修回日期: 2005-06-22
接受日期: 2005-06-30
在线出版日期: 2005-10-15
目的: 探讨环氧合酶-2(COX-2)及其催化产生的前列腺素E2(PGE2)与Barrett食管及食管腺癌的关系.
方法: 采用RT-PCR、免疫组化和RIA等方法, 分别测定Barrett食管组(n = 16), 食管腺癌组(n = 17)和正常对照组(n = 20)食管黏膜中COX-2 mRNA的表达率、COX-2蛋白在组织中的表达率和在细胞中的分布情况, 以及PGE2在组织中的含量.
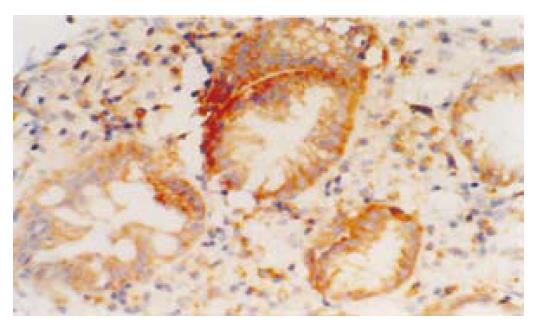
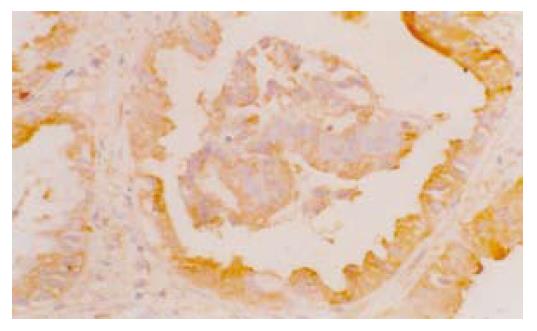
结果: 87.50%(14/16)的Barrett食管和88.24%(15/17)的食管腺癌中COX-2 mRNA呈阳性表达, 与对照组(5/20)比较均有显著差异(P<0.01). 免疫组织化学研究显示: COX-2蛋白在Barrett食管上皮细胞和食管腺癌的癌细胞细胞质中呈阳性表达, 81.25%(13/16)的Barrett食管和76.47%(13/17)的食管腺癌中COX-2蛋白呈阳性表达, 与对照组(4/20)比较均有显著差异(P<0.01), 但Barrett食管组和食管腺癌组COX-2蛋白表达率之间无显著差异(P>0.05). 放射免疫分析测定显示: Barrett食管组(541.41±34.30 pg/mg)和食管腺癌组(559.22±37.77 pg/mg)中PGE2的含量与对照组(357.10±37.58 pg/mg)相比均有显著差异(P<0.05), Barrett食管组和食管腺癌组之间PGE2的含量无显著差异(P>0.05).
结论: COX-2及其mRNA在Barrett食管和食管腺癌中均呈高表达. PGE2的含量在Barrett食管和食管腺癌中均增高. COX-2及其催化产生的PGE2可能与Barrett食管及食管腺癌的形成有关.
引文著录: 刘心娟, 王邦茂, 阎雪燕, 刘文天, 吕宗舜, 张洁. COX-2 在Barrett食管和食管腺癌中的表达及意义. 世界华人消化杂志 2005; 13(19): 2314-2317
Revised: June 22, 2005
Accepted: June 30, 2005
Published online: October 15, 2005
AIM: To explore the significance of cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) level in human Barrett's esophagus (BE) and esophageal adenocarcinoma (EAC).
METHODS: The expression of COX-2 mRNA, the distribution of COX-2 protein, and the content of PGE2 were detected in the endoscopic biopsies of from BE (n = 16), EAC (n = 17), and normal controls (n = 20) by reverse transcription polymerase chain reaction (RT-PCR), immunohistochemistry, and radioimmunoassay (RIA), respectively.
RESULTS: The positive rate of COX-2 mRNA was 87.50% (14/16) and 88.24% (15/17) in BE and EAC patients, respectively, which were significantly higher than that in the normal controls (25%, P < 0.01). Furthermore, COX-2 protein was expressed in the epithelial cytoplasm of BE patients (81.25%, 13/16) and the plasm of cancer cells in EAC patients (76.47%, 13/17), which were significantly higher than that in the normal controls (20.00%, 4/20). The contents of PGE2 (pg/mg) in BE (541.41 ± 34.30) and EAC patients (559.22 ± 37.77) were also significantly higher than those in the normal controls (357.10 ± 37.58) (P < 0.05). No significant difference was found between BE and EAC patients in COX-2 mRNA, protein expression and PGE2 content (P>0.05).
