修回日期: 2005-10-27
接受日期: 2005-10-31
在线出版日期: 2005-12-28
目的: 研究幽门螺杆菌(Helicobacter pylori, H. pylori)在大鼠胃黏膜上皮诱导细胞凋亡中的作用, 并初步探讨其中凋亡相关基因表达的情况, 为胃癌发病机制提供依据.
方法: H. pylori超声提取液来自Sydney SS-1 H. pylori菌株. 大鼠胃黏膜细胞OUMS-37为永生化细胞, 当细胞生长至60%融合时, 加入不同浓度的H. pylori超声提取液, 同时设置空白, 于培养的24-48 h收集细胞进行形态观察. Westhern blotting检测P53蛋白表达, Northern blotting检测bax、bcl-2 mRNA的表达.
结果: 细胞经H. pylori作用48 h后在高倍镜下观察到细胞核碎裂成大小不等的块状, 表现出细胞凋亡如细胞皱缩, 胞浆嗜碱性, 核染色质固缩致密, 核染色质断裂, 形成大小不等的胞内核小体, 部分细胞核膜消失, 核染色质聚集中细胞中央, 呈现分裂期的形态学等形态学特征, 对照组未出现以上特征性改变. 培养细胞经过H. pylori作用后提取DNA, 经15 g/L琼脂糖凝胶电泳, 在紫外线灯下观察呈现不连续的梯状结构电泳条带. 培养细胞经过H. pylori作用后, Westhern blotting显示P53 蛋白表达随H. pylori超声提取液浓度而升高, Northern blotting显示bax mRNA 表达随H. pylori浓度而增加, bcl-2 mRNA表达随H. pylori浓度而降低.
结论: H. pylori超声提取液可在体外诱导鼠胃黏膜上皮细胞凋亡. 其机制可能通过上调野生型P53蛋白和凋亡促进基因bax mRNA表达, 并下调凋亡抑制基因bcl-2 mRNA表达. 提示H. pylori感染可通过干扰胃上皮细胞增殖与凋亡之间的平衡在胃癌病因学中发挥作用.
引文著录: 姜海行, 聂海明, 邓德海, 覃山羽, 陶霖, 黄振宁. 幽门螺杆菌体外诱导大鼠胃黏膜上皮细胞凋亡. 世界华人消化杂志 2005; 13(24): 2838-2841
Revised: October 27, 2005
Accepted: October 31, 2005
Published online: December 28, 2005
AIM: To investigate the effect of Helicobacter pylori (H. pylori) sonicated extract on the apoptosis of rat gastric epithelial cells as well as the expression of apoptosis-related genes in vitro.
METHODS: H. pylori sonicated extract from strain Sydney SS-1 was cultured with OUMS-37, a kind of immortalized rat gastric cell lines. Apoptosis of the cells was confirmed according to specific changes of morphology and DNA ladder 24-48 h after co-incubation. The expression of P53 protein was detected by Western blotting and the expression of bax and bcl-2 mRNA were observed by Northern blotting.
RESULTS: The specific morphology of the cells such as shrinkage, condensation, margination of nuclear chromatin and apoptotic bodies were observed under light microscope. DNA ladder was manifested by fragment analysis. Western blotting showed a dose-dependent increased expression of wild-type P53 protein and Northern blotting showed a dose-pendent increased expression of bax mRNA and reduced expression of bcl-2 mRNA in the treated cells.
CONCLUSION: H. pylori sonicated extract induces the apoptosis in vitro through up-regulation of wild-type P53 protein and bax mRNA expression, and down-regulation of bcl-2 mRNA expression, suggesting that H. pylori infection may interrupt the balance between proliferation and apoptosis of the gastric epithelial cells, which plays a key role in gastric carcinogenesis.
- Citation: Jiang HX, Nie HM, Deng DH, Qin SY, Tao L, Huang ZN. Helicobacter pylori induces apoptosis of rat gastric epithelial cells in vitro. Shijie Huaren Xiaohua Zazhi 2005; 13(24): 2838-2841
- URL: https://www.wjgnet.com/1009-3079/full/v13/i24/2838.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i24.2838
细胞增殖和细胞凋亡间的平衡是多细胞生物体维持自身稳定的重要因素, 当两者发生失平衡时, 将导致细胞恶性病变. 体内外研究显示幽门螺杆菌(Helicobacter pylori, H. pylori)感染可能是胃癌发生的重要因素[1,2], 尽管世界卫生组织将H. pylori定为I类致癌源[3], 但确切机制尚未阐明. 我们在一种新建立的大鼠永生化细胞系(immortalized cell lines)OUMS-37[4]研究H. pylori在胃黏膜上皮诱导细胞凋亡中的作用, 并初步探讨其中凋亡相关基因表达的情况, 为胃癌发生的机制提供理论依据.
