修回日期: 2005-09-25
接受日期: 2005-10-19
在线出版日期: 2005-12-28
目的: 探讨ALA-PDT诱导人结肠癌细胞SW480凋亡和游离钙浓度变化关系.
方法: 将SW480细胞分为四组: 空白对照组、激光照射照组、ALA组和ALA-PDT组, 用DNA片段分析和TUNEL法检测细胞凋亡; 用激光共聚焦显微镜观测各组细胞内游离钙离子浓度的变化.
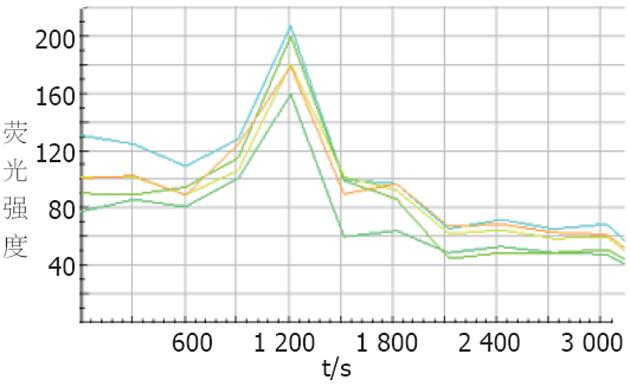
结果: ALA-PDT组的人结肠癌细胞在1、2 h有大量的DNA片段, TUNEL法显示ALA-PDT组的人结肠癌细胞在PDT后30 min AI为25.26%±5.04%, PDT后60 min AI为50.45%±7.85%, 均高于其他3组 (AI均<10%, P<0.01); 激光共聚焦结果为ALA-PDT组细胞内游离钙离子浓度在20 min达高峰 (荧光强度: 185.40±18.90), 与10 min (荧光强度: 100.00±19.83) 相比有显著差异(P<0.01), 之后又逐渐下降.
结论: 细胞内Ca2+浓度的逐渐增加在PDT诱导的细胞凋亡过程中可能起着重要作用.
引文著录: 郑江华, 时德, 陈祖林. ALA-PDT 诱导SW480细胞凋亡和胞内 Ca2+ 浓度变化的关系. 世界华人消化杂志 2005; 13(24): 2828-2832
Revised: September 25, 2005
Accepted: October 19, 2005
Published online: December 28, 2005
AIM: To investigate relationship between intracellular Ca2+ and apoptosis induced by aminolaevulinic acid-photodynamic therapy (ALA-PDT) in SW480 cells.
METHODS: SW480 cells were divided into control, light, ALA and ALA-PDT group. The corresponding treatment was performed in each group. The apoptosis of SW480 cells was detected by DNA fragment assay and TUNEL assay. The changes of intracelluar Ca2+ concentration in each group were observed by confocal laser scanning microscopy.
RESULTS: DNA ladder formation of apoptotic features was demonstrated 1 and 2 h after ALA-PDT treatment. The apoptosis index (AI) of 30 and 60 min after ALA-PDT treatment was 25.26% ± 5.04% and 50.45% ± 7.85%, respectively, which were significantly higher than those in the other 3 groups (all AI <10%, P < 0.01). The concentration of intracelluar Ca2+ 20 min after ALA-PDT treatment was markedly increased than that at 10 min (fluorescent intensity: 185.40 ± 18.90 vs 100.00 ± 19.83, P < 0.01), and then gradually decreased. However, the concentrations of intracelluar Ca2+ had no significant changes in the other 3 groups.
CONCLUSION: The increases of intracellular Ca2+ may play an important role in the ALA-PDT-induced apoptosis in SW480 cells.