CONCLUSION: COX-2 mRNA and its protein are overexpressed, and the level of PGE2 is increased in BE and EAC group. COX-2 and PGE2 may be involved in the development of Barrett's esophagus and esophageal adenocarcinoma.
- Citation: Liu XJ, Wang BM, Yan XY, Liu WT, Lu ZS, Zhang J. Expression of COX-2 in Barrett's esophagus and esophageal adenocarcinoma and its significance. Shijie Huaren Xiaohua Zazhi 2005; 13(19): 2314-2317
- URL: https://www.wjgnet.com/1009-3079/full/v13/i19/2314.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i19.2314
Barrett食管(Barrett's esophagus, BE)是指食管下段正常的复层鳞状上皮被单层柱状上皮替代的一种病理现象[1]. BE具有高度的癌前性质, 已经明确80%食管腺癌的形成与Barrett食管有关[2]. 近年来, 食管腺癌(esophageal adenocarcinoma, EAC)的发病率增加迅速, 增加幅度比其他所有肿瘤都高[3], 且预后极差[4]. 环氧合酶(cyclooxygenase, COX)是催化前列腺素类合成的重要限速酶, 许多研究表明COX-2作为一个早期事件参与消化道肿瘤的形成[5]. COX-2参与肿瘤形成的机制之一就是催化花生四烯酸合成前列腺素E2(PGE2), 后者通过多种途径参与肿瘤的形成[6]. 本文旨在通过测定COX-2在Barrett食管及食管腺癌中的表达和PGE2在Barrett食管及食管腺癌中的含量, 探讨COX-2及其催化产生的PGE2与Barrett食管及食管腺癌的关系.
Barrett食管组(16例, 男8例, 女8例, 31-77岁)、食管腺癌组(17例, 男16例, 女1例, 37-82岁)取自电子胃镜下的病变部位活检组织; 对照组(20例, 男12例, 女8例, 36-77岁)取自正常食管齿状线以上3 cm处活检组织. Barrett食管的诊断标准[1]: 本研究中的Barrett食管均为长节段Barrett食管, 即齿状线由胃食管连接处(GEJ)向口端方向移位长度≥3 cm. 所有黏膜活检组织均经病理学检查证实. 3H标记前列腺素E2放免试剂盒, 2, 5二苯基恶唑, POPOP盒购自解放军总医院销售部.
1.2.1 COX-2 mRNA的表达: 用Trizol一步法提取总RNA, 逆转录为cDNA. 每个PCR循环包括变性(94 ℃, 60 s), 退火(65 ℃, 40 s), 延伸(72 ℃, 30 s), 共35个循环, 最后再延伸72 ℃ 8 min. COX-2引物: (F)5'-TTCAAATGAGATTGTGGGAAAATTGCT-3', (R)5'-GATCATCTCTGCCTGAGTATCTT-3', 304 bp; β-actin(作为外参照)引物: (F)5'-TGTATGCCTCTGGTCGTACCAC-3'(R)5'-ACAGAGTACTTGCGCTCAGGAG-3', 592 bp.
1.2.2 COX-2蛋白在组织中的表达及分布: 石蜡切片经脱蜡水化后, 3 mL/L甲醇过氧化氢封闭30 min; 微波抗原修复; 血清封闭; 加鼠抗人COX-2单克隆抗体(1:100), 4 ℃过夜; 羊抗鼠IgG, 60 min; ABC复合液, 60 min; DAB显色.
1.2.3 PGE2含量的测定: 采用放射免疫分析法(radioi-mmunoassay, RIA).
统计学处理 患者组和对照组进行多个样本率比较的χ2分析, 以及多个样本均数间多重比较的q检验(Newman-Keuls法), P<0.05为有显著性差异.
87.50%(14/16)的Barrett食管和88.24%(15/17)的食管腺癌中COX-2 mRNA呈阳性表达, 与对照组(5/20)比较均有显著差异(P<0.01), 但Barrett食管组和食管腺癌组COX-2 mRNA表达率之间无显著差异(P>0.1).
BE的诊断尚没有统一的标准[7], 新近将其分为长节段和短节段两种[8]. 食管下段鳞状上皮被柱状上皮替代, 即齿状线由GEJ向口端方向移位长度≥3 cm称为长节段BE; 当受累长度≤3 cm, 但组织活检有特殊肠化生黏膜存在, 称为短节段BE[1]. 本文所研究的16例BE均为长节段BE.
