Copyright
©The Author(s) 2005. Published by Baishideng Publishing Group Inc. All rights reserved.
小鼠暴发性肝衰竭肝细胞凋亡形态学及其调控机制
王玉梅, 冯国和, 窦晓光, 刘德刚
王玉梅, 冯国和, 窦晓光, 中国医科大学附属第二医院感染科 辽宁省沈阳市 110004
刘德刚, 鞍山市曙光医院医疗科 辽宁省鞍山市 114031
王玉梅, 女, 1970-02-26生, 山西省大同市人, 2002年中国医科大学博士, 副教授, 主要从事病毒性肝炎发病机制的研究.
ORCID number: $[AuthorORCIDs]
电话: 024-83956961 传真: 024-62124785
收稿日期: 2005-08-03
修回日期: 2005-08-15
接受日期: 2005-08-26
在线出版日期: 2005-11-28
目的: 研究在实验性暴发性肝衰竭(fulminant hepatic failure, FHF)中肝细胞凋亡的形态学变化以及一氧化氮(nitric oxide, NO)、Fas和Bcl-2对肝细胞凋亡的调控作用.
方法: 脂多糖(LPS)和D-氨基半乳糖(D-GalN)联合应用制备FHF小鼠模型; 采用免疫组化方法检测肝组织Fas及Bcl-2表达, 分别采用硝酸还原酶法和RT-PCR法检测血清NO水平及肝组织iNOS mRNA表达; TUNEL法检测肝细胞凋亡; 在用药后动态观察Fas及Bcl-2表达、血清NO水平及肝组织iNOS mRNA表达及肝细胞凋亡的变化, 并对模型鼠给予iNOS的抑制剂L-NMMA, 动态观察上述指标的变化.
结果: 在FHF模型小鼠中, 用药后2 h开始血清NO水平及iNOS mRNA的表达即升高, 于4 h达高峰; 用药后2 h 开始Fas有少量表达, 至8 h和12 h表达均明显增多, 与2 h组比较差异显著(100% vs 20%, P<0.01), 与4 h组比较差异也比较显著(100% vs 40%, P<0.05). 模型组2 h Bcl-2有较多表达, 4 h表达最多, 4 h与2 h比较差异显著(90% vs 60%, P<0.05), 与8, 12 h比较差异非常显著(90% vs 20%, 均P<0.01 ). 8 h可出现典型的肝细胞凋亡表现. 与模型组各时间点比较, 给予iNOS的抑制剂L-NMMA后血清NO水平及iNOS mRNA的表达均为正常水平, Fas及Bcl-2表达均无显著差异(P>0.05), 阻断后8 h亦可见典型的肝细胞凋亡表现, 阻断NO可使病变较模型组更为严重.
结论: 在FHF中, Fas及Bcl-2的表达均增加, Fas的表达与肝细胞凋亡相一致, 而Bcl-2的表达与肝细胞凋亡呈负相关. 单纯应用NO拮抗剂对肝细胞凋亡及肝损伤无保护作用.
关键词: 一氧化氮; Fas抗原; Bcl-2; 暴发性肝衰竭
引文著录: 王玉梅, 冯国和, 窦晓光, 刘德刚. 小鼠暴发性肝衰竭肝细胞凋亡形态学及其调控机制. 世界华人消化杂志 2005; 13(22): 2658-2662
Morphology and regulatory mechanism of hepatocyte apoptosis in experimental fulminant hepatic failure
Yu-Mei Wang, Guo-He Feng, Xiao-Guang Dou, De-Gang Liu
Yu-Mei Wang, Guo-He Feng, Xiao-Guang Dou, Department of Infectious Diseases, the Second Affiliated Hospital of China Medical University, Shenyang 110004, Liaoning Province, China
De-Gang Liu, Department of Medicare, Shuguang Hospital, Anshan 114031, Liaoning Province, China
Correspondence to: Yu-Mei Wang, Department of Infectious Diseases, the Second Affiliated Hospital of China Medical University, Shenyang 110004, Liaoning Province,China. wangym7002@yahoo.com
Received: August 3, 2005
Revised: August 15, 2005
Accepted: August 26, 2005
Published online: November 28, 2005
AIM: To study the morphological changes and the regulation of nitric oxide (NO), Fasand Bcl-2 on hepatocyte apoptosis in mouse model of experimental fulminant hepatic failure (FHF)..