- Citation: Zheng JH, Shi D, Chen ZL. Relationship between intracellular Ca2+ and ALA-PDT induced apoptosis in SW480 cells. Shijie Huaren Xiaohua Zazhi 2005; 13(24): 2828-2832
- URL: https://www.wjgnet.com/1009-3079/full/v13/i24/2828.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i24.2828
δ氨基酮戊酸 (aminolaevulinic acid, ALA)-光动力疗法(photodynamic therapy, PDT) 是指ALA诱导肿瘤细胞产生原卟啉Ⅸ (protoporphyrin,Pp Ⅸ), Pp Ⅸ 接受光照后在细胞内产生活性氧物质, 而导致肿瘤细胞凋亡或坏死的一种治疗方法,又称为内源性光动力疗法[1]. ALA代谢快, 毒副作用小, 是一种很有前途的治疗肿瘤的方法[2]. 细胞分子生物学的发展促进了PDT诱导细胞死亡机制的研究, 1991年, Agarwal et al首次证实了PDT能通过诱导细胞凋亡导致小鼠L5178细胞死亡, 自此, PDT诱导许多不同细胞系细胞发生凋亡等到了证实[3-6]. 我们探讨ALA-PDT诱导SW480发生细胞凋亡期间细胞内Ca2+浓度变化, 分析其作用机制.
ALA(Sigma公司); 培养液RPMI1640(Hyclone公司); D-Hanks(Hyclone公司); Hepes(Sigma公司); 胰蛋白酶(北京鼎国生物技术公司); 小牛血清 (成都哈里公司); TUNEL试剂盒 (Roche公司); Fluo-3/AM(Sigma公司); SW480(第四军医大学动物实验中心提供).
1.2.1 PDT处理[7]: 用含有青霉素和链霉素 (分别100 kU/L) 和100 mL/L小牛血清的RPMI 1640培养基培养人结肠癌细胞 (SW480), 37 ℃, 50 mL/L CO2, 饱和湿度, 单层贴壁生长. 实验分为4组: 空白对照组, 激光照射组, ALA组及ALA-PDT组. 光敏剂ALA用PBS配制成400 mg/L的储存液, 过滤待用, 工作液用无血清培养基稀释为40 mg/L (终浓度). ALA组和ALA-PDT处理组在暗室按预定时间加入ALA, 严格避光条件下继续孵育4 h, 激光照射组和ALA-PDT处理组使用半导体激光仪 (西南师范大学激光所, 波长532 nm, 距光斑3 cm处输出功率12 mw)垂直照射培养板30 min. 空白对照组不加ALA且不受激光照射, ALA组加入ALA不受激光照射.
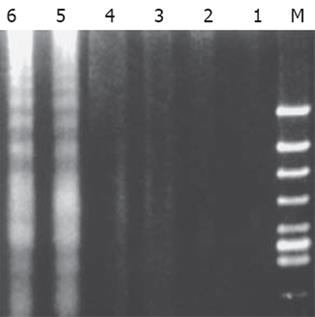
1.2.2 DNA片断分析: SW480细胞 (2-6×109/L)经ALA-PDT作用0、1、2 h后收获细胞, PBS(pH7.4) 洗2遍, 沉淀加入50 μL细胞裂解液 (10 mmol/L Tris-HCl, 10 mmol/L NaCl, 10 mmol/L EDTA, 10 g/L SDS pH8.0) 和终浓度为0.5 g/L的蛋白酶K, 50 ℃水浴2 h, 5 000 r/min离心10 min, 上清移入新Eppendorf管, 以等体积的苯酚/氯仿(1:1)、苯酚/氯仿/异丙醇 (25:24:1) 和氯仿各抽提1次, 取上层水相加1/10体积3 mol/L醋酸钠(NaAc)、2倍体积冷无水乙醇, 颠倒混匀, 置 -20 ℃ 30 min, 13 000 g离心10 min, 烘干乙醇和NaAc, 沉淀加20 μL TE缓冲液 (50 mmol/L Tris-HCl, 10 mmol/L EDTA)溶解, 加RNase A(200 mg/L)于37 ℃ 1 h, 取20 μL样品加6×上样缓冲液4 μL混合上样, 另取一道加入DNA Marker, 15 g/L琼脂糖凝胶电泳 (凝胶含0.5 mg/L EB), 紫外灯下观察并摄影.