关于BE的发生机制尚不十分清楚, 一般认为主要与胃食管反流密切相关[9], 食管下段长期暴露于胃酸、胃酶和胆汁中, 造成食管炎症和破坏, 导致耐酸的柱状上皮替代鳞状上皮, 柱状上皮进一步异型增生, 产生严重的退行性变, 最终将导致癌变[10,11]. 但是, 新近研究[12-14]也认为, 除了反酸以外, 还有更重要的因素参与了Barrett上皮化生的进程.
COX是前列腺素生物合成的限速酶. COX-2是一种诱导型酶[15], 可以为炎症、再生及致癌作用等等多种刺激所诱导[16]. COX-2可能通过影响前列腺素合成、细胞周期、细胞凋亡、细胞分化、影响血管形成[17]等多种途径促进肿瘤的形成[5]. COX-2的表达是食管肿瘤形成的早期事件[18], 发生在癌前的柱状上皮化生阶段. COX-2基因的诱导是恶性表现型持续存在的必要条件, 其可以促进Barrett上皮和腺癌细胞的增殖[19]. 在Barrett食管组织中COX-2水平上调而其他炎症因子却无此现象, 提示在某种程度上, COX-2的上调具有特异性效应而不是简单的炎症反应[20,21]. COX-2基因的诱导是恶性表现型持续存在的必要条件, 其可以促进Barrett上皮和腺癌细胞的增殖[22].
Wilson et al[2]研究中81%BE患者COX-2过度表达, 而5例Barrett食管腺癌患者均有COX-2的过度表达. Zimmermann et al[23]发现77.8%的食管腺癌中COX-2高表达. Buskens et al[16]的研究中, 99%Barrett相关腺癌中COX-2高表达, 并且COX-2高表达的患者生存期较COX-2低表达者短. 我们的结果与以往的报道相一致. 本文在从mRNA水平研究COX-2的同时, 还研究了BE和食管腺癌中COX-2蛋白水平的表达. COX-2 mRNA和蛋白在BE和食管腺癌中的表达率之间均没有统计学差异, 提示COX-2可能在早期参与食管腺癌的形成.
COX-2是前列腺素合成的关键酶, COX-2的过度表达可以导致组织中PGE2水平的增高[24]. 因此, PGE2在组织中的含量在一定程度上反映了COX-2的酶活性[25]. PGE2的抗炎作用部分降低了对肿瘤的免疫反应, 同时作为分化和生长因子, 免疫抑制剂及促血管形成剂[26], 还可能在肿瘤的进展中发挥作用, 因此推测COX-2诱导前列腺素E2在癌基质、血管生成中发挥重要作用[27]. 此外, PGE2可以激活Bcl-2[28], 抑制细胞凋亡; 诱导白介素6(IL-6)的产生, 后者又可以促进结合珠蛋白(haptoglobin)的合成. IL-6与癌细胞的浸润有关, 结合珠蛋白则参与肿瘤种植和血管的形成[29]. COX-2抑制剂可以阻止Barrett食管上皮细胞的增殖, 而给予前列腺素后细胞增殖得以恢复[30,31].
总之, COX-2的表达与Barrett食管及食管腺癌的发生有关, 提示COX-2抑制剂的应用可能成为Barrett食管及食管腺癌防治的一个研究方向.
电编: 张敏 编辑:张海宁
| 2. | Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929-2934. [PubMed] |
| 3. | Shaheen NJ. Advances in Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology. 2005;128:1554-1566. [PubMed] [DOI] |
| 4. | Morris CD, Armstrong GR, Bigley G, Green H, Attwood SE. Cyclooxygenase-2 expression in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96:990-996. [PubMed] |
| 5. | Shamma A, Yamamoto H, Doki Y, Okami J, Kondo M, Fujiwara Y, Yano M, Inoue M, Matsuura N, Shiozaki H. Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res. 2000;6:1229-1238. [PubMed] |
| 6. | Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101:591-596. [PubMed] [DOI] |
| 7. | Attwood SE, Morris CD. Who defines Barrett's oesophagus: endoscopist or pathologist? Eur J Gastroenterol Hepatol. 2001;13:97-99. [PubMed] [DOI] |
| 8. | Sharma P. Short segment Barrett esophagus and specialized columnar mucosa at the gastroesophageal junction. Mayo Clin Proc. 2001;76:331-334. [PubMed] [DOI] |
| 9. | Mafune K. [Barrett's adenocarcinoma]. Nihon Rinsho. 2005;63:1463-1469. [PubMed] |
| 10. | Buttar NS, Wang KK, Sebo TJ, Riehle DM, Krishnadath KK, Lutzke LS, Anderson MA, Petterson TM, Burgart LJ. Extent of high-grade dysplasia in Barrett's esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630-1639. [PubMed] [DOI] |
| 11. | Cheng P, Gong J, Wang T, Chen J, Liu GS, Zhang R. Gene expression in rats with Barrett's esophagus and esophageal adenocarcinoma induced by gastroduodenoesophageal reflux. World J Gastroenterol. 2005;11:5117-5122. [PubMed] [DOI] |
| 12. | Fitzgerald RC. Barrett's oesophagus and oesophageal adenocarcinoma: how does acid interfere with cell proliferation and differentiation? Gut. 2005;54 Suppl 1:i21-i26. [PubMed] [DOI] |
| 13. | Brabender J, Marjoram P, Lord RV, Metzger R, Salonga D, Vallböhmer D, Schäfer H, Danenberg KD, Danenberg PV, Selaru FM. The molecular signature of normal squamous esophageal epithelium identifies the presence of a field effect and can discriminate between patients with Barrett's esophagus and patients with Barrett's-associated adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14:2113-2117. [PubMed] [DOI] |