bcl-2, bax cDNA探针由日本冈山大学医学部细胞生物部门迁俊也惠赠. P53单抗为Santa Cruz产品; RediprimerⅡ随机引物标记系统购自Amersham Pharmacia公司. H. pylori菌株Sydney SS-1由日本冈山大学医学部细菌科横田副教授惠赠, Hela细胞空泡试验证实为VacA阳性. H. pylori在Brucella broth培养基 (Difco, 美国) 微氧环境下振荡中培养7 d后收获, 以6 000 g离心30 min, 弃上清, 用20 mL冰PBS (pH7.4)洗涤2次, 然后将其置于超声粉碎仪 (Astrason W-380, INC) 破碎细菌20 min, 在4 ℃以20 000 g离心20 min, 收集上清, 经0.4 μm滤纸过滤, 测定蛋白质浓度后, 置-80 ℃保存.
大鼠胃黏膜细胞OUMS-37由日本冈山大学医学部细胞生物部门难波教授惠赠. 将1.5×105个细胞接种于35 mm培养皿中, 培养基为含100 mL/L小牛血清的DMED液 (Nissui, 日本), 在37 ℃, 50 mL/L CO5饱和湿度的恒温培养箱内培养, 当细胞生长至60%融合时, 更换培养基并加入浓度700, 900, 1100, 1300 mg/L的H. pylori超声提取液, 同时设置空白对照组, 于培养的48 h收集细胞进行形态观察和其他相关基因检测. 细胞经H. pylori处理后定时用胰酶消化收集, 细胞离心涂片, 甲醇室温固定, Wright-Giemsa染色3 min, 高倍镜下观察. 用胰酶消化收集每组6×106细胞, PBS缓冲液洗涤2次, 细胞溶于500 μL含5 mmol/L Tris-HCL, 20 mmol/L EDTA和50 g/L TritonBX-100溶液中, 置于冰上30 min, 离心提取上清液, 上清液经酚/氯仿/异戊醇 (25:24:1)提取和酒精沉淀, RNA酶消化, 重新提取和沉淀. DNA淀物溶解于10 μL TE缓冲液 (pH8.0) 中, 以50 g/L琼脂糖凝胶电泳, DNA条带在紫外线灯下观察摄片.
1.2.1 Northern blotting分析: 采用硫氰酸胍-酚-氯仿一步法提取方法提取细胞总RNA, RNA定量后再取20 μg, 经甲醛-琼脂糖凝胶电泳分离, 应用虹吸迹法将RNA转移至Hybond N+尼龙膜 (Amersham, UK) 上. 杂交液含5×SSC, 500 g/L甲酰胺, 1×Denhart's溶液, 20 mol/L磷酸钠 (pH6.8), 5 mol/L EDTA, 2 g/LSDS及100 mg/L 热变性蛙精DNA, bcl-2及bax探针浓度为30 μg/L, 探针cDNA用P-dCTP随机引物反应标记, 于42 ℃旋转杂交24 h, 洗膜后凉干, 置-80 ℃曝光7 d.
1.2.2 Western blotting分析: 取上述刺激细胞, 以每107细胞加入60 μL细胞裂碎液10 min (150 mol/L NaCl, 1 mol/L EGTA, 1 mol/L EDTA, 10 g/L Triton-100, 1 g/L CHAPS, 2 mg/L aprotinin, 2 mg/L leupeptin)12 000 g离心15 min, 取上清, 以Braford法测定蛋白质浓度后, 每孔蛋白含量为40 μg, 煮沸变性5 min, 经150 g/L SDS-PAGE电泳, 过夜, 然后电转移到Hybond ECL硝酸纤维膜 (Amersham, UK) 上, 100 g/L脱脂奶/PBST (含0.1% Tween20) 溶液封闭1 h, 加入1:1000 P53 单抗, PBS-T洗涤后以1:1000 Bio-HRP (Amersham, UK) 二抗与膜孵育1 h, PBS-T洗涤5次, 采用ECL系统曝光显迹.
