修回日期: 2006-04-15
接受日期: 2006-04-20
在线出版日期: 2006-06-18
目的: 体外研究不同浓度拉米夫定(lamivu-dine, LAM)对慢性乙型肝炎(chronic hepatitis B, CHB)患者树突状细胞(dendritic cell, DC)功能影响.
方法: 分离慢乙肝患者外周血单核细胞, 在含GM-CSF+IL-4及不同浓度LAM (0, 0.125, 0.25, 0.5, 1, 2 mmol/L)培养条件下制备DC. 观察DC形态学变化并用MTT法测定DC刺激同种异体淋巴细胞增殖能力, 流式细胞仪(FCM)测定其细胞表型分子CD1a, CD83, CD80及HLA-DR的表达, ELISA法检测培养上清中IL-12和IL-6含量.
结果: 在LAM 0.5 mmol/L组, DC表型分子CD83, CD1a, HLA-DR的表达最高, CD80与LAM未处理组相比无明显差异. 与LAM未处理组相比, LAM 0.5 mmol/L处理组DC膜表面分子CD1a, CD83, HLA-DR表达增高(CD1a: 54.0±9.2 vs 33.6±10.1, P<0.05; CD83: 20.3±6.1 vs 11.8±6.2, P<0.05; HLA-DR: 74.5±7.1 vs 52.9±7.7, P<0.05); 其上清液中IL-12分泌水平增高(810.0±91.5 ng/L vs 268.0±34.3 ng/L, P<0.05), IL-6则显著降低(28.1±2.6 ng/L vs 55.3±7.4 ng/L, P<0.05); 刺激同种异体淋巴细胞增殖能力(SI)增强(1.9±0.6 vs 1.2±0.5, P<0.05).
结论: LAM体外可增强慢乙肝患者树突状细胞活性.
引文著录: 张冬云, 郑鹏远, 张晓琴, 刘光辉, 娄惠萍, 唐芙爱, 白经修, 祁元明. 拉米夫定体外对慢性乙型肝炎患者树突状细胞功能的影响. 世界华人消化杂志 2006; 14(17): 1693-1698
Revised: April 15, 2006
Accepted: April 20, 2006
Published online: June 18, 2006
AIM: To investigate the effects of lamivudine on the function of dendritic cells derived from patients with chronic hepatitis B (CHB) in vitro.
METHODS: Dentritic cells (DCs) derived from the monocytes of CHB patients were cultured with interleukin-4 (IL-4) plus granulocyte-macrophage colony-stimulating factor (GM-CSF). Three days after being cultured, the cells were divided group A and B. Group A was treated with different concentrations of lamivudine (0, 0.125, 0.25, 0.5, 1, 2 mmol/L), while group B served as the control. Cell morphology was observed under light microscope and cell surface molecules including HLA-DR, CD80, CD83, and CD1a were assayed by flow cytometry. The concentrations of IL-6 and IL-12 in the supernatant were measured by enzyme-linked immunosorbent assay (ELISA). T cell proliferation induced by DC was detected by methyl thiazolyl tetrazolium (MTT).
RESULTS: The cells treated with 0.5 mmol/L lamivudine had the highest expression of CD1a, CD83 and HLA-DR, and the expression of CD80 was not significantly different between the cells with and without lamivudine treatment. After treatment with 0.5 mmol/L lamivudine, the expression of CD1a, CD83 and HLA-DR were significantly higher than those of DCs without lamivudine treatment (CD1a: 54.0 ± 9.2 vs 33.6 ± 10.1, P < 0.05; CD83: 20.3 ± 6.1 vs 11.8 ± 6.2, P < 0.05; HLA-DR: 74.5 ± 7.1 vs 52.9 ± 7.7, P < 0.05); the secretion of IL-12 was significantly increased in comparison with that of the control group (810.0 ± 91.5 ng/L vs 268.0 ± 34.3 ng/L, P < 0.05), while the level of IL-6 was lowered markedly (28.1 ± 2.6 ng/L vs 55.3 ± 7.4 ng/L, P < 0. 05); the stimulatory capacity of DCs in the allogeneic mixed leukocyte reaction was markedly enhanced as compared with that of the control group (stimulatory index: 1.9 ± 0.6 vs 1.2 ± 0.5, P < 0.05).
