修回日期: 2006-04-25
接受日期: 2006-05-08
在线出版日期: 2006-06-18
目的: 探讨实验性急性胰腺炎(acute pancreatitis, AP)合并肺损伤的发生机制及前列腺素E1 (PGE1)的保护作用.
方法: 健康成年SD大鼠78只, 随机平均分为假手术组(SO组)、AP组和PGE1组, 采用十二指肠闭袢法建立大鼠AP模型. PGE1组制模后即刻经颈静脉持续每分钟输入PGE1 60 ng/kg. 观察胰腺和肺组织的病理组织学改变, 测定血清淀粉酶、肺组织中性粒细胞髓过氧化物酶(MPO)活性、脂质过氧化产物(LPO)水平及肺毛细血管通透性(LCP), 免疫组织化学ABC法检测肺组织细胞黏附分子-1(ICAM-1)的表达.
结果: 制模后12和24 h, AP组大鼠胰腺和肺组织病理损伤持续加重, 肺组织MPO (12 h: 5.65±0.80 vs 1.22±0.71 kat/g, P<0.01; 24 h: 7.22±1.05 vs 1.48±0.57 kat/g, P<0.01)和LPO (12 h: 1.44±0.63 vs 0.38±0.07 μmol/g, P<0.01; 24 h: 3.64±0.83 vs 0.44±0.15 μmol/g, P<0.01)水平以及LCP (12 h: 145.4±23.0 vs 47.3±5.5 μg/g组织湿重, P<0.01)明显高于SO组, AP组大鼠肺组织ICAM-1表达呈阳性或强阳性, 而SO组呈阴性; 与AP组比较, PGE1组的胰腺病理损伤虽未减轻, 但肺组织MPO (12 h: 2.96±1.04 vs 5.65±0.80 kat/g, P<0.05; 24 h: 3.68±1.15 vs 7.22±1.05 kat/g, P<0.05)和LPO (12 h: 0.86±0.34 vs 1.44±0.63 μmol/g, P<0.05; 24 h: 1.69±0.45 vs 3.64±0.83 μmol/g, P<0.05)水平以及LCP (12 h: 105.9±23.9 vs 145.4±23.0 μg/g组织湿重, P<0.05)明显降低, ICAM-1表达下调. 肺间质出血、水肿和中性粒细胞(PMN)浸润明显减轻.
结论: 肺组织ICAM-1过度表达、PMN浸润和氧自由基大量释放与AP早期肺损伤的发生关系密切. PGE1通过降低肺组织ICAM-1表达, 抑制PMN活化和氧自由基释放, 从而减轻AP早期肺损伤.
引文著录: 尹勇, 李兆丽. 大鼠急性胰腺炎早期肺损伤机制及前列腺素E1的保护作用. 世界华人消化杂志 2006; 14(17): 1688-1692
Revised: April 25, 2006
Accepted: May 8, 2006
Published online: June 18, 2006
AIM: To explore the mechanism of lung injury in experimental acute pancreatitis (AP) and the protective effect of prostaglandin E1 (PGE1).
METHODS: Seventy-eight rats were averagely and randomly divided into sham operation, AP, and PGE1 group. AP model was induced by creating a closed duod enal loop in rats. The rats in PGE1 group were intravenously injected with PGE1 (60 ng/kg). The histopathological changes of pancreatic and pulmonary tissues were examined by microscopy. The serum level of amylase, the activity of myeloperoxidase (MPO), the pulmonary level of lipid peroxidation (LPO), and lung capillary permeability (LCP) were measured. The expression of pulmonary intercellular adhesion molecule-1 (ICAM-1) was determined by immunohistochemical technique (ABC).