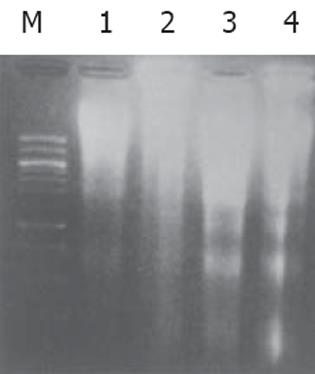
细胞经H. pylori (400 mg/L) 处理48 h后, 主要形态变化为细胞固缩、胞质嗜碱性, 核染色质固缩致密, 或者核染色质断裂, 形成大小不等的胞内核小体, 部分细胞核膜消失, 核染色质聚集中细胞中央, 胞质略嗜酸性, 呈现分裂期的形态学表现. DNA沉淀物溶解于10 μL TE缓冲液 (pH8.0) 中, 经15 g/L琼脂糖凝胶电泳, 在紫外线灯下观察呈现不连续呈梯状结构电泳条带, 对照组未见梯状条带(图1).
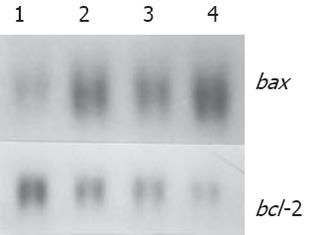
随H. pylori超声提取液浓度而增强, bcl-2 mRNA表达随H. pylori浓度而减弱 (图2).
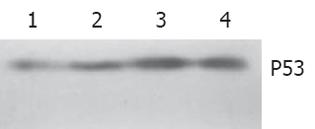
随H. pylori超声提取液浓度而增强(图3).
细胞凋亡是一种多基因调控的自身程序性细胞死亡, 有关人体H. pylori感染伴随细胞凋亡已经有许多报道[5-10], 多数结果显示感染H. pylori后细胞凋亡指数显著增加, 推测细胞凋亡可能是细胞增生过度的一种代偿性机制, 可作为潜在的癌前反应. Chen et al[5]报道H. pylori感染患者细胞增殖指数和细胞凋亡指数显著高于非感染患者, 在根除H. pylori感染成功的患者, 细胞凋亡指数可恢复正常[8-10]. 在体外研究中, 不仅在培养的胃上皮细胞中加入H. pylori能诱导细胞凋亡[11-16], 而且在分离出的单核细胞或巨噬细胞中H. pylori也能诱导细胞凋亡[17-19]. 我们将H. pylori超生提取液在一种新建立起的具有分泌胃蛋白酶原的永生细胞系 (OUMS-37) 中诱导细胞凋亡, 与以往报道结果一致. 初期研究认为全菌接触细胞是诱导凋亡的条件之一, 以后研究表明H. pylori的脂多糖成份以及H. pylori的CagA和VacA状态也与细胞凋亡有关[20-24]. 一组前瞻性研究显示只有CagA阳性菌感染才伴随胃黏膜上皮细胞凋亡增加[21], 但Peek et al[25]报道CagA+ VacA s1 阳性菌感染伴随细胞增殖系数的增加, 而细胞凋亡无显著改变, 推测这种不伴随细胞凋亡的过度增殖可能是CagA+ VacA s1阳性菌致病的机制之一. 有关H. pylori VacA毒素状态与细胞凋亡相关性研究报道较多, 多数研究认为VacA毒素本身能诱导细胞凋亡, 采用基因重组[22]或VacA阳性菌株培养上清液[23,24]提取获得的VacA均在胃黏膜上皮细胞系中成功诱导细胞凋亡, 本研究H. pylori虽然为VacA阳性菌株, 但鉴于VacA毒素一般存在于细菌的培养液中, 从菌体提取的超声粉碎物中VacA含量是非常低的, 故本文诱导细胞凋亡是非依赖VacA毒素的. 本研究还显示, H. pylori诱导细胞凋亡不需全菌黏附, 进一步研究显示菌体超声提取液通过100 ℃加热10 min后其诱导凋亡作用减弱或消失, 提示可能是菌体超声提取液中的活性蛋白成份起到作用, 该结果与shibayama et al[26]报道一致.