METHODS: Mouse model of experimental FHF was established by combination of lipopolysaccharide (LPS) and D-galactosamin (D-GalN). The expression of Fas and Bcl-2 in the liver tissues was tested by immunohistochemistry. The level of serum NO and iNOS mRNA expression in liver were tested by nitrate reductase method and reverse transcription polymerase chain reaction (RT-PCR), respectively. The hepatocyte apoptosis was examined by TUNEL method. In addition, the changes of the above items were observed after pretreatment with L-NMMA, an inhibitor of iNOS.
RESULTS: The level of serum NO and expression of iNOS mRNA in the liver tissues were increased at 2 h in model group, reaching the peak at 4 h. There was a little Fas expression at 2 h in model group. The expression of Fas was increased significantly at 8 and 12 h, which was distinctly higher than that at 2 h (100% vs 20%, P < 0.01) and 4 h (100% vs 40%, P < 0.05). The expression of Bcl-2 started to increase at 2 h, reaching the peak at 4 h, which was markedly higher than that at 2 h (90% vs 60%, P < 0.05). The expression of Bcl-2 at 4 h was also significantly higher than that at 8 or 12 h (90% vs 20%, both P < 0.01). Typical features of hepatocyte apoptosis were observed at 8 h. The level of serum NO and liver iNOS mRNA expression were normal and the Fas, Bcl-2 expression did not change notably after L-NMMA administration in comparison with those in model group (P > 0.05). Typical hepatocyte apoptosis was also observed at 8 h after L-NMMA administration, and the pathological changes of the liver tissues were more severe.
CONCLUSION: Both expression of Fas and Bcl-2 are increased in FHF. Fas expression is consistent with hepatocyte apoptosis, while Bcl-2 expression is negatively correlated with hepatocyte apoptosis. Single administration of iNOS inhibitor can not protect hepatocytes against apoptosis and injury.
Key Words: Nitric oxide; Fas; Bcl-2; Fulminant hepatic failure
0 引言
细胞凋亡有赖于各种因素的调控, 如Fas系统、Bcl-2家族、一氧化氮(NO)等[1-8], 而暴发性肝衰竭(FHF)又与肝细胞凋亡的发生密切相关[9-13], 故研究以上各因素在FHF时对肝细胞凋亡的作用十分必要, 这将有助于进一步了解FHF时肝细胞凋亡的途径, 并对各因素的作用及影响因素进行探讨.
1 材料和方法
1.1材料
NO试剂盒购自南京建成生物公司, iNOS mRNA引物购自北京奥科生物公司, cDNA合成试剂盒购自宝生物工程有限公司(TaKaRa产品), LPS购自Sigma公司, D-GalN购自重庆医科大学, L-NMMA(Cayman公司产品)购自深圳晶美生物公司, TUNEL试剂盒、Bcl-2免疫组化试剂盒及Fas免疫组化试剂盒均购自武汉博士德生物公司, ♂昆明小鼠(中国医科大学附属二院动物实验室提供)100只, 清洁级, 体质量20-25 g, 随机分成12组, 取4组(每组10只)给予LPS 10 μg/kg皮内注射, D-GalN 800mg/kg ip(模型组), 另取4组(每组10只)除注射LPS和D-GalN外, 同时ip NO合酶抑制剂L-NMMA 7 mg/鼠(抗NO组), 余4组动物(每组5只)注射同样体积的生理盐水(正常对照组), 对上述小鼠分别于注药后2, 4, 8, 12 h处死, 每个时间点为1组动物, 留取血清及肝脏标本, 将血清于-20℃冻存待检, 肝组织一部分于40 g/L中性甲醛固定, 一部分于-80℃冻存待检. 注射LPS+D-GalN后 2, 4, 8, 12 h分别编号为T2, T4, T8, T12组, 注射生理盐水后2, 4, 8, 12 h分别编号为N2, N4, N8, N12组, 注射LPS+D-GalN+L-NMMA后2, 4, 8, 12 h分别编号为L2, L4, L8, L12组.
