修回日期: 2005-10-01
接受日期: 2005-10-11
在线出版日期: 2005-10-28
目的: 检测大肠癌中转录因子Ets-1, 基质金属蛋白酶-1(MMP-1)和血管内皮生长因子(VEGF)的表达, 探讨Ets-1在大肠癌血管生成和浸润转移中的作用.
方法: 应用免疫组化SP法检测61例大肠癌组织和21例正常大肠组织中Ets-1, MMP-1和VEGF蛋白的表达水平.
结果: Ets-1, MMP-1和VEGF在正常大肠黏膜中表达均为阴性. 在大肠癌组织中表达的阳性率分别为75.4%, 78.7%和82.0%. 其表达水平与肿瘤大小和分化程度无关(P>0.05), 与Duke's分期(χ2 = 10.718, P<0.01; χ2 = 8.323, P<0.01; χ2 = 6.145, P<0.05)、浸润深度(χ2 = 7.705, P<0.01; χ2 = 19.101, P<0.01; χ2 = 14.707, P<0.01)、淋巴结转移(χ2 = 9.333, P<0.01; χ2 = 3.965, P<0.05; χ2 = 4.638, P<0.05)和远处转移(χ2 = 5.472, P<0.05; χ2 = 4.125, P<0.05; χ2 = 5.034, P<0.05)密切相关. 在大肠癌中, Ets-1的表达与MMP-1和VEGF的表达呈正相关(r = 0.447, P<0.01; r = 0.425, P<0.05).
结论: Ets-1在大肠癌中高表达, 与临床分期、浸润深度、转移密切相关.Ets-1的表达与MMP-1和VEGF的表达呈正相关, 三者的表达水平可作为判定大肠癌恶性生物学行为的参考指标.
引文著录: 洪玮, 刘南植, 张庆, 李秀梅, 倪志. 大肠癌组织Ets-1,MMP-1和VEGF的表达及意义. 世界华人消化杂志 2005; 13(20): 2441-2445
Revised: October 1, 2005
Accepted: October 11, 2005
Published online: October 28, 2005
AIM: To study the expression of E26 transformation-specific-1 (Ets-1), matrix metalloproteinases-1 (MMP-1) and vascular endothelial growth factor (VEGF) in human colorectal carcinoma, and to explore the role of Ets-1 in the angiogenesis and metastasis of carcinoma.
METHODS: The expression of Ets-1, MMP-1 and VEGF were detected in colorectal carcinoma (n = 61) and normal colon tissues (n = 21) by the immunohistochemical method respectively.
RESULTS: Ets-1, MMP-1 and VEGF were negatively expressed in all normal mucosal tissues. The positive rates of Ets-1, MMP-1 and VEGF expression were 75.4%, 78.7% and 82.0% in colorectal carcinoma respectively. No significant correlation was found between their positive rates and tumor′s size as well as the differentiation (P >0.05). The expression of Ets-1, MMP-1 and VEGF were significantly correlated with Duke's staging (χ2 = 10.718, P <0.01; χ2 = 8.323, P <0.01; χ2 = 6.145, P <0.05), the depth of invasion (χ2 = 7.705, P <0.01; χ2 = 19.101, P <0.01; χ2 = 14.707, P <0.01), lymphatic invasion (χ2 = 9.333, P <0.01; χ2 = 3.965, P <0.05; χ2 = 4.638, P <0.05) and distant metastasis (χ2 = 5.472, P <0.05; χ2 = 4.125, P <0.05; χ2 = 5.034, P <0.05). Ets-1 expression was positively associated with MMP-1 and VEGF level (r = 0.447, P <0.01; r = 0.425, P <0.05).
CONCLUSION: Ets-1 was over-expressed in colorectal carcinoma, and its expression was related to clinical staging, invasion and metastasis. Ets-1 expression was also positively related to MMP-1 and VEGF level. Their expression can become referential indexes to predict the malignant behavior of colorectal carcinoma.