关于H. pylori诱导细胞凋亡的基因调控机制仍在研究之中.体外研究中Kurosawa et al[27]报道细胞凋亡伴有P53和bax表达增强, Zhang et al[28]报道P53阳性时细胞凋亡作用更强, Cho et al[22]报道细胞凋亡伴有P53, P21和bax表达增强, 而Ashktorab et al报道细胞凋亡伴有P53和P14表达增强[29], Unger et al在体内研究也证实了以上观点[30]. Choi et al[31]结果显示H. pylori诱导细胞凋亡伴bcl-2表达下调. 一般认为bcl-2家族由一组高度同源蛋白组成, 经bcl-2家族成员或其他蛋白之间的互相作用来调节细胞内信号传导, 进而调节细胞凋亡效应通路之下游共同的调定点. 在bcl-2家族中, bax, bak, bclxs等促进细胞凋亡, 而bcl-2, bfl-1, bclxl等则抑制凋亡. 在肿瘤研究过程中也表明, 野生型P53可诱导bcl-2表达的减少和bax表达的增加, P53是上游调控基因, bcl-2和bax是下游调控基因, bcl-2和bax的比例决定细胞是否存在还是凋亡. 本研究显示, H. pylori呈现剂量依赖性地诱导野生型P53表达, 而且bax mRNA表达逐渐增强, bcl-2 mRNA表达逐渐减弱, 与H. pylori诱导细胞凋亡结果一致, 支持以上结论.
本结果说明, H. pylori超声提取物可在体外诱导鼠胃黏膜上皮细胞凋亡, 其机制可能通过在转录水平上调野生型P53和凋亡促进基因bax表达, 并下调凋亡抑制基因bcl-2表达, 说明H. pylori在干扰胃黏膜细胞增殖和细胞凋亡间的平衡中发挥重要因素.
致谢: 感谢日本冈山大学医学部细胞生物部门难波教授惠赠大鼠OUMS-37细胞系; 日本冈山大学医学部细菌科横田副教授惠赠幽门螺杆菌菌株.
电编: 张勇 编辑: 潘伯荣 审读: 张海宁
| 1. | Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16-26. [PubMed] [DOI] |
| 2. | Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521-8525. [PubMed] [DOI] |
| 3. | Schistosomes, liver flukes and helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 4. | Jiang HX, Pu H, Huh NH, Yokota K, Oguma K, Namba M. Helicobacter pylori induces pepsinogen secretion by rat gastric cells in culture via a cAMP signal pathway. Int J Mol Med. 2001;7:625-629. [PubMed] [DOI] |
| 5. | Chen X, Wang MW, You WD. Relationship between Helicobacter pylori infection and proliferation and apoptosis of gastric epithelial dysplasia cell. Ai Zheng. 2003;22:244-247. [PubMed] |
| 6. | Shirin H, Hibshoosh H, Kawabata Y, Weinstein IB, Moss SF. p16Ink4a is overexpressed in H. pylori-associated gastritis and is correlated with increased epithelial apoptosis. Helicobacter. 2003;8:66-71. [PubMed] [DOI] |
| 7. | Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and P53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779-782. [PubMed] [DOI] |
| 8. | Unger Z, Molnar B, Szaleczky E, Torgyekes E, Muller F, Zagoni T, Tulassay Z, Pronai L. Effect of Helicobacter pylori infection and eradication on gastric epithelial cell proliferation and apoptosis. J Physiol Paris. 2001;95:355-360. [PubMed] [DOI] |
| 9. | Ohara T, Kanoh Y, Higuchi K, Arakawa T, Morisita T. Eradication therapy of Helicobacter pylori directly induces apoptosis in inflammation-related immunocytes in the gastric mucosa--possible mechanism for cure of peptic ulcer disease and MALT lymphoma with a low-grade malignancy. Hepatogastroenterology. 2003;50:607-609. [PubMed] |
| 10. | Satoh K, Kawata H, Tokumaru K, Kumakura Y, Ishino Y, Kawakami S, Inoue K, Kojima T, Satoh Y, Mutoh H. Change in apoptosis in the gastric surface epithelium and glands after eradication of Helicobacter pylori. Dig Liver Dis. 2003;35:78-84. [PubMed] [DOI] |
| 11. | Maeda S, Yoshida H, Mitsuno Y, Hirata Y, Ogura K, Shiratori Y, Omata M. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Mol Pathol. 2002;55:286-293. [PubMed] [DOI] |
| 12. | Chen Y, Wang Y, Xu W, Zhang Z. Analysis on the mechanism of Helicobacter pylori-induced apoptosis in gastric cancer cell line BGC-823. Int J Mol Med. 2005;16:741-745. [PubMed] |
| 13. | Martin JH, Potthoff A, Ledig S, Cornberg M, Jandl O, Manns MP, Kubicka S, Flemming P, Athmann C, Beil W. Effect of H. pylori on the expression of TRAIL, FasL and their receptor subtypes in human gastric epithelial cells and their role in apoptosis. Helicobacter. 2004;9:371-386. [PubMed] [DOI] |
| 14. | Wu YY, Tsai HF, Lin WC, Chou AH, Chen HT, Yang JC, Hsu PI, Hsu PN. Helicobacter pylori enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in human gastric epithelial cells. World J Gastroenterol. 2004;10:2334-2339. [PubMed] [DOI] |