CONCLUSION: Pulsed with lamivudine in certain concentration, the biological activity of DCs derived from CHB patients can be efficiently enhanced.
- Citation: Zhang DY, Zheng PY, Zhang XQ, Liu GH, Lou HP, Tang FA, Bai JX, Qi YM. In vitro effects of lamivudine on function of dendritic cells derived from patients with chronic hepatitis B infection. Shijie Huaren Xiaohua Zazhi 2006; 14(17): 1693-1698
- URL: https://www.wjgnet.com/1009-3079/full/v14/i17/1693.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i17.1693
慢性乙型肝炎(chronic hepatitis B, CHB)造成免疫耐受的重要原因之一是患者自身树突状细胞(dendritic cell, DC)功能低下, 抗原提呈功能缺陷, 不能有效地把病毒抗原的信号传递给机体免疫系统[1-2]. DC是唯一能激活初始化T淋巴细胞的抗原提呈细胞(antigen-presenting cell, APC), 在抗病毒的细胞免疫治疗中起重要作用[3-4]. 如何加强DC的抗原提呈能力, 发挥其免疫功能已成为治疗CHB急需解决的问题之一. 拉米夫定(lamivudine, LAM)为一口服核苷类药物, 在细胞内经磷酸化后, 与脱氧胞嘧啶核苷(dCTP)竞争进入病毒合成中的DNA链, 终止病毒DNA的复制[5-7]. 国外研究发现LAM在抗病毒同时对DC也有一定作用[8-9]. 国内尚未见相关报道. 我们以不同浓度LAM与CHB的DC共孵育, 观察LAM对CHB的DC生物学特性的影响, 为临床上LAM与DC疫苗联合治疗CHB寻找理论依据.
rhGM-CSF, rhIL-4, 抗人CD1a、CD80、CD83、HLA-DR均购自美国R&D公司; RPMI 1640培养基, 美国GIBCO公司; 胎牛血清, 杭州四季青公司; MTT购自美国Sigma公司; 淋巴细胞分离液, 天津市津脉基因测绘技术有限公司; IL-12, IL-6 ELISA试剂盒, 上海森雄科技有限公司; LAM购自葛兰素史克(苏州)制药有限公司. 按中华医学会传染病与寄生虫学分会、肝病学分会联合修订的《病毒性肝炎防治方案》(2000-09西安)为诊断标准, 从门诊选出HBeAg(+)慢性乙型病毒性肝炎患者15例, 同时设健康对照者15例.
常规方法分离CHB外周血单核细胞, 悬浮后加入24孔板, 37℃, 50 mL/L CO2孵箱温育2 h, 吸弃培养上清, 洗涤去除非贴壁细胞, 即获得贴壁细胞. 每孔加入含100 mL/L自体血清, GM-CSF 10 μg/L, IL-4 5 μg/L的RPMI 1640培养基1 mL, 于37℃, 50 mL/L CO2孵箱培养[10], 并于第4天将DC分成2组: 一组加入LAM (0.125, 0.25, 0.5, 1, 2 mmol/L)作为实验组即LAM处理组; 一组继续培养作为对照组即LAM未处理组. 各组均隔天半量换液. 第8天收集细胞行相关检测.
1.2.1 DC形态学及表型检测: 倒置相差显微镜动态观察DC形态学变化并收集8 d的各组DC, 调细胞密度为1×108/L, 各取细胞悬液0.5 mL加入FCM测定管, 分别加入FITC标记的鼠抗人CD1a和鼠抗人CD83, PE标记的鼠抗人CD80和鼠抗人HLA-DR各2 μL, 轻轻摇匀2 s, 室温避光反应45 min, 生理盐水洗涤1次, PBS重悬细胞后上机检测DC表型分子CD1a, CD83, CD80及HLA-DR的表达.