RESULTS: In AP group, the progressive pathological damages in the pancreas and lung tissues were clearly observed. The activity of pulmonary MPO (12 h: 5.65 ± 0.80 vs 1.22 ± 0.71 kat/g, P < 0.01; 24 h: 7.22 ± 1.05 vs 1.48 ± 0.57 kat/g, P < 0.01), the level of LPO (12 h: 1.44 ± 0.63 vs 0.38 ± 0.07 μmol/g, P < 0.01; 24 h: 3.64 ± 0.83 vs 0.44 ± 0.15 μmol/g, P < 0.01) and LCP (12 h: 145.4 ± 23.0 vs 47.3 ± 5.5 μg/g wet weight, P < 0.01), as well as pulmonary ICAM-1 expression were markedly increased as compared with those in sham operation group. In comparison with those in AP group, the activity of MPO (12 h: 2.96 ± 1.04 vs 5.65 ± 0.80 kat/g, P < 0.05; 24 h: 3.68 ± 1.15 vs 7.22 ± 1.05 kat/g, P < 0.05) and the level of LPO (12 h: 0.86 ± 0.34 vs 1.44 ± 0.63 μmol/g, P < 0.05; 24 h: 1.69 ± 0.45 vs 3.64 ± 0.83 μmol/g, P < 0.05) in the lung tissues were significantly decreased, and the level of LCP (12 h: 105.9 ± 23.9 vs 145.4 ± 23.0 μg/g wet weight, P < 0.05), as well as pulmonary ICAM-1 expression was down-regulated (12 h: P < 0.05; 24 h: P < 0.01) in PGE1 group. Pathological examination revealed that intra-alveolar hemorrhage, edema and polymorphonuclear leukocytes (PMN) infiltration in the lung tissues were attenuated after PGE1 treatment, although pancreatic damages were not alleviated.
CONCLUSION: The over-expression of ICAM-1, PMN infiltration and the release of free oxygen radicals in lung tissues may be closely related to pancreatitis-associated lung injury. PGE1 can ameliorate lung injury by reducing ICAM-1 expression and inhibiting PMN activation and the release of free oxygen radicals.
- Citation: Yin Y, Li ZL. Mechanism of lung injury and protective effect of prostaglandin E1 in experimental acute pancreatitis. Shijie Huaren Xiaohua Zazhi 2006; 14(17): 1688-1692
- URL: https://www.wjgnet.com/1009-3079/full/v14/i17/1688.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i17.1688
急性肺损伤是重症胰腺炎早期主要合并症之一, 在发病1 wk内死亡的病例中有60%归因于急性肺损伤[1], 目前治疗尚无良策. 前列腺素E1(PGE1)是一种活性较强的二十碳烯酸代谢产物, 研究发现, PGE1对急性胰腺炎(acute pancreatitis, AP)早期肺损伤具有防治作用[2], 我们旨在探究AP早期肺损伤的发生机制, 并观察PGE1的治疗作用.
PGE1针剂为重庆药友制药有限公司产品; 戊巴比妥钠, Serva进口分装; 伊文思蓝, Fluka进口分装; 髓过氧化物酶(MPO)、脂质过氧化产物(LPO)丙二醛检测试剂盒由南京建成生物工程研究所提供, 小鼠抗大鼠细胞间黏附分子-1(ICAM-1)mAb、SABC免疫组化检测试剂盒及DAB显色试剂盒购自武汉博士德生物工程有限公司. 健康成年Sprague-Dawley大鼠78只, 雄雌不拘, 体质量180-250 g.