- Citation: Hong W, Liu NZ, Zhang Q, Li XM, Ni Z. Expression of E26 transformation-specific-1, matrix metalloproteinases-1 and vascular endothelial growth factor in colorectal carcinoma. Shijie Huaren Xiaohua Zazhi 2005; 13(20): 2441-2445
- URL: https://www.wjgnet.com/1009-3079/full/v13/i20/2441.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i20.2441
众所周知, 肿瘤的恶性生物学行为-侵袭转移是导致癌症患者死亡的主要原因, 研究证实, 肿瘤灶的血管生成加速了实体瘤的生长, 侵袭, 转移[1,2], Ets-1是与肿瘤血管发生和侵袭转移有关的转录因子[3,4]. 近来关于转录因子Ets-1在血管发生和浸润转移中的作用受到了国内外学者的关注[5-8], 但在大肠癌中关于Ets-1与MMP-1, VEGF的相关研究较少. 我们用免疫组化SP法检测Ets-1和MMP-1, VEGF在大肠癌中的表达水平及关系, 探讨Ets-1与大肠癌血管发生和浸润转移的关系, 为大肠癌的早期发现和判断预后提供理论依据.
2003-10/2004-10手术切除大肠癌标本61例, 男30例, 女31例, 平均年龄52.6(26-81)岁, 肿瘤平均直径为5.5 cm. 病理组织学诊断高分化腺癌21例, 中分化腺癌28例, 低分化腺癌12例. Duke's A期14例, B期18例, C期17例, D期12例. 淋巴结转移29例, 未转移者32例. 远处转移12例, 未转移49例. 所有患者术前均未经过任何抗癌治疗. 另21例正常大肠组织为对照(取自肠镜标本). 标本均经40 g/L甲醛固定, 常规石蜡切片厚3-5 µm. 兔抗人Ets-1多克隆抗体购自美国Santa Cruz公司, 兔抗人MMP-1多克隆抗体和VEGF多克隆抗体购自武汉博士德生物工程有限公司, 即用型SP试剂盒和DAB显色剂购自北京中山试剂公司, 其他常规试剂均为国产分析纯试剂.
采用免疫组化SP法染色, 每批染色均设立对照组, 以PBS代替一抗为阴性空白对照, 用已知阳性切片为阳性对照. 简要步骤如下: 组织切片常规脱蜡脱水后, 使用3 mL/L H2O2甲醇阻断内源性酶, 柠檬酸抗原修复液热水浴30 min, 用15 mL/L的正常山羊血清以减少非特异性着色, 再滴入相应抗体(Ets-1, MMP-1和VEGF的滴度均为1‥100), 4℃冰箱过夜, 继而滴入1‥200生物素标记的第二抗体, 30 min后清洗切片后再滴入1‥200稀释的链霉素抗生素蛋白-过氧化物酶(SP), 孵育20 min后经1 g/L DAB-H2O2显色后, 苏木素复染, 常规封片, 镜检并摄像. 细胞质出现棕黄色颗粒者为阳性, 高倍镜下(×200)对每张切片随机选择5个视野, 计数200个细胞/视野, 按阳性细胞数占视野总细胞数的百分比分为3级: 无阳性细胞或阳性细胞数<5%为阴性(-), 阳性细胞数在5-50%之间为阳性(+), 阳性细胞数>50%为强阳性(++).
统计学处理 采用SPSS11.0分析软件进行统计学处理, 根据数据性质, 分别应用χ2检验, fishers精确概率法以及Spearman等级相关分析, 设P<0.05为差异显著性标准.
