修回日期: 2005-08-08
接受日期: 2005-08-10
在线出版日期: 2005-09-28
目的: 探讨血小板活化因子(platelet activating factor, PAF)对肠黏膜分泌型IgA(secretory IgA, SIgA)的影响.
方法: 用50 µg/kg和65 µg/kg PAF对大鼠进行腹腔注射(1 µL/g), 不同时间点处死动物, 应用双抗体-PEG放射免疫法测定肠黏膜中SIgA含量, 常规苏木精-伊红染色, 光镜观察形态学改变.
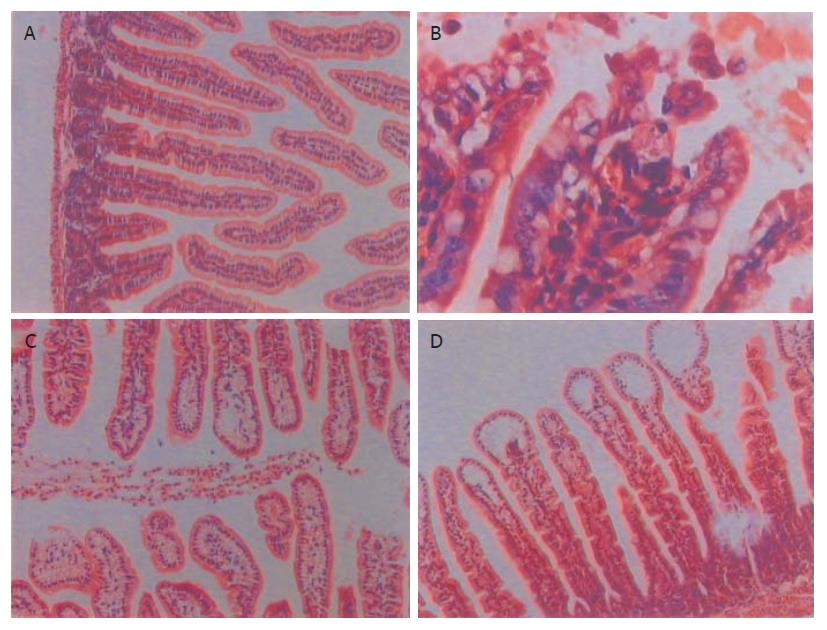
结果: PAF65组回肠0.5, 1.5, 3 h可见绒毛水肿, 固有层血管充血, 间质淋巴管扩张, 肠腔炎性渗出, 上皮脱落, 6, 24 h绒毛水肿. PAF50组0.5, 1.5 h可见绒毛水肿, 固有层血管充血, 3, 6, 24 h绒毛水肿. 实验组0.5, 1.5, 3, 6 h SIgA 均较对照组显著降低(PAF50组分别为0.31±0.03 mg/L, 0.40±0.10 mg/L, P<0.01, 0.43±0.13 mg/L, 0.46±0.11 mg/L,P<0.05; PAF65组分别为0.28±0.07 mg/L, 0.36±0.08 mg/L, P<0.01, 0.40±0.11 mg/L, 0.42±0.06 mg/L, P<0.05 vs0.66±0.10 mg/L). 0.5 h下降幅度最大, 随时间推移有逐渐升高趋势.
结论: PAF可损害肠黏膜的免疫屏障功能, 使SIgA降低.
引文著录: 王丽杰, 刘冬妍, 孙梅, 赵恂. 血小板活化因子对幼鼠肠道免疫屏障功能的影响. 世界华人消化杂志 2005; 13(18): 2266-2268
Revised: August 8, 2005
Accepted: August 10, 2005
Published online: September 28, 2005
AIM: To investigate the effect of platelet activating factor (PAF) on the content of secretory IgA (SIgA) in intestinal mucosa.
METHODS: The rats were intraperitoneally injected with different concentrations of PAF (50 and 65 µg/kg) at a dose of 1 µL/g. Double antibody-PEG radioimmunoassay was used to determine the amount of SIgA in the intestinal mucosa. The histological changes were detected by hematoxylin and Eosin staining under light microscope.