大鼠随机分为假手术组(SO组)、AP组和PGE1组, 每组各26只. 参照Nevalainen et al[3]的十二指肠闭袢法制备大鼠AP模型, 术前禁食24 h, 自由饮水, 30 g/L戊巴比妥钠(30 mg/kg) ip麻醉, 颈静脉插管成功后经皮下隧道引至背部与微量输液泵连接, 消毒腹部皮肤, 正中切口入腹, 胃窦部切开将长3 cm内径0.4 cm无菌塑料软管插入十二指肠, 结扎十二指肠形成闭袢, 袢长2 cm, 缝合胃窦部切口及腹壁. SO组仅作胃窦切开术. PGE1组制模后即刻经颈静脉持续每分钟输入PGE1 60 ng/kg, SO组和AP组给予等量生理盐水. 术后12, 24 h每组各取10只大鼠采血用碘比色法测定血清淀粉酶(AMY), 处死大鼠迅速取胰腺和肺组织固定于40 g/L甲醛溶液中, 包埋制片, 采用盲法由病理医师在光镜下观察胰腺和肺组织的病理改变和肺组织ICAM-1蛋白的表达, 另取数块肺组织-70℃保存待测. (1)取肺组织约100 mg, 制备肺组织匀浆, 超声粉碎亚细胞成分, 然后按照MPO检测试剂盒说明书进行操作. (2)肺组织LPO含量的测定采用硫代巴比妥酸法, 按照试剂盒说明书进行操作, 考马斯亮兰法测定组织蛋白含量. (3)肺组织石蜡切片ICAM-1表达采用辣根过氧化物酶标记链霉卵白素(ABC)免疫组织化学法, 一抗工作浓度为1∶50, 以PBS液代替一抗孵育作为阴性对照. 判断标准: 根据细胞染色的深浅和分布进行半定量分析. 无染色-0分, 淡黄色-1分, 棕黄色-2分, 深棕黄色-3分; 视野中显色细胞所占的比例<20%: 0分, 20%-40%: 1分, 40%-60%: 2分, >60%: 3分, 取10个视野的平均值. 将两项评分的乘积作为积分数, 根据积分将ICAM-1表达分为4级: 0分: 阴性, 1-3分: 弱阳性, 4-6分: 阳性, >6分: 强阳性. (4)肺脏毛细血管通透性(LCP)的测定. 每组各取6只大鼠, 于术后12 h经尾静脉穿刺注入20 g/L伊文思蓝(20 mg/kg), 15 min后脱颈处死动物, 迅速剖胸, 由右心室匀速注入生理盐水10 mL冲洗血管内染料, 取出肺脏, 将肺表面水分吸尽后称取湿质量, 浸入甲酰胺4 mL中, 37℃放置24 h, 提取组织中染料, 过滤, 染液620 nm处读取吸光度(A值), 通过标准曲线计算提取出的染料量, 以单位组织中染料提取量反映肺脏毛细血管通透性. 伊文思蓝标准曲线的建立方法: 分别取1 g/L伊文思蓝标准品0, 5, 15, 20, 30, 40 mL, 加入甲酰胺4 mL中, 620 nm比色, 数据进行线性回归分析, 回归方程为: A = 0.011x-0.001 (r = 0.99).
统计学处理 用SPSS 10.0软件进行统计学分析, 组间比较采用t检验, ICAM-1表达的积分数比较采用秩和检验.
术后12, 24 h AP组和PGE1组大鼠AMY水平均显著高于SO组(P<0.01, 表1), 而PGE1组与AP组比较差异无显著性. 术后12 h AP组和PGE1组肺组织MPO, LPO水平均显著高于SO组(P<0.01, 表1), 至术后24 h, 两组MPO, LPO水平继续升高; 而PGE1组两时间点MPO, LPO水平明显低于AP组(P<0.05, 表1).