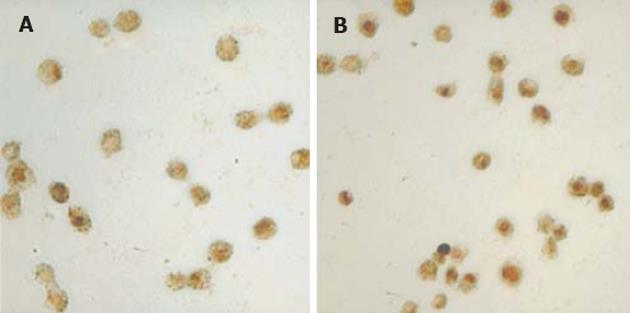
1.2.3 末端标记法(TUNEL)检测细胞凋亡[8]: 采用盖玻片培养法, 取对数生长细胞1×108/L, 接种到含有高压灭菌盖玻片 (22 mm×22 mm, 100 g/L多聚赖氨酸处理)的6孔培养板中, 48 h后行ALA-PDT处理, 分别在PDT后30 min和60 min后, 取出盖玻片, 用PBS冲洗3次, 16 g/L多聚甲醛固定30 min, 其余步骤按试剂盒说明进行,光镜下分别记数明显的5个高倍视野内的凋亡细胞数和细胞总数, 计算凋亡指数 (apoptosis index, AI). AI = 凋亡细胞数/细胞总数×100%.
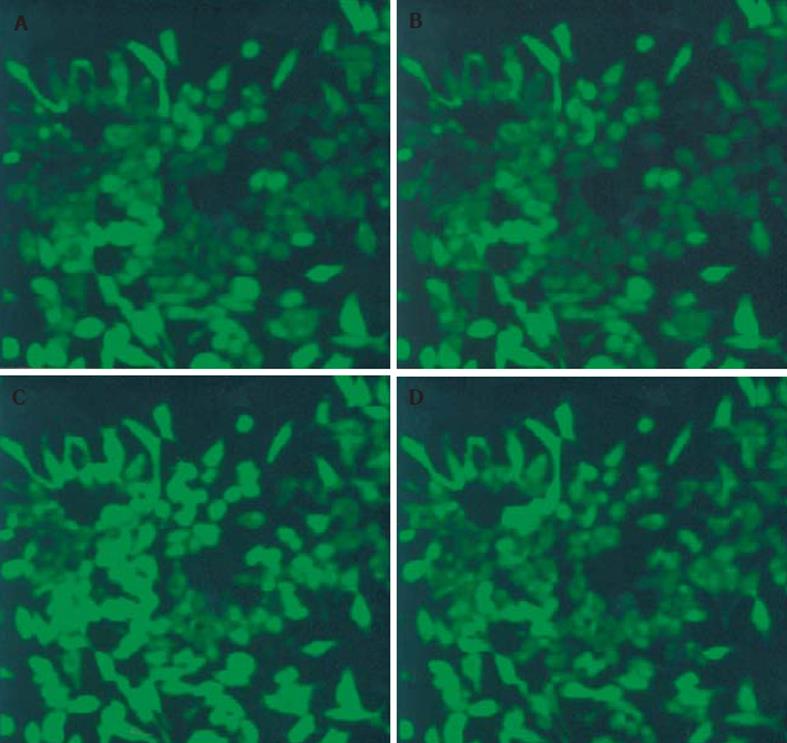
1.2.4 激光共聚焦显微镜动态观察[9]: 取对数生长细胞1×108/L, 接种到含有高压灭菌盖玻片 (22 mm×22 mm, 100 g/L多聚赖氨酸处理) 的6孔培养板中, 孵育48 h后, 用D-Hanks液漂洗3次, PDT处理后, 各组细胞均用 D-Hanks液漂洗三次, 用终浓度4.4 μm/L的Fluo-3/AM溶液于37 oC避光条件下染色30 min, D-Hanks液漂洗3次, 最后再用D-Hanks液酯化15 min, 激光照射后, 将盖玻片置于激光共聚焦显微镜载物台上, 选择激发波长488 nm、发射波长526 nm, 动态观察各组细胞的荧光强度, 每5 min扫描1次, 共60 min. 因为Fluo-3/AM与细胞内游离钙离子结合显示荧光, 其荧光强度与所结合的Fluo-3/AM细胞内游离钙离子浓度成正比, 所以测得的荧光强度可作为反映细胞内游离钙离子浓度的指标.
统计学处理 数据以mean±SD表示, 显著性检验采用χ2检验, 采用SPSS10.0统计软件进行数据处理.
