crateri P, Befani O, Martone A, Agostinelli E. Enzymatic oxidation products of spermine induce greater cytotoxic effects on human multidrug-resistant colon carcinoma cells (LoVo) than on their wild-type counterparts. Int J Cancer. 2002;99:43-52. [PubMed] [DOI] |