1.2 方法
NO水平采用硝酸还原酶法检测(试剂盒购自南京建成生物公司), 按试剂盒说明书进行, 于酶标仪550 nm处测定各管吸光度值, 计算公式如下: NO(mmol/L)=(样品管吸光度£空白管吸光度)/(标准管吸光度£空白管吸光度)×100 mmol/L标准品浓度×样品测试前稀释倍数. iNOS mRNA的相对含量的检测采用异硫氰酸胍-酚-氯仿方法, 提取肝组织内RNA, 采用TNF-a-2(antisense primer, 序列见后), 在30-65℃, 35 min条件下逆转录合成TNF-a mRNA cDNA后进行PCR反应, 引物序列如下: iNOS-1 5'-TCGGTGCAGTCTTTTCCTATGG-3'; iNOS-2 5'-TATTAGAGCGGCGGCATGGT-3'(产物长404 bp), 同时设内参照(产物长592 bp), 引物序列: b-actin-1 5'-TGTATGCCTCTGGTCGTACCAC-3'; b-actin-2 5'-ACAGAGTACTTGCGCTCAGGAG-3'. PCR反应条件: 94℃变性3 min, 然后94℃ 30 s, 58℃ 1 min, 72℃ 1 min进行30个循环, 72℃延伸5 min. 将PCR产物在20 g/L琼脂糖凝胶上电泳, 用Kodak 1D型凝胶成像分析系统分析检测各扩增带的产物含量, 用下列公式表示: iNOS mRNA的相对含量=iNOS mRNA密度/b-actin密度×100. Bcl-2免疫组化染色: 实验步骤按试剂盒说明书进行, 染色后细胞浆呈棕褐色染色的为阳性细胞, 细胞浆无棕褐色染色的为阴性细胞. Fas免疫组化染色: 实验步骤按试剂盒说明书进行, 染色后细胞浆呈棕褐色染色的为阳性细胞, 细胞浆无棕褐色染色的为阴性细胞. 缺口原位末端标记技术(TUNEL)按试剂盒说明书进行检测, 对石蜡包埋的肝组织切片进行缺口原位末端标记后DAB显色, 染色后细胞核呈棕褐色染色的为阳性细胞, 细胞核无棕褐色染色的为阴性细胞. TUNEL、Bcl-2免疫组化染色及Fas免疫组化染色阳性细胞计数分三级: 低倍(10×)镜1 cm 2视野阳性细胞数小于1/3为(+), 1/3-2/3为(++), 大于2/3为(+++). 肝组织石蜡切片用HE染色于光镜下观察肝组织学变化.
统计学处理: 采用mean±SD表示血清NO水平及iNOS mRNA相对含量, 使用SAS软件包作方差分析, 并进行两两比较, 采用χ2检验进行Bcl-2免疫组化染色及Fas免疫组化染色阳性表达率及TUNEL法检测肝细胞凋亡的比较.
2 结果
模型组2 h组肝组织基本上无异常所见, 4 h组可见肝细胞细胞质疏松化, 呈气球样变, 并可见散在的嗜酸变性, 8 h组可见点片状肝细胞坏死, 其中有较多的嗜酸变性和坏死, 12 h组则见肝细胞呈大片坏死, 核碎裂或溶解. 给予L-NMMA后肝组织HE染色结果表明, 8 h组可见较多的点片状肝细胞坏死, 嗜酸变性和坏死明显, 12 h组则见肝细胞坏死较模型组更为严重.
2.1 血清NO水平及肝组织iNOS mRNA表达的变化
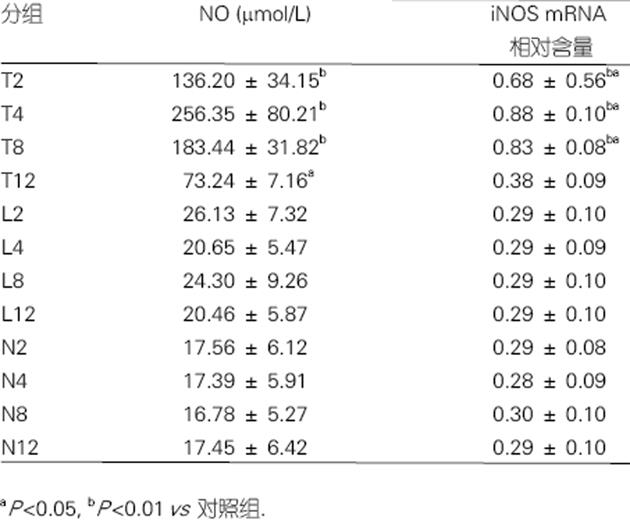
NO在模型组2 h即升高, 4 h达高峰, 以后逐渐下降. 在模型组iNOS mRNA的表达于2 h即升高, 4, 8 h达高峰, 12 h基本降至正常, 与对照组2, 4, 8 h相比差异显著(P<0.01). 模型组2, 4, 8 h iNOS mRNA的表达均较12 h显著增高(P<0.01). 抗NO组各时间点NO水平及iNOS mRNA的表达均为正常水平, 与模型组相比, 抗NO组各时间点ALT, TBil水平无显著差异, 但较模型组有所升高(图1).