1.2.2 混合淋巴细胞反应[11]: 取健康者外周血, 分离出单核细胞, 37℃, 50 mL/L CO2孵箱温育2 h后吸取未黏附细胞为淋巴细胞, 调密度为1×108/L; 收集培养第8天的各组DC并用丝裂霉素C (50 mg/L)孵育30 min, 调密度为1×107/L. 按DC与淋巴细胞比例为1∶10混合作为实验孔种植于96孔板, 200 μL/孔, DC组及T淋巴细胞组为对照孔, 设3复孔, 用MTT法在570 nm处测各孔A570值, 并计算刺激指数(SI) = 实验组A值/(反应细胞对照组A值+刺激细胞对照组A值). 另按ELISA试剂盒说明书操作, 测定培养第8天各组DC上清中IL-12和IL-6含量.
统计学处理 用SPSS 10.0统计软件进行处理, 全部数据均采用mean±SD表示, 以α = 0.05为检验性水准, P<0.05具有统计学意义.
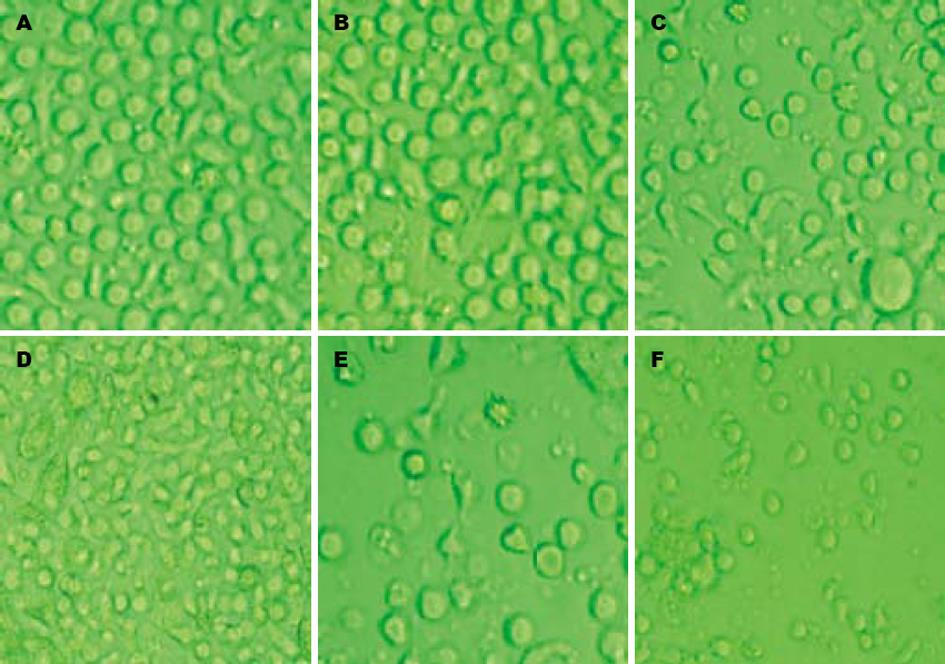
倒置显微镜下, 培养24 h后可见细胞呈集落生长, 2 d后细胞数目明显增多,体积开始逐渐增大, 胞体拉长; 第5天观察部分细胞有多个短突起, 有的胞体四周细胞膜扩展成多方向长突起, 呈树枝状, 面纱状, 第7, 8天时可见培养液中漂浮着颜色较深、云雾状的大细胞或细胞团. LAM处理小剂量组(图1B)与LAM未处理组(图1A)相比, 变形细胞较少, 细胞数目变化不大. LAM处理大剂量组(图1F)于加药第2天镜下观察可发现培养液中出现大量颗粒状物质, 细胞数量减少, 考虑为受药物毒性作用所致裂解的细胞碎片(图1). 在DC+LAM 0.5 mmol/L组, DC表型分子CD83, CD1a, HLA-DR的表达最高, CD80与LAM未处理组相比无明显差异, 但其膜分子表达量与健康人DC相比仍有所降低; 而其他组别与LAM未处理组相比, 虽有差异但无统计学意义. 说明CHB的DC体外与LAM (0.5 mmol/L)共孵育后, 其递呈抗原及刺激T细胞增殖能力相对增强(表1).