| 15. | Neu B, Randlkofer P, Neuhofer M, Voland P, Mayerhofer A, Gerhard M, Schepp W, Prinz C. Helicobacter pylori induces apoptosis of rat gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G309-G318. [PubMed] [DOI] |
| 16. | Yang Y, Deng CS, Peng JZ, Wong BC, Lam SK, Xia HH. Effect of Helicobacter pylori on apoptosis and apoptosis related genes in gastric cancer cells. Mol Pathol. 2003;56:19-24. [PubMed] [DOI] |
| 17. | Galgani M, Busiello I, Censini S, Zappacosta S, Racioppi L, Zarrilli R. Helicobacter pylori induces apoptosis of human monocytes but not monocyte-derived dendritic cells: role of the cag pathogenicity island. Infect Immun. 2004;72:4480-4485. [PubMed] [DOI] |
| 18. | Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA Jr, Wilson KT. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161-40173. [PubMed] [DOI] |
| 19. | Menaker RJ, Ceponis PJ, Jones NL. Helicobacter pylori induces apoptosis of macrophages in association with alterations in the mitochondrial pathway. Infect Immun. 2004;72:2889-2898. [PubMed] [DOI] |
| 20. | Kawahara T, Teshima S, Kuwano Y, Oka A, Kishi K, Rokutan K. Helicobacter pylori lipopolysaccharide induces apoptosis of cultured guinea pig gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G726-G734. [PubMed] |
| 21. | Moss SF, Sordillo EM, Abdalla AM, Makarov V, Hanzely Z, Perez-Perez GI, Blaser MJ, Holt PR. Increased gastric epithelial cell apoptosis associated with colonization with cagA+Helicobacter pylori strains. Cancer Res. 2001;61:1406-1411. [PubMed] |
| 22. | Cho SJ, Kang NS, Park SY, Kim BO, Rhee DK, Pyo S. Induction of apoptosis and expression of apoptosis related genes in human epithelial carcinoma cells by Helicobacter pylori VacA toxin. Toxicon. 2003;42:601-611. [PubMed] [DOI] |
| 23. | Cover TL, Krishna US, Israel DA, Peek RM Jr. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951-957. [PubMed] |
| 24. | Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080-5087. [PubMed] [DOI] |
| 25. | Peek RM Jr, Moss SF, Tham KT, Perez-Perez GI, Wang S, Miller GG, Atherton JC, Holt PR, Blaser MJ. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863-868. [PubMed] [DOI] |
| 26. | Shibayama K, Kamachi K, Nagata N, Yagi T, Nada T, Doi Y, Shibata N, Yokoyama K, Yamane K, Kato H. A novel apoptosis-inducing protein from Helicobacter pylori. Mol Microbiol. 2003;47:443-451. [PubMed] [DOI] |
| 27. | Kurosawa A, Miwa H, Hirose M, Tsune I, Nagahara A, Sato N. Inhibition of cell proliferation and induction of apoptosis by Helicobacter pylori through increased phosphorylated p21 and Bax expression in endothelial cells. J Med Microbiol. 2002;51:385-391. [PubMed] [DOI] |
| 28. | Zhang ZW, Patchett SE, Farthing MJ. Role of Helicobacter pylori and P53 in regulation of gastric epithelial cell cycle phase progression. Dig Dis Sci. 2002;47:987-995. [PubMed] [DOI] |
| 29. | Ashktorab H, Ahmed A, Littleton G, Wang XW, Allen CR, Tackey R, Walters C, Smoot DT. P53 and p14 increase sensitivity of gastric cells to H. pylori-induced apoptosis. Dig Dis Sci. 2003;48:1284-1291. [PubMed] [DOI] |
| 30. | Unger Z, Molnar B, Pronai L, Szaleczky E, Zagoni T, Tulassay Z. Mutant P53 expression and apoptotic activity of Helicobacter pylori positive and negative gastritis in correlation with the presence of intestinal metaplasia. Eur J Gastroenterol Hepatol. 2003;15:389-393. [PubMed] [DOI] |
| 31. | Choi IJ, Kim JS, Kim JM, Jung HC, Song IS. Effect of inhibition of extracellular signal-regulated kinase 1 and 2 pathway on apoptosis and bcl-2 expression in Helicobacter pylori-infected AGS cells. Infect Immun. 2003;71:830-837. [PubMed] [DOI] |