RESULTS: In the PAF65 group, the histological examination showed edema of the villus, capillary congestion of the lamina propria, extension of the subepithelial lymphatic channel, polymorphonuclear infiltration in enteric cavity, and shedding of the epithelial layer at 0.5, 1.5, and 3 h. Edema of the villus were still shown at 6 and 24 h. In the PAF50 group, edema of the villus, capillary congestion of the lamina propria were showed at 0.5 and 1.5 h. Edema of the villus were still shown at 3, 6 and 24 h. The content of SIgA was obviously decreased in the experimental group than that in the control group (0.31±0.03 mg/L, 0.40±0.10 mg/L, P < 0.01; 0.43±0.13 mg/L, 0.46±0.11mg/L, P < 0.05, in PAF50 group; 0.28±0.07 mg/L, 0.36±0.08 mg/L, P < 0.01, 0.40±0.11 mg/L, 0.42±0.06 mg/L, P < 0.05, in PAF65 group vs 0.66±0.10 mg/L in the control) at 0.5, 1.5, 3 and 6 h, respectively. The SIgA content decreased most at 0.5 h, but then gradually increased.
CONCLUSION: PAF can lead to damages of the intestinal immunologic barrier by decreasing the SIgA content.
- Citation: Wang LJ, Liu DY, Sun M, Zhao X. Effect of platelet activating factor on function of intestinal immunological barrier in young rats. Shijie Huaren Xiaohua Zazhi 2005; 13(18): 2266-2268
- URL: https://www.wjgnet.com/1009-3079/full/v13/i18/2266.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i18.2266
肠源性感染是机体遭受严重损伤后发生顽固性休克、全身感染及多器官功能衰竭的重要潜在因素, 而肠黏膜屏障功能损害则是肠源性感染发生的关键. 近年来血小板活化因子(platelet activating factor, PAF)在胃肠黏膜损害中的作用日益受到重视. 我们对PAF对幼鼠肠黏膜免疫屏障功能损害作一初步研究.
健康18日龄Wistar大鼠, 平均体质量32.21±6.67 g, 与母鼠共同饲养, 由中国医科大学附属第二临床学院实验动物中心提供. PAF(1-O-hexadecyl-2-acetyl-sn- glycero-3-phosphocholine)(美国Sigma公司产品). 全自动γ放免计数器FJ-2008PS(西安检测仪器厂).SIgA试剂盒由中国原子能科学研究所提供.
随机分为对照组8只, 实验组分为PAF50和PAF65两组, 每一时相点(0.5,1.5,3,6,24,48,72 h)各8只. PAF 2 g/L溶于氯仿中, 再溶于含2.5 mL/L小牛血清白蛋白盐水中, 于实验前配成浓度为50 mg/L的溶液. 按Bhatia[1]的方法, 实验组用微量加样器分别以PAF 50 µg/kg和65 µg/kg腹腔注射(1 µL/g), 对照组按1 µL/g注入无菌生理盐水. 用药后各组均放回鼠笼, 继续哺乳, 直至实验结束. 按时间点分别处死动物, 距回盲部3 cm处取回肠5 cm, 加入生理盐水200 µL, 4 ℃过夜, 2 000 r/min离心15 min, 取上清-20 ℃保存. 应用双抗体-PEG放射免疫法测定肠黏膜中SIgA含量. 实验严格按说明书要求操作. 再取部分回肠于40 g/L甲醛中固定, 石蜡包埋, 作4-5 mm连续切片, 常规苏木精-伊红染色, 光镜观察形态学改变.
统计学处理 采用SPSS 10.0 For Windows数据分析软件, 所有数据用mean±SD表示, 组间比较采用配对t检验, P<0.05为有统计学意义.