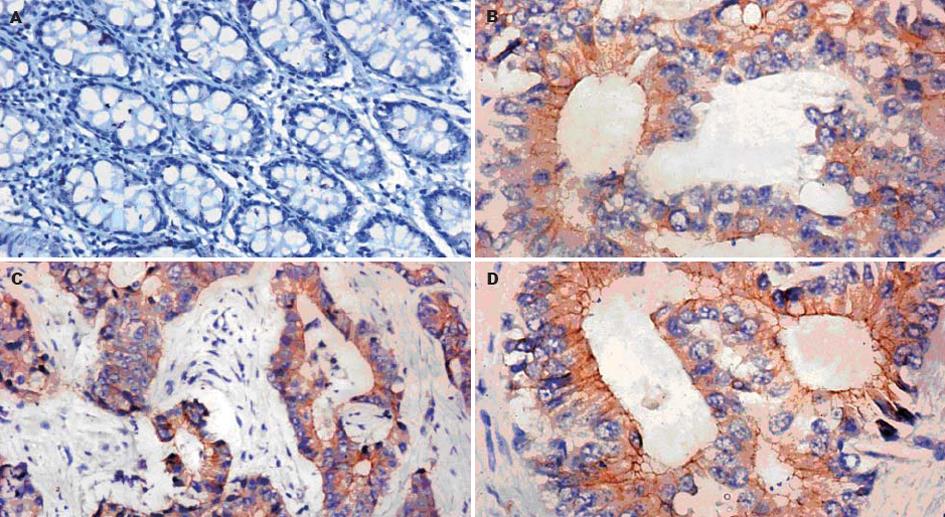
Ets-1, MMP-1和VEGF在大肠癌中阳性率分别为75.4%, 78.7%和82.0%, 显著高于癌旁正常大肠黏膜(χ2 = 18.983, P<0.01; χ2 = 22.285, P<0.01; χ2 = 23.963, P<0.01), Ets-1, MMP-1和VEGF在癌旁正常大肠黏膜中的表达均为阴性. Ets-1阳性染色颗粒以癌细胞胞质为主, 少数可见癌细胞胞核染色. MMP-1阳性染色颗粒以癌细胞胞质为主, 主要表达在侵袭前沿, 和Ets-1的表达是共定位的, 在部分间质细胞也可见阳性表达. VEGF阳性染色颗粒以癌细胞胞质为主, 少量可见癌细胞胞膜染色, 在部分血管内皮细胞可见阳性表达(图1).
Ets-1的表达与肿瘤大小和分化程度无关(P>0.05),与Duke's分期、浸润深度、淋巴结转移和远处转移相关(χ2 = 10.718, P<0.01; χ2 = 7.705, P<0.01; χ2 = 9.333, P<0.01; χ2 = 5.472, P<0.05). MMP-1的表达与肿瘤大小和分化程度无关(P>0.05),与Duke's分期、浸润深度、淋巴结转移和远处转移有关(χ2 = 8.323, P<0.01; χ2 = 19.101, P<0.01; χ2 = 3.965, P<0.05; χ2 = 4.125, P<0.05). VEGF的表达与肿瘤大小和分化程度无关(P>0.05), 与Duke's分期、浸润深度、淋巴结转移和远处转移有关(χ2 = 6.145, P<0.05; χ2 = 14.707, P<0.01; χ2 = 4.638, P<0.05; χ2 = 5.034, P<0.05, 表1).
肿瘤的生长和转移依赖于肿瘤血管形成.在新生血管形成之前, 由于被动供氧和营养扩散的限制, 肿瘤灶仅以一种小的, 无症状的病损存在. 而新生血管形成之后, 肿瘤灶局部快速播散, 增强肿瘤灶的远处转移能力[9-11]. 因此, 恶性肿瘤的生长转移与其间质血管的生成密切相关, 如果能找到有效调节血管生成的途径, 则有望控制肿瘤的生长和转移, 从而阻止肿瘤的恶性生物学行为, 延长肿瘤患者的生命. Ets-1是从白血病病毒E26分离出来的v-ets同源的原癌基因c-ets-1的表达产物, 它是Ets家族中具有代表性的转录因子. Ets家族是一组转录因子群, 具有由85个氨基酸构成的winged helix-turn-helix构造的DNA结合区(称作ETS区), 可以识别、结合嘌呤丰富的DNA核心序列GGAA/T, 这一序列存在于与细胞外基质降解以及血管生成有关的许多基因的5'-侧翼调节区, 如MMP-1, MMP-3, MMP-9和尿激酶型纤溶酶原激活物(uPA), 从而调节这些基因的转录[12-14]. Vandenbunder et al[15]用鸡胚进行原位杂交分析中发现, 血管形成(vasculogenesis)和血管新生(angiogenesis)时, 处于血管形成期的内皮细胞中都高表达Ets-1 mRNA. Khatun et al[16]发现Ets-1可以上调MMP-1, MMP-3, MMP-9, 整合素β和uPA的表达, 使内皮细胞转化为成血管表型, 从而诱导了癌组织的血管生成, 促进了癌的侵袭转移. 本研究发现, Ets-1在大肠癌中呈高表达, 有淋巴结和远处转移者Ets-1阳性表达率显著高于无淋巴结和无远处转移者, 且随着Duke's分期和浸润深度的进展, Ets-1的表达显著增加.