| 14. | Chang JT, Katzka DA. Gastroesophageal reflux disease, Barrett esophagus, and esophageal adenocarcinoma. Arch Intern Med. 2004;164:1482-1488. [PubMed] [DOI] |
| 15. | Abdalla SI, Lao-Sirieix P, Novelli MR, Lovat LB, Sanderson IR, Fitzgerald RC. Gastrin-induced cyclooxygenase-2 expression in Barrett's carcinogenesis. Clin Cancer Res. 2004;10:4784-4792. [PubMed] [DOI] |
| 16. | Buskens CJ, Ristimäki A, Offerhaus GJ, Richel DJ, van Lanschot JJ. Role of cyclooxygenase-2 in the development and treatment of oesophageal adenocarcinoma. Scand J Gastroenterol Suppl. 2003;87-93. [PubMed] [DOI] |
| 17. | von Rahden BH, Stein HJ, Pühringer F, Koch I, Langer R, Piontek G, Siewert JR, Höfler H, Sarbia M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005;65:5038-5044. [PubMed] [DOI] |
| 18. | Kuramochi H, Vallböhmer D, Uchida K, Schneider S, Hamoui N, Shimizu D, Chandrasoma PT, DeMeester TR, Danenberg KD, Danenberg PV. Quantitative, tissue-specific analysis of cyclooxygenase gene expression in the pathogenesis of Barrett's adenocarcinoma. J Gastrointest Surg. 2004;8:1007-1016; discussion 1016-1017. [PubMed] [DOI] |
| 19. | Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487-496. [PubMed] [DOI] |
| 20. | Konturek PC, Nikiforuk A, Kania J, Raithel M, Hahn EG, Mühldorfer S. Activation of NFkappaB represents the central event in the neoplastic progression associated with Barrett's esophagus: a possible link to the inflammation and overexpression of COX-2, PPARgamma and growth factors. Dig Dis Sci. 2004;49:1075-1083. [PubMed] [DOI] |
| 21. | Raj A, Jankowski J. Acid suppression and chemoprevention in Barrett's oesophagus. Dig Dis. 2004;22:171-180. [PubMed] [DOI] |
| 22. | Souza RF, Shewmake K, Pearson S, Sarosi GA Jr, Feagins LA, Ramirez RD, Terada LS, Spechler SJ. Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett's adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G743-G748. [PubMed] [DOI] |
| 23. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] |
| 24. | Kaur BS, Triadafilopoulos G. Acid- and bile-induced PGE(2) release and hyperproliferation in Barrett's esophagus are COX-2 and PKC-epsilon dependent. Am J Physiol Gastrointest Liver Physiol. 2002;283:G327-G334. [PubMed] [DOI] |
| 26. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [PubMed] [DOI] |
| 27. | Jang TJ, Min SK, Bae JD, Jung KH, Lee JI, Kim JR, Ahn WS. Expression of cyclooxygenase 2, microsomal prostaglandin E synthase 1, and EP receptors is increased in rat oesophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut. 2004;53:27-33. [PubMed] [DOI] |
| 28. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [PubMed] [DOI] |
| 29. | Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3-21. [PubMed] |
| 30. | Buttar NS, Wang KK, Anderson MA, Dierkhising RA, Pacifico RJ, Krishnadath KK, Lutzke LS. The effect of selective cyclooxygenase-2 inhibition in Barrett's esophagus epithelium: an in vitro study. J Natl Cancer Inst. 2002;94:422-429. [PubMed] [DOI] |
| 31. | Kaur BS, Khamnehei N, Iravani M, Namburu SS, Lin O, Triadafilopoulos G. Rofecoxib inhibits cyclooxygenase 2 expression and activity and reduces cell proliferation in Barrett's esophagus. Gastroenterology. 2002;123:60-67. [PubMed] [DOI] |