光镜下观察(×100,×400), PAF65组回肠0.5,1.5,3 h可见绒毛水肿, 固有层血管充血, 间质淋巴管扩张, 肠腔炎性渗出, 上皮脱落,6,24 h绒毛水肿, 以0.5,1.5 h改变最明显. 48,72 h无明显改变. PAF50组0.5,1.5 h可见绒毛水肿, 固有层血管充血, 3,6,24 h绒毛水肿, 48,72 h无改变. 对照组各时相点回肠结构正常(图1).
实验组0.5,1.5,3,6 h SIgA均较对照组(0.66±0.10 mg/L)显著降低(PAF50组分别为0.31±0.03 mg/L, 0.40±0.10 mg/L, P<0.01, 0.43±0.13 mg/L,0.46±0.11 mg/L, P<0.05;PAF65组分别为0.28±0.07 mg/L,0.36±0.08 mg/L, P<0.01, 0.40±0.11 mg/L,0.42±0.06 mg/L, P<0.05), 24,48,72 h较前略升高, 与对照组比较无统计学意义. 0.5 h下降幅度最大, 随时间推移有逐渐升高趋势. PAF65组各时相点SIgA较PAF50组略低, 但无统计学差异.
血小板活化因子是第一个被发现的具有强大生物活性的磷脂, 能调节多种细胞活性, 如调节细胞因子网络、免疫反应、聚集血小板、舒缩小血管等, 从而在内毒素血症及休克等病理过程中起到重要作用.
有研究认为PAF在诸多参与胃肠黏膜损害的炎症介质中可能起到"中心放大"的介导作用[2]. 目前国内外多应用PAF受体拮抗剂可减轻细菌移位或应用静脉注射PAF等方法证实PAF对胃肠的损伤作用[3,6],本文采用直接腹腔注射的方法研究PAF对胃肠的损伤[1]. 实验中应用PAF 0.5 h后即可见肠水肿, 点片状出血, 甚至坏死, 以空回肠明显. 随时间推移, 肠损伤逐渐减轻. 至24 h无或仅有轻度水肿, 无出血. 大体所见PAF65组较PAF50组改变稍重, 与病理改变相符. 本文所测SIgA与病情严重程度相关, 即大体与病理改变愈明显,SIgA愈低. SIgA的下降程度与PAF剂量有关, 剂量越大,SIgA下降越多, 但无统计学差异.
肠道是人体最大的淋巴器官, 人的肠腔不断与病毒、细菌和外来异物等微生物接触, 为阻止有害物质的入侵, 肠腔表面被覆的黏膜起了重要作用. 肠黏膜形成黏膜免疫系统, 在机体担负第一线的局部防御任务, SIgA是肠黏膜主要的免疫球蛋白, 长期以来一直被认为是第一线的免疫防御[7,8], 对黏膜固有的和入侵的病原体具有保护作用. 可抑制由IgM免疫复合物介导的抗体依赖式细胞毒作用对肠道局部的免疫损伤[9].SIgA具有杀菌、抑菌、阻止细菌对肠上皮细胞的黏附, 破坏及中和毒素等多种保护作用[10-12].SIgA分泌减少与细菌移位关系密切[13-15].SIgA分泌减少, 一方面使其不能有效包被革兰氏阴性杆菌, 使肠道抵御病原体和毒素侵袭的能力下降, 对机体有害的代谢产物增加, 细菌对肠上皮的吸附、穿透能力增加, 导致肠源性感染; 另一方面使其对某些抗原物质的封闭作用减弱, 导致部分T淋巴细胞活化, 激活炎性细胞因子, 产生过度炎症反应, 进一步损害肠黏膜, 引起全身炎症反应综合征(systemic inflammatory response syndrome, SIRS)[16].
本文研究结果证实, PAF可损害肠黏膜的免疫屏障功能, 因此如何维护肠道免疫屏障功能的稳定, 防治肠源性感染以减少对机体的继发损伤, 成为基础及临床工作者必须面对的课题.