图1 鼠血清NO水平及肝组织iNOS mRNA表达的变化(mean±SD).
2.2 肝细胞凋亡的检测结果
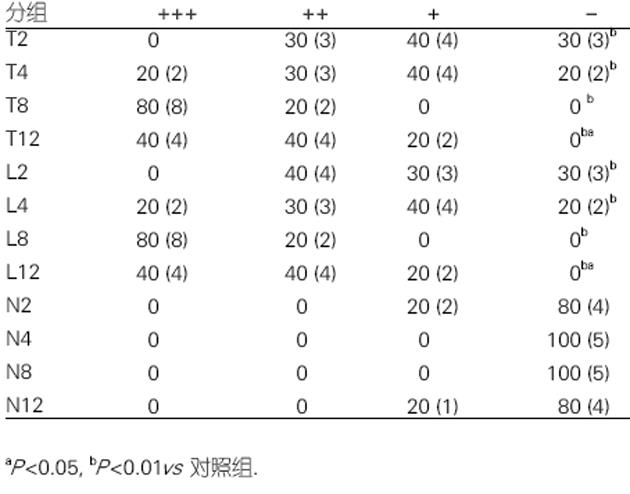
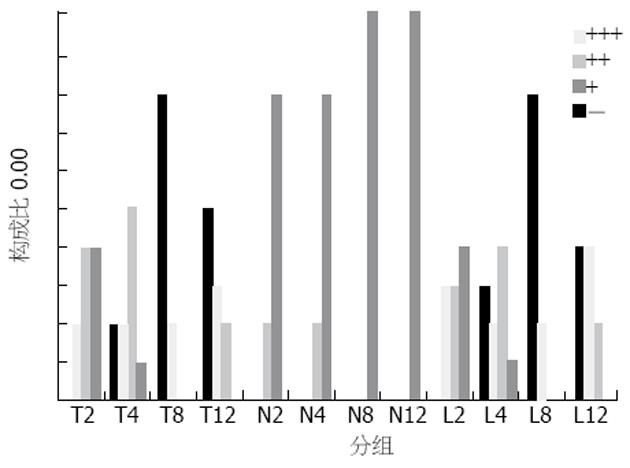
肝组织TUNEL检测结果显示, 模型组在用药后2 h, 肝组织可出现少量阳性细胞, 4 h阳性细胞有所增加, 8 h阳性细胞明显增加, 12 h亦存在凋亡细胞, 且肝组织出现明显的坏死表现. 3/4阻断NO后, 各时间点阳性细胞变化与模型组相一致, 二者比较差异无显著性(图2).
2.3 肝组织中Fas和Bcl-2的表达
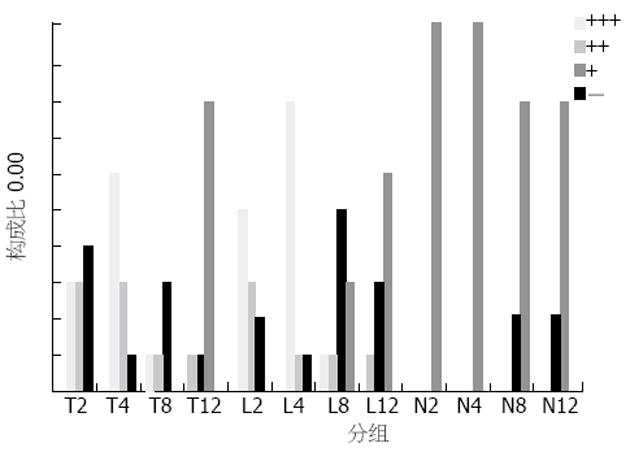
各时间点的对照组无Fas表达, 模型组2 h Fas有少量表达, 4 h表达较多, 8 h和12 h时表达均很多, 8 h和12 h比较无显著差异, 与2 h比较P<0.01, 与4 h比较P<0.05. 抗NO组并未出现Fas表达的明显变化, 与模型组各时间点比较P>0.05(图3). 各时间点的对照组无Bcl-2表达, 模型组2 h Bcl-2有较多表达, 4 h表达最多, 8 h明显减少, 12 h则很少表达, 4 h与2 h比较, P<0.05, 与8, 12 h比较P<0.01. 抗NO组并未出现Bcl-2表达的明显变化, 与模型组各时间点比较P>0.05(图4).