SW480细胞经ALA-PDT分别处理1、2 h后可见明显的DNA梯形条带 (DNA ladder), PDT即刻、激光照射组、空白对照组以及ALA组未见DNA梯形条带 (图1).
光镜下阳性细胞明显皱缩, 呈圆形, 核染色质致密, 染成棕色. ALA-PDT后细胞有明显凋亡, PDT后30 min AI为25.26%+5.04%, PDT后60 min AI为50.45%+7.85%, 其余各组, 包括激光照射组, 空白对照组以及ALA组AI均<10%, 有显著差异(P<0.01, 图2).
PDT作用过程中, 光敏剂特异聚集在肿瘤细胞或组织中, 在适当波长 (通常是红光或近紫外光或与光敏剂的吸光谱相近的光) 的光照下, 光敏剂吸收特定波长的光后, 转变为激发三重态光敏剂, 此三重态光敏剂可经Ⅰ或Ⅱ型反应型反应, 产生光敏剂的自由基离子或单线态氧, 自由基离子进一步与周围的氧反应生成氧化产物; 单线态氧是一种高反应性物质, 能与脂肪酸、蛋白质及核酸等物质反应而产生损伤效应, 最终导致细胞凋亡或死亡[10]. 由于PDT处理的细胞可见膜去极化[11-13], 细胞内K+外流, Na+-K+依赖式ATP酶抑制[14], 因此PDT诱导细胞凋亡主要靶点可能细胞膜[15-18]. 有作者认为位于细胞膜上的磷脂酶A2 (PLA2) 和磷脂酶C(PLC) 的激活是PDT诱导细胞凋亡的早期机制, 理由是Ca2+作为细胞信号的第二信使受大量刺激因素的影响, 并与PLA2和PLC激活有关[19]. 激活的PLC水解磷脂酰肌醇-4,5-二磷酸 (PIP2) 产生三磷酸肌醇 (IP3) 和二酰甘油 (DG), IP3促进细胞内Ca2+浓度增加, DG激活蛋白酶C(PKC), 从而分别形成IP3/Ca2+和DG/PKC信号传递途径[20-22]. 在IP3/Ca2+途径中, Ca2+与钙调蛋白(CaM)结合激活calcineurin(CN), 由CN将磷酸化的NF-AT(nuclear factor of activated T cell) 脱磷酸[23], NF-AT因而进入核内, 并与转录因子AP-1(activator protein-1)结合, 从而诱导IL-2基因转录[24-26]. 因此, 本实验的目的是探讨PDT后细胞早期凋亡和细胞内钙浓度的关系.
在我们的实验中, DNA断裂片段出现在PDT后1-2 h,凋亡形态学 (TUNEL) 结果显示: PDT后20 min AI为25.26%+5.04%, PDT后40 min AI为50.45%+7.85%, 在其他细胞系, PDT后细胞早期也发生凋亡, Kuzalova et al[27]用 ALA-PDT 处理K562细胞, 发现2 h后细胞出现凋亡特征. 另外, 激光共聚焦显微镜动态观察结果表明PDT后10 min时荧光强度为100.00+19.83, 之后逐渐增加, 20 min时达185.40+18.90, 之后又逐渐下降, 30 min后继续降至基线水平以下, 而空白对照组、激光照射组以及ALA组细胞内钙无明显变化, 在实验过程中, 采用D-hanks液平衡SW480细胞, 避开细胞外钙离子内流途径, 说明ALA-PDT对SW480细胞内的钙库有促进其开放的作用, 从而引起细胞内钙离子浓度升高; 30 min后游离钙继续降至基线水平以下可能是由于ALA-PDT 后细胞质膜系统受到破坏, 细胞内钙离子流向细胞外造成的. Tajiri et al[28]用卟菲尔钠 (porfimer sodium)-PDT处理人鳞状细胞癌HSC-2, 发现细胞内钙离子在PDT后2 h达高峰. Zhou et al[29]用竹红菌素A-PDT处理人胃癌细胞MGC803, 结果表明细胞内钙离子浓度在PDT后60 s达高峰. 造成实验结果不一致的原因可能是由于光敏剂、细胞类型、光照剂量以及观察时间不同[30]. PDT后20 min, 细胞出现凋亡, 而PDT后10-20 min细胞内Ca2+浓度逐渐增加, 20 min达高峰, 提示了细胞内Ca2+浓度的逐渐增加在PDT诱导的细胞凋亡过程中可能起重要作用, 达高峰时立即引起 PDT诱导细胞凋亡. 为了证实ALA-PDT诱导SW480细胞凋亡中是否存在IP3/Ca2+信号传递途径, 有必要对CaM, CN, NF-AT以及AP-1等蛋白表达进一步研究.