MMPs是一大类锌依赖性内肽酶家族, 活性部位都含有一个Zn2+, 均能降解一种或几种细胞外基质, 在基质降解过程中起主导作用. MMP-1(亦称胶原酶)能降解Ⅰ,Ⅱ, Ⅲ型胶原, 把Ⅰ型胶原分解成1/4和3/4片段, 破坏基底膜, 通过对细胞外基质的改建, 促进肿瘤新生血管的形成, 利于肿瘤的浸润与转移[17-20]. 目前认为, 肿瘤侵袭转移的进程依赖于肿瘤细胞蛋白水解活性的增加[21]. 己知Ⅰ和Ⅲ型胶原是胃肠道间质的主要结构组分, 故MMP-1在降解肠道组织基底膜, 以利于肿瘤的进一步侵袭中起了重要作用[22]. 基因分析表明, MMPs的启动子区域PEA-3位点是Ets基因产物的结合点, 是一个功能性的转录元件[23]. 我们发现MMP-1主要表达在大肠癌细胞的胞质里, 且主要表达在侵袭前沿, 和Ets-1的表达是共定位的, 相关分析也显示Ets-1和MMP-1显著正相关, 从而表明Ets-1和MMP-1都在大肠癌的侵袭中起了重要作用. VEGF是目前引起大家关注的最主要的一种促血管生长的因子, 亦称血管渗透因子(vascular permeability factor, VPF)[24], 它是一个有效的, 多功能的细胞因子, 特异作用于血管内皮细胞, 促进内皮细胞的有丝分裂和趋化作用, 还增加血管通透性, 使管内的纤维蛋白原等外渗[25]. 在肿瘤细胞, VEGF通过直接刺激内皮细胞增殖和迁移发挥重要作用. 它也活化很多蛋白酶降解周围的基质, 促进肿瘤的侵袭转移[26]. Iwasaka et al[27]发现VEGF, bFGF可诱导内皮细胞表达Ets-1, 表达的Ets-1进一步诱导uPA, MMP-1等在血管新生中所必要的基因的表达. 另一方面, VEGF诱导产生的Ets-1可调节VEGFR-1(Flt-1)的表达, 从而促进VEGF与内皮细胞的结合[28]. 我们发现, VEGF与Ets-1的表达呈明显正相关, 支持VEGF诱导Ets-1基因表达的观点, 从而提出VEGF和Ets-1可能在血管发生中起协同作用.
Hahne et al[29]报道VEGF可诱导内皮细胞表达Ets-1基因, 而后者上调MMP-1, -3, -9和uPA等蛋白水解酶类的表达, 促使基底膜分解, 参与血管形成过程. 在这个通路中, Ets-1处于中间环节, 如果阻断Ets-1的表达则有可能达到阻断肿瘤血管生成的目的[30]. Kitange et al[31]报道, 用Ets-1的反义寡核苷酸处理神经胶质瘤细胞后, 可抑制细胞的迁移和侵入, 同时伴有Ets-1和uPA表达的下调. 应用Ets-1的反义寡核苷酸可有效抑制人内皮细胞和血管平滑肌细胞中VEGF, HGF和c-met的表达[32]. 提示Ets-1反义寡核苷酸有望成为一个有效的抗肿瘤药物.
总之, 本实验证实Ets-1在大肠癌中高表达, 与临床分期、浸润深度、转移密切相关, 在大肠癌血管发生和侵袭转移中都起了重要作用. Ets-1和MMP-1, VEGF均参与肿瘤浸润和淋巴转移过程, 检测三者的表达可做为判定大肠癌恶性生物学行为的参考指标, 为大肠癌的早期发现和判断预后提供理论依据.