3 讨论
肝细胞的凋亡主要通过Fas/FasL途径介导所致[1,3,4,14-20]. 在病毒性肝病中, 肝细胞的死亡主要通过Fas抗原介 导[1,21-25], 在重型肝炎、慢性肝病患者的肝细胞表面均有Fas抗原的表达[1,21-25]. 对暴发性肝衰竭模型小鼠ip Fas受体的抗体可加重肝细胞凋亡和肝功能衰竭. 本结果表明, 在LPS+GalN用药后Fas表达增加, 且其表达水平的变化与肝细胞凋亡的检测结果相一致, 提示在此种FHF模型中肝细胞凋亡的发生与Fas的表达增加有关. Bcl-2为重要的抗凋亡因子, 定位于线粒体膜的Bcl-2蛋白可通过调节线粒体功能而制约着凋亡的发生. 以往的研究认为, Bcl-2可通过抑制内膜Ca2+的释放、抗氧化、阻止胞内细胞色素C 的释放、抑制白细胞介素-1β转化酶的自身催化等机制而抑制凋亡. 近年来的研究显示肝细胞是否发生凋亡受Bcl-2家族的调控[5,6,26-28]. 将Bcl-2转基因表达于重型肝炎模型小鼠的肝细胞, 可保护小鼠肝脏功能. 在肝缺血-再灌注损伤后进行肝移植术的大鼠中Bcl-2表达增加可提高存活率 [27]. Bcl-2的上调可减轻肝细胞的纤维化程度[28]. 本结果表明, 在LPS+GalN用药后Bcl-2表达增加, 但随着肝细胞凋亡的增加, Bcl-2的表达反而下降, 提示Bcl-2的表达与肝细胞凋亡呈负相关.
在炎症环境下NO及其反应产物在生理和病理方面起着关键的作用, 在不同的情况下NO对肝细胞可能起着保护和损伤的双重作用[2,8,11,29,30]. 研究表明, LPS诱导的NO在体内发挥细胞保护作用, 而在体外却表现为细胞毒性[11]. 目前关于NO对肝细胞作用的报道仍有争议. 一些研究者认为NO促进肝细胞凋亡[12,13,31,32], 可启动Fas凋亡途径, 抑制NO的过量产生可上调Bcl-2 mRNA的表达, 从而起到抑制肝细胞凋亡的作用[31]. LPS 通过刺激NO大量产生而诱导肝细胞凋亡[32]. 给予NOS抑制剂可减轻损伤[12]. iNOS的强烈上调可能有助于FHF时的炎症过程[13]. 但也有相反的报道, 认为NO对肝细胞凋亡起抑制作用[2,7,10,33-36]. 近年来的研究表明, 在部分肝切除术后NO的持续存在发挥着促进肝脏再生和有效地保护肝脏免于发生细胞凋亡的作用 [2]. NO可减少肝缺血-再灌注损伤后肝酶的释放, 抑制肝细胞的凋亡, 改善肝缺血-再灌注损伤[33]. 给予NO供体可减轻由APAP引起的凋亡并阻断caspase活性[10].NO可保护肝细胞免于acetaminophen引起的损伤, 减轻肝脏毒性[35]. 本结果表明, 在由LPS+D-GalN造成的FHF动物模型中血清NO水平及肝组织iNOS mRNA表达均显著增加. 应用iNOS的抑制剂L-NMMA后, Fas、Bcl-2的表达无明显变化, 拮抗NO作用不能阻断肝细胞凋亡和肝脏组织学变化, 提示在本实验条件下, 外源性地给予iNOS的抑制剂不能抑制肝细胞凋亡的发生, 单纯应用NO拮抗剂对肝细胞凋亡及肝损伤无保护作用.
总之, 从细胞凋亡的角度研究FHF发病机制有很重要的意义, 同时, 对其调控机制的研究可能会对临床治疗提供依据, 此方面的研究还待进一步深入.