电编: 张勇 编辑: 潘伯荣 审读: 张海宁
| 1. | Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ. Photodynamic therapy in oncology. Expert Opin Pharmacother. 2001;2:917-927. [PubMed] [DOI] |
| 2. | Haddad R, Kaplan O, Greenberg R, Siegal A, Skornick Y, Kashtan H. Photodynamic therapy of murine colon cancer and melanoma using systemic aminolevulinic acid as a photosensitizer. Int J Surg Investig. 2000;2:171-178. [PubMed] |
| 3. | Almeida RD, Manadas BJ, Carvalho AP, Duarte CB. Intracellular signaling mechanisms in photodynamic therapy. Biochim Biophys Acta. 2004;1704:59-86. [PubMed] |
| 4. | Saczko J, Kulbacka J, Chwilkowska A, Drag-Zalesiniska M, Wysocka T, Lugowski M, Banas T. The influence of photodynamic therapy on apoptosis in human melanoma cell line. Folia Histochem Cytobiol. 2005;43:129-132. [PubMed] |
| 5. | Sarissky M, Lavicka J, Kocanova S, Sulla I, Mirossay A, Miskovsky P, Gajdos M, Mojzis J, Mirossay L. Diazepam enhances hypericin-induced photocytotoxicity and apoptosis in human glioblastoma cells. Neoplasma. 2005;52:352-359. [PubMed] |
| 6. | Huang HF, Chen YZ, Wu Y. ZnPcS2P2-based photodynamic therapy induces mitochondria-dependent apoptosis in K562 cells. Acta Biochim Biophys Sin. 2005;37:488-494. [PubMed] [DOI] |
| 7. | Tsai T, Hong RL, Tsai JC, Lou PJ, Ling IF, Chen CT. Effect of 5-aminolevulinic acid-mediated photodynamic therapy on MCF-7 and MCF-7/ADR cells. Lasers Surg Med. 2004;34:62-72. [PubMed] [DOI] |
| 8. | Houston A, Waldron-Lynch FD, Bennett MW, Roche D, O'Sullivan GC, Shanahan F, O'Connell J. Fas ligand expressed in colon cancer is not associated with increased apoptosis of tumor cells in vivo. Int J Cancer. 2003;107:209-214. [PubMed] [DOI] |
| 9. | Kai L, Wang ZF, Shi YL, Liu LM, Hu DY. Opioid receptor antagonists increase [Ca2+]i in rat arterial smooth muscle cells in hemorrhagic shock. Acta Pharmacol Sin. 2004;25:395-400. [PubMed] |
| 10. | Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem Photobiol. 2002;75:382-391. [PubMed] [DOI] |
| 11. | Marchal S, Bezdetnaya L, Guillemin F. Modality of cell death induced by Foscan-based photodynamic treatment in human colon adenocarcinoma cell line HT29. Biochemistry. 2004;69:45-49. [PubMed] [DOI] |
| 12. | Teiten MH, Marchal S, D'Hallewin MA, Guillemin F, Bezdetnaya L. Primary photodamage sites and mitochondrial events after Foscan photosensitization of MCF-7 human breast cancer cells. Photochem Photobiol. 2003;78:9-14. [PubMed] [DOI] |
| 13. | Dolgachev V, Nagy B, Taffe B, Hanada K, Separovic D. Reactive oxygen species generation is independent of de novo sphingolipids in apoptotic photosensitized cells. Exp Cell Res. 2003;288:425-436. [PubMed] [DOI] |
| 14. | Ben-Hur E, Dubbelman TM, Van Steveninck. Effect of fluoride on inhibition of plasma membrane functions in Chinese hamster ovary cells photosensitized by aluminum phthalocyanine. Radiat Res. 1992;131:47-52. [PubMed] [DOI] |
| 15. | Uzdensky A, Juzeniene A, Ma LW, Moan J. Photodynamic inhibition of enzymatic detachment of human cancer cells from a substratum. Biochim Biophys Acta. 2004;1670:1-11. [PubMed] |