电编: 张勇 编辑: 潘伯荣 审读: 张海宁
| 1. | Costa C, Soares R, Schmitt F. Angiogenesis: now and then. APMIS. 2004;112:402-412. [PubMed] [DOI] |
| 2. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [PubMed] [DOI] |
| 3. | Hahne JC, Okuducu AF, Kaminski A, Florin A, Soncin F, Wernert N. Ets-1 expression promotes epithelial cell transformation by inducing migration, invasion and anchorage-independent growth. Oncogene. 2005;24:5384-5388. [PubMed] [DOI] |
| 4. | Rothhammer T, Hahne JC, Florin A, Poser I, Soncin F, Wernert N, Bosserhoff AK. The Ets-1 transcription factor is involved in the development and invasion of malignant melanoma. Cell Mol Life Sci. 2004;61:118-128. [PubMed] [DOI] |
| 5. | Tsutsumi S, Kuwano H, Nagashima N, Shimura T, Mochiki E, Asao T. Ets-1 expression in gastric cancer. Hepatogastroenterology. 2005;52:654-656. [PubMed] |
| 6. | Alipov G, Nakayama T, Ito M, Kawai K, Naito S, Nakashima M, Niino D, Sekine I. Overexpression of Ets-1 proto-oncogene in latent and clinical prostatic carcinomas. Histopathology. 2005;46:202-208. [PubMed] [DOI] |
| 7. | Katayama S, Nakayama T, Ito M, Naito S, Sekine I. Expression of the ets-1 proto-oncogene in human breast carcinoma: differential expression with histological grading and growth pattern. Histol Histopathol. 2005;20:119-126. [PubMed] |
| 8. | Lincoln DW 2nd, Bove K. The transcription factor Ets-1 in breast cancer. Front Biosci. 2005;10:506-511. [PubMed] [DOI] |
| 9. | Sivridis E, Giatromanolaki A, Koukourakis MI. The vascular network of tumours-what is it not for? J Pathol. 2003;201:173-180. [PubMed] [DOI] |
| 10. | Verheul HM, Voest EE, Schlingemann RO. Are tumours angiogenesis-dependent? J Pathol. 2004;202:5-13. [PubMed] [DOI] |
| 11. | Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005;25:3327-3333. [PubMed] |
| 12. | Lelievre E, Lionneton F, Soncin F, Vandenbunder B. The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391-407. [PubMed] [DOI] |
| 13. | Reddy SY, Obika S, Bruice TC. Conformations and dynamics of Ets-1 ETS domain-DNA complexes. Proc Natl Acad Sci USA. 2003;100:15475-15480. [PubMed] [DOI] |
| 14. | Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11-34. [PubMed] [DOI] |
| 15. | Vandenbunder B, Pardanaud L, Jaffredo T, Mirabel MA, Stehelin D. Complementary patterns of expression of c-ets 1,c-myb and c-myc in the blood-forming system of the chick embryo. Development. 1989;107:265-274. [PubMed] |
| 16. | Khatun S, Fujimoto J, Toyoki H, Tamaya T. Clinical implications of expression of ETS-1 in relation to angiogenesis in ovarian cancers. Cancer Sci. 2003;94:769-773. [PubMed] [DOI] |
| 17. | Nishikawa A, Iwasaki M, Akutagawa N, Manase K, Yamashita S, Endo T, Kudo R. Expression of various matrix proteases and Ets family transcriptional factors in ovarian cancer cell lines: correlation to invasive potential. Gynecol Oncol. 2000;79:256-263. [PubMed] [DOI] |
| 18. | Ozaki I, Mizuta T, Zhao G, Zhang H, Yoshimura T, Kawazoe S, Eguchi Y, Yasutake T, Hisatomi A, Sakai T. Induction of multiple matrix metalloproteinase genes in human hepatocellular carcinoma by hepatocyte growth factor via a transcription factor Ets-1. Hepatol Res. 2003;27:289-301. [PubMed] [DOI] |