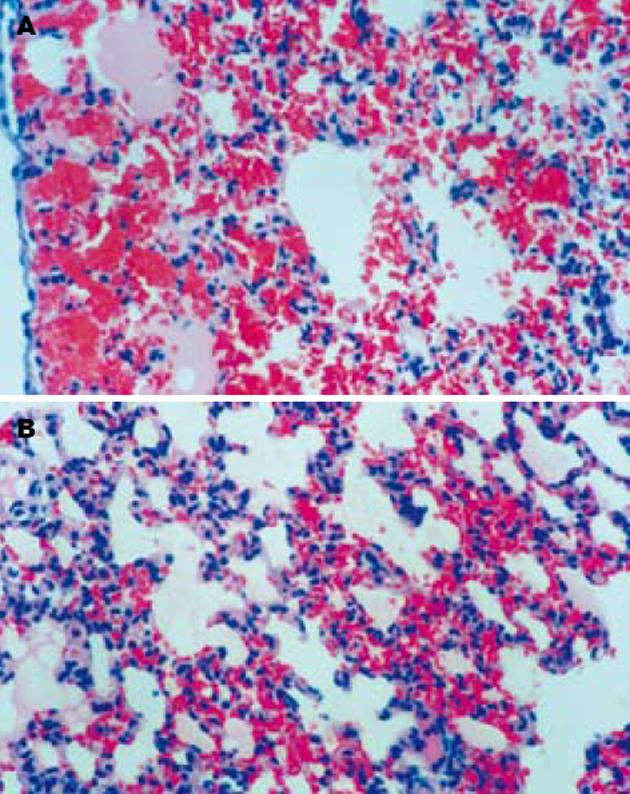
SO组胰腺和肺脏组织结构清晰, 无异常改变. AP组术后12 h胰腺组织间质水肿, 可见红细胞漏出, 血管扩张充血, 腺泡间隔增宽, 部分腺泡组织坏死伴PMN浸润, 24 h腺泡坏死范围扩大, 间质出血、PMN浸润更加明显. AP组术后12 h肺间质毛细血管扩张、充血, 肺泡壁塌陷, 肺泡隔PMN浸润明显, 部分肺泡腔有红细胞漏出, 24 h肺组织出血明显加重, 肺泡腔内可见淡红色液体渗出(肺水肿). PGE1组胰腺的病理损伤与AP组无明显差异, 但两时间点肺脏间质充血、出血、水肿和PMN浸润均较AP组明显减轻(图1).
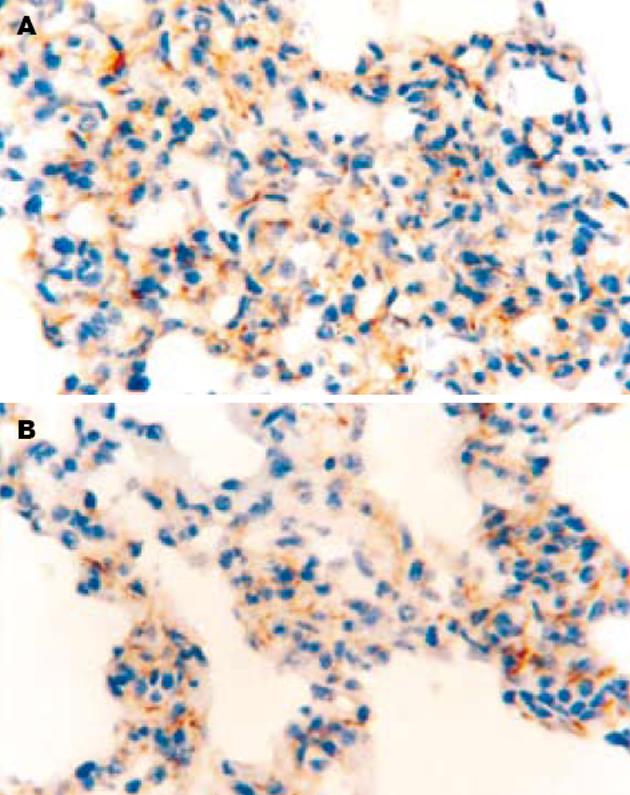
SO组肺泡上皮细胞和肺泡隔血管内皮细胞表面均无染色, ICAM-1的表达为阴性. AP组术后12 h肺泡隔血管内皮细胞表面出现淡黄色或棕黄色颗粒, 部分肺泡Ⅰ, Ⅱ型上皮细胞表面也可见淡黄色颗粒, ICAM-1表达为阳性; 24 h肺泡隔血管内皮细胞和肺泡上皮细胞表面出现深棕黄色颗粒, ICAM-1表达呈现强阳性. PGE1组12, 24 h肺组织ICAM-1表达的分布与AP组基本一致, 但其表达强度的积分数明显低于AP组(12 h: P<0.05; 24 h: P<0.01)(图2).
术后12 h SO组大鼠LCP值为47.3±5.5 μg/g组织湿质量, AP组和PGE1组的LCP值分别为145.4±23.0 μg/g和105.9±23.9 μg/g, 均显著高于SO组(P<0.01), 而PGE1组LCP值与AP组相比明显降低(P<0.05).