| CD83 | CD80 | CD1a | HLA-DR | |
| LAM 0 mmol/L (CHB)(A) | 12.8±2.1 | 35.8±2.4 | 33.6±3.1 | 52.8±2.5 |
| 0.125 mmol/L | 13.4±2.2 | 37.7±3.2 | 40.3±2.1 | 53.7±3.3 |
| 0.25 mmol/L | 18.3±3.2 | 41.1±3.6 | 53.1±2.8 | 67.8±4.2 |
| 0.5 mmol/L (B) | 20.2±2.5ae | 41.7±4.2 | 54.1±4.2ae | 74.5±5.2ae |
| 1 mmol/L | 12.8±2.3 | 25.7±3.1 | 16.6±1.9 | 42.1±3.9 |
| 2 mmol/L | 11.9±1.3 | 24.7±2.6 | 16.5±1.8 | 41.0±3.5 |
| 0 mmol/L (健康人)(C) | 61.4±6.6c | 49.3±6.9 | 71.1±6.7c | 87.3±6.5c |
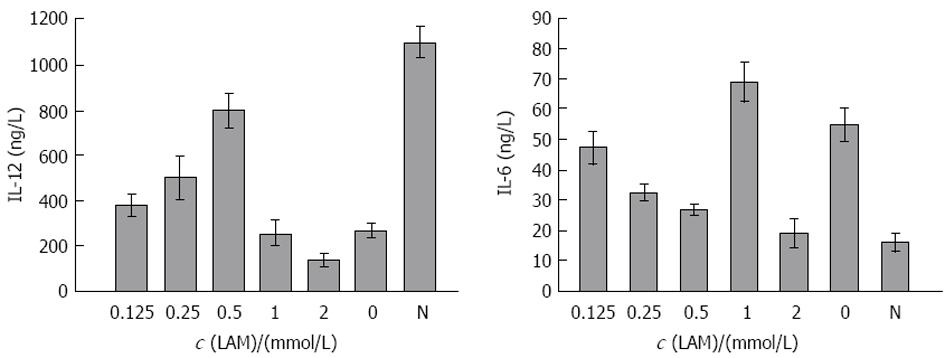
在DC+LAM 0, 0.125, 0.25, 0.5, 1, 2 mmol/L组DC刺激同种异体T淋巴细胞增殖能力分别为1.2±0.5, 1.3±0.6, 1.5±0.5, 1.9±0.6, 0.9±0.4, 0.6±0.3; DC+LAM 2 mmol/L组, 由于大量细胞裂解刺激淋巴细胞增殖能力减弱; 而DC+LAM 0.5 mmol/L组其刺激指数(SI)值与对照组相比有差异. 另经ELISA检测, 在LAM浓度依次为0, 0.125, 0.25, 0.5, 1, 2 mmol/L时, 与其共孵育的DC培养上清液中IL-12含量分别为268.1±34.3, 380.3±51.2, 500.4±89.4, 810.2±91.5, 255.1±73.1, 138.0±27.9 ng/L; IL-6含量依次为55.2±7.4, 47.1±5.2, 32.2±2.7, 28.4±2.6, 69.1±8.7, 67.3±8.4 ng/L; 健康人DC所分泌的IL-12, IL-6含量依次为1075.3±92.6, 21.2±6.7 ng/L(图2). 其中IL-12含量在DC+LAM 0.5 mmol/L组较高; IL-6含量在此浓度下较低, 与LAM未处理组及健康者DC分泌的IL-12, IL-6相比均有差异. DC+LAM 2 mmol/L组, 由于大量细胞裂解分泌IL-12, IL-6含量下降.