| 19. | Torlakovic EE, Bilalovic N, Nesland JM, Torlakovic G, Florenes VA. Ets-1 transcription factor is widely expressed in benign and malignant melanocytes and its expression has no significant association with prognosis. Mod Pathol. 2004;17:1400-1406. [PubMed] [DOI] |
| 20. | Ozaki I, Zhao G, Mizuta T, Ogawa Y, Hara T, Kajihara S, Hisatomi A, Sakai T, Yamamoto K. Hepatocyte growth factor induces collagenase (matrix metalloproteinase-1) via the transcription factor Ets-1 in human hepatic stellate cell line. J Hepatol. 2002;36:169-178. [PubMed] [DOI] |
| 21. | Nakayama T, Ito M, Ohtsuru A, Naito S, Sekine I. Expression of the ets-1 proto-oncogene in human colorectal carcinoma. Mod Pathol. 2001;14:415-422. [PubMed] [DOI] |
| 22. | Horiuchi S, Yamamoto H, Min Y, Adachi Y, Itoh F, Imai K. Association of ets-related transcriptional factor E1AF expression with tumour progression and overexpression of MMP-1 and matrilysin in human colorectal cancer. J Pathol. 2003;200:568-576. [PubMed] [DOI] |
| 23. | Singh S, Barrett J, Sakata K, Tozer RG, Singh G. ETS proteins and MMPs: partners in invasion and metastasis. Curr Drug Targets. 2002;3:359-367. [PubMed] [DOI] |
| 24. | Mukhopadhyay D, Zeng H, Bhattacharya R. Complexity in the vascular permeability factor/vascular endothelial growth factor (VPF/VEGF)-receptors signaling. Mol Cell Biochem. 2004;264:51-61. [PubMed] [DOI] |
| 25. | Chodorowska G, Chodorowski J, Wysokinski A. Vascular endothelial growth factor (VEGF) in physiological and pathological conditions. Ann Univ Mariae Curie Sklodowska. 2004;59:8-14. |
| 26. | Ferroni P, Spila A, Martini F, D'Alessandro R, Mariotti S, Del Monte G, Graziano P, Buonomo O, Guadagni F, Roselli M. Prognostic value of vascular endothelial growth factor tumor tissue content of colorectal cancer. Oncology. 2005;69:145-153. [PubMed] [DOI] |
| 27. | Iwasaka C, Tanaka K, Abe M, Sato Y. Ets-1 regulates angio-genesis by inducing the expression of urokinase-type plasmin-ogen activator and matrix metalloproteinase-1 and the migrati-on of vascular endothelial cells. J Cell Physiol. 1996;169:522-531. [PubMed] [DOI] |
| 28. | Mukherjee T, Kumar A, Mathur M, Chattopadhyay TK, Ralhan R. Ets-1 and VEGF expression correlates with tumor angiogenesis, lymph node metastasis, and patient survival in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2003;129:430-436. [PubMed] [DOI] |
| 29. | Hahne JC, Okuducu AF, Kaminski A, Florin A, Soncin F, Wernert N. Ets-1 expression promotes epithelial cell transformation by inducing migration, invasion and anchorage-indepen-dent growth. Oncogen. 2005;1124:5384-5388. [PubMed] [DOI] |
| 30. | Konno S, Iizuka M, Yukawa M, Sasaki K, Sato A, Horie Y, Nanjo H, Fukushima T, Watanabe S. Altered expression of angiogenic factors in the VEGF-Ets-1 cascades in inflammatory bowel disease. J Gastroenterol. 2004;39:931-939. [PubMed] [DOI] |
| 31. | Kitange G, Shibata S, Tokunaga Y, Yagi N, Yasunaga A, Kishikawa M, Naito S. Ets-1 transcription factor-mediated urokinase-type plasminogen activator expression and invasion in glioma cells stimulated by serum and basic fibroblast growth factors. Lab Invest. 1999;79:407-416. [PubMed] |
| 32. | Hashiya N, Jo N, Aoki M, Matsumoto K, Nakamura T, Sato Y, Ogata N, Ogihara T, Kaneda Y, Morishita R. In vivo evidence of angiogenesis induced by transcription factor Ets-1: Ets-1 is located upstream of angiogenesis cascade. Circulation. 2004;109:3035-3041. [PubMed] [DOI] |