急性胰腺炎(AP)是临床常见的消化系急症, 尤其重症胰腺炎发病急骤、凶险, 死亡率高达40%[1]. AP早期易并发急性肺损伤, 其病理特点与急性呼吸窘迫综合征(ARDS)十分相似, 表现为肺泡毛细血管壁通透性增高, 肺泡内皮细胞和肺间质损伤, 肺水肿及中性粒细胞(PMN)聚集. 文献报道95%的重症胰腺炎早期死亡病例存在肺水肿和肺淤血的表现, 发病1 wk内的死亡病例中有60%与急性肺损伤关系密切[1,4], 可见防治急性肺损伤是降低重症胰腺炎早期死亡率的重要措施. 目前对于AP合并肺损伤的发生机制尚未完全阐明, 但研究发现, 大量PMN过度激活并聚集于肺组织是急性肺损伤早期的基本病理改变, 也是导致急性肺损伤的关键因素[5-12]. 过度激活的PMN跨内皮细胞迁移、浸润, 直接释放弹性蛋白酶、促炎细胞因子和氧自由基等大量细胞毒性物质, 使肺内皮细胞损伤、变性, 血管通透性增加, 肺间质水肿, 氧交换障碍[4,13-17]; 另外, 聚集的PMN机械堵塞肺毛细血管致微循环障碍, 并可产生缩血管活性物质, 使肺血管收缩, 产生肺动脉高压, 导致通气/血流比例失调、低氧血症, 发生ARDS[18].
在PMN跨越内皮细胞屏障、聚集浸润于局部受损区域之前, 首先须完成与血管内皮细胞的黏附. 细胞黏附是一个复杂、多步骤的过程, 需要多种细胞表面黏附分子的参与. 其中, PMN表面的整合素CD11b/CD18β2复合体与血管内皮细胞表面的细胞间黏附分子-1(ICAM-1)以受体-配体的形式相结合是细胞黏附过程中的关键环节. 实验研究发现, AP鼠的胰腺、肺脏及血清中ICAM-1水平均明显升高, 胰腺、肺脏有大量PMN浸润, 而敲除ICAM-1基因或应用抗ICAM-1抗体后AP鼠胰腺、肺脏中PMN积聚数目明显减少, 微血管通透性降低, 组织损伤也明显减轻[14,19-22]. 临床研究也发现, AP早期患者血浆可溶性ICAM-1水平高于正常对照组, 且与胰腺坏死性组织损伤程度密切相关[23-24]. 本实验结果显示, AP组大鼠术后12 h肺组织出现明显的肺泡壁塌陷、充血、出血和PMN浸润等病理改变, 至24 h肺组织损伤程度更为严重. 同时发现, 术后12 h肺泡隔血管内皮细胞和部分肺泡上皮细胞表面即可见ICAM-1表达, 术后24 h肺组织ICAM-1表达强度和范围进一步增大, 肺组织MPO和LPO水平明显高于对照组. 以上结果进一步表明, AP早期肺损伤与肺组织ICAM-1高表达和大量PMN浸润密切相关. 因此, 早期阻断或抑制细胞黏附分子的表达, 降低PMN的黏附能力, 减少活化PMN的迁移和各种炎症介质的释放, 可能成为防治AP早期急性肺损伤的有效手段.
PGE1是一种活性较强的二十碳烯酸代谢产物, 他具有扩张血管, 改善组织血供, 抑制血小板聚集等作用. 此外, PGE1通过扩张血管, 增加血流速度, 机械性阻止血液中PMN在微血管内皮细胞表面的滚动、紧密黏附及穿越过程, 从而抑制PMN活化和毒性介质的释放, 并诱导具有保护效应因子的生成[2,25-26]. 但在AP时, PGE1对各种细胞黏附分子的表达有无直接影响目前并不十分清楚. 本研究结果显示, 术后12和24 h, PGE1组大鼠肺组织中ICAM-1表达较AP组明显减低, MPO活性和LPO水平明显下降. 术后12 h, PGE1组肺毛细血管通透性明显低于AP组. PGE1组大鼠胰腺的病理损伤虽无改善, 但肺组织出血、水肿和PMN浸润等病理改变明显减轻. 我们认为, PGE1可下调大鼠肺脏ICAM-1的表达, 降低PMN的黏附和聚集, 从而减少氧自由基及脂质过氧化产物的释放, 对肺泡上皮细胞和肺泡隔血管内皮细胞起到一定的保护作用. PGE1的抗黏附作用为AP合并肺损伤的治疗提供了一条新的思路.