HBV转基因小鼠试验中证明, 在清除乙肝病毒过程中, 机体免疫及细胞因子发挥了重要作用[12]. 感染后首先启动一系列非特异性早期应答, 包括干扰素, NK细胞及枯否氏细胞的活化; 彻底清除病毒则有赖于HBV特异性T细胞[13-14]. HBV分泌的各种抗原可刺激机体产生多克隆的、HBV抗原特异性的CTL应答, 杀伤携带HBV的靶细胞. 若不能对病毒进行有效的清除, 则造成感染的慢性化, 诱导机体内HBV抗原特异性的CD8+T细胞大幅度下降, 导致机体内抗原特异性T细胞免疫耐受, 造成CHB的发生[15-16]. 机体内的APC, 尤其是DC是特异性免疫应答的关键细胞, 启动和介导特异性T细胞免疫应答[17]. HBV感染慢性化与被感染者机体免疫功能低下尤其与其体内DC低表达黏附分子, 递呈抗原及刺激T细胞增殖能力低下有关[18-19]. 选择性修饰DC, 有效激活机体抗HBV特异性免疫反应, 已成为CHB治疗中的一条重要途径[20-22]. Beckebaum et al[9]报道, LAM在抗HBV同时, 能提高DC表面HLA-DR表达. 在此基础上, 我们参考LAM的临床应用剂量, 用不同浓度的LAM与培养第4天的DC共孵育, 第8天收获各组细胞进行了检测, 观察LAM对CHB的DC生物学活性的影响.
DC摄取抗原并将其处理成具有免疫原性的多肽, 以MHC-抗原肽复合物的形式表达于其表面的同时, 其膜表面的协同刺激分子与T细胞表面相应配体结合, 进而激活抗原特异性T细胞, 促其活化增殖, 产生免疫应答[23-25]. 实验中分别检测了DC表面CD1a, CD83, HLA-DR及CD80的表达. CD1a是DC的特异性表面标志, CD1a阳性细胞数的多少可反映DC的数量; CD83为DC的成熟性标志; HLA-DR为MHC-Ⅱ类分子之一, 主要参与抗原提呈; CD80为DC表面的共刺激分子之一, 通过与淋巴细胞表面的共刺激分子受体(如CD28)相结合, 形成强烈的共刺激信号, 参与T细胞的活化[26-27]. 本实验结果表明, LAM (0.5 mmol/L)与DC共孵育后提高了DC表面CD1a, CD83, HLA-DR的表达. 其CD80的表达与LAM未处理组相比有所增高, 但统计学上无差异. 与健康者DC相比, 其膜分子表达量仍有所降低. 而适当浓度LAM (0.5 mmol/L)孵育的DC淋巴细胞增殖能力增强, 这初步提示淋巴细胞的活化增殖不是单纯依赖于传统的双信号机制, 可能有其他机制参与. DC通过分泌IL-6, IL-12, IFN-γ等来控制Th0细胞向Th1和Th2细胞分化[28]. IL-12为成熟DC所分泌, 调节Th0细胞向Th1细胞分化, 促进Th1细胞分泌IFN-γ, IL-2等刺激T细胞活化增殖, 介导细胞免疫应答; IL-6主要由未成熟DC分泌, 主要促使Th0细胞向Th2细胞分化, 通过抑制Th1细胞的功能来抑制细胞免疫, 诱导免疫耐受[29-30]. 实验发现LAM可影响DC分泌IL-12, IL-6水平. 在DC+LAM 0.5 mmol/L组细胞培养上清液中IL-12分泌水平较高, IL-6分泌量较低. 故选择合适浓度LAM处理DC可促使T淋巴细胞增殖能力增强并向Th1分化, 增强其细胞免疫学活性. 实验结果表明, LAM可以提高CHB的DC表面分子CD1a, CD83, HLA-DR的表达, 提高DC分泌细胞因子IL-12水平, 降低IL-6分泌, 增强DC刺激淋巴细胞增殖能力. 表明LAM除了作为抗病毒药物外, 还可作为免疫调节剂, 一定程度上改善CHB的DC生物学活性. 这为临床上LAM与DC疫苗相结合进行慢性乙型肝炎的免疫治疗提供了理论依据.