| 16. | Sharma M, Bansal H, Gupta PK. Photodynamic action of merocyanine 540 on carcinoma of cervix cells. Indian J Exp Biol. 2002;40:252-257. [PubMed] |
| 17. | Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379-47386. [PubMed] [DOI] |
| 18. | Kriska T, Korytowski W, Girotti AW. Hyperresistance to photosensitized lipid peroxidation and apoptotic killing in 5-aminolevulinate-treated tumor cells overexpressing mitochondrial GPX4. Free Radic Biol Med. 2002;33:1389-1402. [PubMed] [DOI] |
| 19. | Molgo J, Colasantei C, Adams DS, Jaimovich E. IP3 receptors and Ca2+ signals in adult skeletal muscle satellite cells in situ. Biol Res. 2004;37:635-639. [PubMed] [DOI] |
| 20. | Hanson CJ, Bootman MD, Roderick HL. Cell signalling: IP3 receptors channel calcium into cell death. Curr Biol. 2004;14:R933-R935. [PubMed] [DOI] |
| 21. | van Dijk M, Muriana FJ, van Der Hoeven PC, de Widt J, Schaap D, Moolenaar WH, van Blitterswijk WJ. Diacylglycerol generated by exogenous phospholipase C activates the mitogen-activated protein kinase pathway independent of Ras- and phorbol ester-sensitive protein kinase C: dependence on protein kinase C-zeta. Biochem J. 1997;323:693-699. [PubMed] [DOI] |
| 22. | Jiang G, Wei Q. Function and structure of N-terminal and C-terminal domains of calcineurin B subunit. Biol Chem. 2003;384:1299-1303. [PubMed] [DOI] |
| 23. | Sancho R, Macho A, de La Vega L, Calzado MA, Fiebich BL, Appendino G, Munoz E. Immunosuppressive activity of endovanilloids: N-arachidonoyl-dopamine inhibits activation of the NF-kappa B, NFAT, and activator protein 1 signaling pathways. J Immunol. 2004;172:2341-2351. [PubMed] [DOI] |
| 24. | Faubert Kaplan BL, Kaminski NE. Cannabinoids inhibit the activation of ERK MAPK in PMA/Io-stimulated mouse splenocytes. Int Immunopharmacol. 2003;3:1503-1510. [PubMed] [DOI] |
| 25. | He KL, Ting AT. Essential role for IKKgamma/NEMO in TCR-induced IL-2 expression in Jurkat T cells. Eur J Immunol. 2003;33:1917-1924. [PubMed] [DOI] |
| 26. | Agarwal ML, Larkin HE, Zaidi SI, Mukhtar H, Oleinick NL. Phospholipase activation triggers apoptosis in photosensitized mouse lymphoma. Cancer Res. 1993;53:5897-5902. [PubMed] |
| 27. | Kuzalova K, Grebenova D, Pluskanova M, Marinov I, Hrkal Z. Early apoptotic features of K562 cell death induced by 5-aminolaevulinic acid-based photodynamic therapy. Photochem Photobiol. 2004;73:67-78. [PubMed] [DOI] |
| 28. | Tajiri H, Hayakawa A, Matsumoto Y, Yokoyama I, Yoshida S. Changes in intracellular Ca2+ concentrations related to PDT-induced apoptosis in photosensitized human cancer cell. Cancer Lett. 1998;128:205-210. [PubMed] [DOI] |
| 29. | Zhou ZH, Yang HG, Zhang ZY. Role of calcium in phototoxicity of 2-butylamino-2-demethoxy-hypocrellin A to human gastric cancer MGC-803. Biochim Biophys Acta. 2003;1593:191-200. [PubMed] [DOI] |
| 30. | Wyld L, Reed MW, Brown NJ. Differential cell death response to photodynamic therapy is dependent on dose and cell type. Br J Cancer. 2001;84:1384-1386. [PubMed] [DOI] |