急性肺损伤是重症胰腺炎早期的严重并发症, 文献报道95%的重症胰腺炎早期死亡病例存在肺水肿和肺淤血的表现, 在发病7 d内的死亡病例中有60%与急性肺损伤关系密切, 可见防治急性肺损伤对于降低重症胰腺炎早期死亡率至关重要. 研究发现, 大量中性粒细胞聚集于肺组织是急性肺损伤早期的基本病理改变, 也是导致急性肺损伤的重要因素.
本实验研究显示, 肺组织ICAM-1过度表达、中性粒细胞浸润和氧自由基大量释放与AP早期肺损伤的发生关系密切. PGE1能够降低肺组织ICAM-1表达, 抑制中性粒细胞活化和氧自由基释放, 减轻AP早期肺损伤. 这一结果为临床防治重症胰腺炎早期肺损伤提供了理论依据.
本研究立题依据充分, 实验动物选择标准, 模型建立和实验操作规范, 结论可靠, 文章简洁、论点明确, 条理性强, 组织学图谱清晰漂亮.
电编: 张敏 编辑:潘伯荣
| 1. | Renner IG, Savage WT 3rd, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005-1018. [PubMed] [DOI] |
| 2. | Yamanaka K, Saluja AK, Brown GE, Yamaguchi Y, Hofbauer B, Steer ML. Protective effects of prostaglandin E1 on acute lung injury of caerulein-induced acute pancreatitis in rats. Am J Physiol. 1997;272:G23-30. [PubMed] |
| 3. | Nevalainen TJ, Seppa A. Acute pancreatitis caused by closed duodenal loop in the rat. Scand J Gastroenterol. 1975;10:521-527. [PubMed] |
| 4. | Werner J, Z'graggen K, Fernandez-del Castillo C, Lewandrowski KB, Compton CC, Warshaw AL. Specific therapy for local and systemic complica-tions of acute pancreatitis with monoclonal antibo-dies against ICAM-1. Ann Surg. 1999;229:834-840; discussion 841-842. [PubMed] [DOI] |
| 5. | Banerjee AK, Haggie SJ, Jones RB, Basran GS. Respiratory failure in acute pancreatitis. Postgrad Med J. 1995;71:327-330. [PubMed] [DOI] |
| 6. | Willemer S, Feddersen CO, Karges W, Adler G. Lung injury in acute experimental pancreatitis in rats. I. Morphological studies. Int J Pancreatol. 1991;8:305-321. [PubMed] |
| 7. | Donnelly SC, Haslett C. Cellular mechanisms of acute lung injury: implications for future treatment in the adult respiratory distress syndrome. Thorax. 1992;47:260-263. [PubMed] [DOI] |
| 8. | Wittel UA, Rau B, Gansauge F, Gansauge S, Nussler AK, Beger HG, Poch B. Influence of PMN leukocyte-mediated pancreatic damage on the systemic immune response in severe acute pancreatitis in rats. Dig Dis Sci. 2004;49:1348-1357. [PubMed] [DOI] |
| 9. | Hartwig W, Werner J, Warshaw AL, Antoniu B, Castillo CF, Gebhard MM, Uhl W, Buchler MW. Membrane-bound ICAM-1 is upregulated by trypsin and contributes to leukocyte migration in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1194-1199. [PubMed] [DOI] |
| 10. | Kyriakides C, Jasleen J, Wang Y, Moore FD Jr, Ashley SW, Hechtman HB. Neutrophils, not complement, mediate the mortality of experimental hemorrhagic pancreatitis. Pancreas. 2001;22:40-46. [PubMed] [DOI] |
| 11. | Liu XM, Xu J, Wang ZF. Pathogenesis of acute lung injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2005;4:614-617. [PubMed] |
| 12. | Liu XM, Liu QG, Xu J, Pan CE. Microcirculation disturbance affects rats with acute severe pancrea-titis following lung injury. World J Gastroenterol. 2005;11:6208-6211. [PubMed] [DOI] |
| 13. | Wang X, Sun Z, Borjesson A, Andersson R. Inhibition of platelet-activating factor, intercellular adhesion molecule 1 and platelet endothelial cell adhesion molecule 1 reduces experimental pancrea-titis-associated gut endothelial barrier dysfunction. Br J Surg. 1999;86:411-416. [PubMed] [DOI] |