DC是功能最强的抗原提呈细胞, 能激活初始化T细胞, 诱发T细胞免疫应答. CHB体内DC功能低下, 不能充分提呈抗原给T细胞, 使T细胞活化. 如何增强DC的抗原提呈功能是CHB治疗中需解决的关键性问题. LAM为一口服核苷类药物. 在细胞内经磷酸化后, 与dCTP竞争进入病毒合成中的DNA链, 终止病毒DNA的复制. 国外研究发现LAM在抗病毒同时能提高DC表面HLA-DR的表达, 国内尚未见相关文献报道.
本实验首次以不同浓度LAM与CHB的DC共孵育, 通过一系列指标检测来观察LAM对CHB的DC生物学特性的影响, 为临床上LAM与DC疫苗相结合治疗慢乙肝寻找理论依据.
本文题目确切, 结果明确, 对于3TC可能存在的对于DC的免疫调节作用有一定的阐释作用.
电编: 张敏 编辑:潘伯荣
| 1. | Hilleman MR. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine. 2003;21:4626-4649. [PubMed] [DOI] |
| 2. | Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439-1449. [PubMed] [DOI] |
| 3. | Liu SR, Zhang Y, Xie Q, Li ZL, Yu ZY, Kong XP. Effect of HBsAg pulsed dendritic cells on the functions of cytokine-induced killer cells. Xibao Yu Fenzi Mianyixue Zazhi. 2005;21:634-636. [PubMed] |
| 4. | Huang Y, Chen Z, Jia H, Wu W, Zhong S, Zhou C. Induction of Tc1 response and enhanced cytotoxic T lymphocyte activity in mice by dendritic cells transduced with adenovirus expressing HBsAg. Clin Immunol. 2006;119:280-290. [PubMed] [DOI] |
| 5. | Zhang HH, He Y, Zhao H, Piao WH, Liu MC, Xi HL, Yu M, Wang GQ. The effect of stimulation of dendritic cell on HBV-epitopic cytotoxic T-lympho-cyte. Zhonghua Yixue Zazhi. 2005;85:1171-1176. [PubMed] |
| 6. | Wang FS, Xing LH, Liu MX, Zhu CL, Liu HG, Wang HF, Lei ZY. Dysfunction of peripheral blood dendritic cells from patients with chronic hepatitis B virus infection. World J Gastroenterol. 2001;7:537-541. [PubMed] [DOI] |
| 7. | Li HW, Wang HF, Wang FS, Jin B, Duan XZ. Clinical profiles of circulating dendritic cell phenotype and lymphocyte subsets in patients chronically infected with HBV during lamivudine treatment. Zhonghua Shiyan He Linchuangbingduxue Zazhi. 2006;20:43-46. [PubMed] |
| 8. | Feng B, Wei L, Chen M, Han FZ, Wang LP. Dyna-mic variations of hepatitis B virus polymerase gene in lamivudine-treated hepatitis B patients. Zhonghua Ganzangbing Zazhi. 2006;14:327-330. [PubMed] |
| 9. | Beckebaum S, Cicinnati VR, Zhang X, Ferencik S, Frilling A, Grosse-Wilde H, Broelsch CE, Gerken G. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology. 2003;109:487-495. [PubMed] [DOI] |
| 10. | Duan XZ, Wang M, Li HW, Zhuang H, Xu D, Wang FS. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol. 2004;24:637-646. [PubMed] [DOI] |
| 11. | Kranz B, Vester U, Bonzel KE, Paul A, Gerken G, Hoyer PF. Antiviral treatment of chronic hepatitis B with lamivudine in pediatric renal transplantation. Pediatr Transplant. 2006;10:384-389. [PubMed] [DOI] |
| 12. | Wolters LM, Niesters HG, de Man RA. Nucleoside analogues for chronic hepatitis B. Eur J Gastroenterol Hepatol. 2001;13:1499-1506. [PubMed] [DOI] |
| 13. | Arima S, Akbar SM, Michitaka K, Horiike N, Nuriya H, Kohara M, Onji M. Impaired function of antigen-presenting dendritic cells in patients with chronic hepatitis B: localization of HBV DNA and HBV RNA in blood DC by in situ hybridization. Int J Mol Med. 2003;11:169-174. [PubMed] [DOI] |
| 14. | Kurose K, Akbar SM, Yamamoto K, Onji M. Produc-tion of antibody to hepatitis B surface antigen (anti-HBs) by murine hepatitis B virus carriers: neonatal tolerance versus antigen presentation by dendritic cells. Immunology. 1997;92:494-500. [PubMed] [DOI] |