电编: 张勇 编辑:张海宁
| 1. | Bhatia AM, Ramos CT, Scott SM, Musemeche CA. Developmental susceptibility to intestinal injury by platelet-activating factor in the newborn rat. J Invest Surg. 1996;9:351-358. [PubMed] [DOI] |
| 2. | Anderson BO, Bensard DD, Harken AH. The role of platelet activating factor and its antagonists in shock, sepsis and multiple organ failure. Surg Gynecol Obstet. 1991;172:415-424. [PubMed] |
| 3. | de Souza LJ, Sampietre SN, Assis RS, Knowles CH, Leite KR, Jancar S, Monteiro Cunha JE, Machado MC. Effect of platelet-activating factor antagonists (BN-52021, WEB-2170, and BB-882) on bacterial translocation in acute pancreatitis. J Gastrointest Surg. 2001;5:364-370. [PubMed] [DOI] |
| 4. | Leveau P, Wang X, Sun Z, Börjesson A, Andersson E, Andersson R. Severity of pancreatitis-associated gut barrier dysfunction is reduced following treatment with the PAF inhibitor lexipafant. Biochem Pharmacol. 2005;69:1325-1331. [PubMed] [DOI] |
| 5. | Sun X, Caplan MS, Liu Y, Hsueh W. Endotoxin-resistant mice are protected from PAF-induced bowel injury and death. Role of TNF, complement activation, and endogenous PAF production. Dig Dis Sci. 1995;40:495-502. [PubMed] [DOI] |
| 6. | Han XB, Liu X, Hsueh W, De Plaen IG. Macrophage inflammatory protein-2 mediates the bowel injury induced by platelet-activating factor. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1220-G1226. [PubMed] [DOI] |
| 7. | Keren DF, Brown JE, McDonald RA, Wassef JS. Secretory immunoglobulin A response to Shiga toxin in rabbits: kinetics of the initial mucosal immune response and inhibition of toxicity in vitro and in vivo. Infect Immun. 1989;57:1885-1889. [PubMed] |
| 8. | Kunisawa J, Kiyono H. A marvel of mucosal T cells and secretory antibodies for the creation of first lines of defense. Cell Mol Life Sci. 2005;62:1308-1321. [PubMed] [DOI] |
| 9. | Punthuprapasa P, Thammapalerd N, Chularerk U, Charoenlarp K, Bhaibulaya M. Diagnosis of intestinal amebiasis using salivary IgA antibody detection. Southeast Asian J Trop Med Public Health. 2001;32 Suppl 2:159-164. [PubMed] |
| 10. | Chen LW, Hsu CM, Huang JK, Chen JS, Chen SC. Effects of bombesin on gut mucosal immunity in rats after thermal injury. J Formos Med Assoc. 2000;99:491-498. [PubMed] |
| 11. | Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer's patches. J Virol. 2001;75:10870-10879. [PubMed] [DOI] |
| 12. | Favre L, Spertini F, Corthésy B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J Immunol. 2005;175:2793-2800. [PubMed] [DOI] |
| 13. | Wang ZT, Yao YM, Xiao GX, Sheng ZY. Risk factors of development of gut-derived bacterial translocation in thermally injured rats. World J Gastroenterol. 2004;10:1619-1624. [PubMed] [DOI] |
| 14. | Wang ZT, Yao YM, Xiao GX, Sheng ZY. [The protective effect of supplementation of probiotics combined with riboflavin on the intestinal barrier of the rats after scald injury]. Zhonghua Shaoshang Zazhi. 2004;20:202-205. [PubMed] |
| 15. | Bollinger RR, Everett ML, Palestrant D, Love SD, Lin SS, Parker W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology. 2003;109:580-587. [PubMed] [DOI] |
| 16. | Ikeda S, Zarzaur BL, Johnson CD, Fukatsu K, Kudsk KA. Total parenteral nutrition supplementation with glutamine improves survival after gut ischemia/reperfusion. JPEN J Parenter Enteral Nutr. 2002;26:169-173. [PubMed] [DOI] |