| 14. | Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, Steer ML. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694-701. [PubMed] [DOI] |
| 15. | Lundberg AH, Granger N, Russell J, Callicutt S, Gaber LW, Kotb M, Sabek O, Gaber AO. Temporal correlation of tumor necrosis factor-alpha release, upregulation of pulmonary ICAM-1 and VCAM-1, neutrophil sequestration, and lung injury in diet-induced pancreatitis. J Gastrointest Surg. 2000;4:248-257. [PubMed] [DOI] |
| 16. | Frossard JL, Hadengue A, Spahr L, Morel P, Pastor CM. Natural history of long-term lung injury in mouse experimental pancreatitis. Crit Care Med. 2002;30:1541-1546. [PubMed] [DOI] |
| 17. | Steer ML. Relationship between pancreatitis and lung diseases. Respir Physiol. 2001;128:13-16. [PubMed] [DOI] |
| 18. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [PubMed] [DOI] |
| 19. | Lundberg AH, Fukatsu K, Gaber L, Callicutt S, Kotb M, Wilcox H, Kudsk K, Gaber AO. Blocking pulmonary ICAM-1 expression ameliorates lung injury in established diet-induced pancreatitis. Ann Surg. 2001;233:213-220. [PubMed] [DOI] |
| 20. | Zaninovic V, Gukovskaya AS, Gukovsky I, Mouria M, Pandol SJ. Cerulein upregulates ICAM-1 in pancreatic acinar cells, which mediates neutrophil adhesion to these cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G666-676. [PubMed] |
| 21. | Foitzik T, Eibl G, Buhr HJ. Therapy for microcircu-latory disorders in severe acute pancreatitis: compa-rison of delayed therapy with ICAM-1 antibodies and a specific endothelin A receptor antagonist. J Gastrointest Surg. 2000;4:240-246; discussion 247. [PubMed] [DOI] |
| 22. | Zhao X, Dib M, Wang X, Widegren B, Andersson R. Influence of mast cells on the expression of adhesion molecules on circulating and migrating leukocytes in acute pancreatitis-associated lung injury. Lung. 2005;183:253-264. [PubMed] [DOI] |
| 23. | Kaufmann P, Demel U, Tilz GP, Krejs GJ. Time course of plasma soluble intercellular adhesion molecule-1 (sICAM-1) is related to severity of acute pancreatitis. Hepatogastroenterology. 1999;46:2565-2571. [PubMed] |
| 24. | Nakae H, Endo S, Sato N, Wakabayashi G, Inada K, Sato S. Involvement of soluble adhesion molecules in acute pancreatitis. Eur Surg Res. 2001;33:377-382. [PubMed] [DOI] |
| 25. | de Perrot M, Fischer S, Liu M, Jin R, Bai XH, Waddell TK, Keshavjee S. Prostaglandin E1 protects lung transplants from ischemia-reperfusion injury: a shift from pro- to anti-inflammatory cytokines. Transplantation. 2001;72:1505-1512. [PubMed] [DOI] |
| 26. | Matsuo K, Togo S, Sekido H, Morita T, Kamiyama M, Morioka D, Kubota T, Miura Y, Tanaka K, Ishikawa T. Pharmacologic preconditioning effects: prostaglandin E1 induces heat-shock proteins immediately after ischemia/reperfusion of the mouse liver. J Gastrointest Surg. 2005;9:758-768. [PubMed] [DOI] |