| 15. | Akbar SM, Inaba K, Onji M. Upregulation of MHC class II antigen on dendritic cells from hepatitis B virus transgenic mice by interferon-gamma: abrogation of immune response defect to a T-cell-dependent antigen. Immunology. 1996;87:519-527. [PubMed] [DOI] |
| 16. | Accapezzato D, Visco V, Francavilla V, Molette C, Donato T, Paroli M, Mondelli MU, Doria M, Torrisi MR, Barnaba V. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med. 2005;202:817-828. [PubMed] [DOI] |
| 17. | Akbar SM, Horiike N, Onji M. Immune therapy including dendritic cell based therapy in chronic hepatitis B virus infection. World J Gastroenterol. 2006;12:2876-2883. [PubMed] [DOI] |
| 18. | Hasebe A, Akbar SM, Furukawa S, Horiike N, Onji M. Impaired functional capacities of liver dendritic cells from murine hepatitis B virus (HBV) carriers: relevance to low HBV-specific immune responses. Clin Exp Immunol. 2005;139:35-42. [PubMed] [DOI] |
| 19. | Tavakoli S, Schwerin W, Rohwer A, Hoffmann S, Weyer S, Weth R, Meisel H, Diepolder H, Geissler M, Galle PR. Phenotype and function of monocyte derived dendritic cells in chronic hepatitis B virus infection. J Gen Virol. 2004;85:2829-2836. [PubMed] [DOI] |
| 20. | Kolb-Maurer A, Brocker EB. The role of dendritic cells during infection. J Dtsch Dermatol Ges. 2003;1:438-442. [PubMed] [DOI] |
| 21. | Duan XZ, Zhuang H, Wang M, Li HW, Liu JC, Wang FS. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2). J Gastroenterol Hepatol. 2005;20:234-242. [PubMed] [DOI] |
| 22. | Untergasser A, Zedler U, Langenkamp A, Hosel M, Quasdorff M, Esser K, Dienes HP, Tappertzhofen B, Kolanus W, Protzer U. Dendritic cells take up viral antigens but do not support the early steps of hepatitis B virus infection. Hepatology. 2006;43:539-547. [PubMed] [DOI] |
| 23. | Kunitani H, Shimizu Y, Murata H, Higuchi K, Watanabe A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J Hepatol. 2002;36:734-741. [PubMed] [DOI] |
| 24. | Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995;154:5851-5861. [PubMed] |
| 25. | Rodriguez-Fernandez JL, Corbi AL. Adhesion molecules in human dendritic cells. Curr Opin Investig Drugs. 2005;6:1103-1111. [PubMed] |
| 26. | Rouard H, Leon A, Klonjkowski B, Marquet J, Tenneze L, Plonquet A, Agrawal SG, Abastado JP, Eloit M, Farcet JP. Adenoviral transduction of human 'clinical grade' immature dendritic cells enhances costimulatory molecule expression and T-cell stimulatory capacity. J Immunol Methods. 2000;241:69-81. [PubMed] [DOI] |
| 27. | Seager Danciger J, Lutz M, Hama S, Cruz D, Castrillo A, Lazaro J, Phillips R, Premack B, Berliner J. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288:123-134. [PubMed] [DOI] |
| 28. | Foti M, Granucci F, Ricciardi-Castagnoli P. Dendritic cell interactions and cytokine production. Ernst Schering Res Found Workshop. 2006;61-80. [PubMed] |
| 29. | Reis E Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476-483. [PubMed] [DOI] |
| 30. | Talmor M, Mirza A, Turley S, Mellman I, Hoffman LA, Steinman RM. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998;28:811-817. [PubMed] [DOI] |