修回日期: 2005-05-22
接受日期: 2005-06-08
在线出版日期: 2005-07-28
目的: 构建表达人内皮抑素的重组真核表达载体, 并将其转染人肝癌SMMC-7721细胞, 观察其体外抑制血管生成的活性.
方法: 将含有IL-2分泌肽的人endostatin全长cDNA插入真核表达载体pcDNA3.0产生重组质粒pCD-sEndo, 利用阳离子脂质体介导将其体外转染人肝癌细胞SMMC-7721细胞, G418筛选后得到阳性克隆, 并被命名为SMMC/sEndo, 采用Western-blot检测转染细胞上清中endostatin蛋白的表达, 鸡胚绒毛尿囊膜(CAM)血管生成实验检测转染细胞上清中内皮抑素蛋白的生物活性.
结果: 成功构建endostatin基因的真核表达质粒pCD-sEndo并经酶切和序列测定证实, 通过阳离子脂质体转染SMMC-7721细胞, Western-blot显示转染细胞上清有人endostatin蛋白的表达, Mr 20 000, 而转然空质粒对照组未检测到目的蛋白. CAM实验显示在给予内皮抑素蛋白的鸡胚绒毛膜血管稀疏, 血管密度明显减少, 并且随着蛋白量的增加, 血管减少程度加强.
结论: 重组endostatin真核表达质粒构建正确, 转染SMMC-7721细胞后可有效表达具有生物学活性的人endostatin蛋白并能分泌到细胞外.
引文著录: 邵俊伟, 刘然义, 易继林, 卢绮萍, 黄文林. 重组人内皮抑素真核表达载体pCD-sEndo的构建和表达. 世界华人消化杂志 2005; 13(14): 1679-1683
Revised: May 22, 2005
Accepted: June 8, 2005
Published online: July 28, 2005
AIM: To construct the recombinant eukaryotic expression vector for human endostatin and express it in human liver cancer cell line SMMC-7721, and to observe its anti-angiogenesis activity in vitro.
METHODS: Human endostatin cDNA containing interleukin-2 (IL-2) secreting peptide was cloned into eukaryotic expression plasmid pcDNA3.0 to construct recombinant plasmid pCD-sEndo. The plasmid pCD-sEndo was transfected intoSMMC-7721 cells by cationic liposome. The positive cell clones were selected by G418 and named SMMC/sEndo. The expression of endostatin protein was analyzed by Western-blot. The activity of endostatin protein in the supernatant of SMMC/sEndo cells was explored by the angiogenesis experiment of chicken chorioallantoic membrane (CAM).
RESULTS: The eukaryotic expression vector pCD-sEndo was successfully constructed and was confirmed by enzyme digestion and sequence analysis. The endostatin protein was expressed in the supernatant of SMMC/sEndo cells, about 20 ku in size. No expression of endostatin protein was found in the control cells. There were fewer blood vessels in the CAM treated with endostatin protein, and the blood vessel density markedly decreased. Furthermore, the density decreased with the increase of endostatin protein.
CONCLUSION: The recombinant eukaryotic expression vector is correctly constructed. The human endostatin protein with the activity of anti-angiogenesis can be expressed and secreted in the supernatant of SMMC-7721 cells tranfected with pCD-sEndo.
- Citation: Shao JW, Liu RY, Yi JL, Lu JP, Huang WL. Construction of recombinant eukaryotic expression vector for human endostatin and its expression in liver cancer cell line SMMC-7721. Shijie Huaren Xiaohua Zazhi 2005; 13(14): 1679-1683
- URL: https://www.wjgnet.com/1009-3079/full/v13/i14/1679.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i14.1679
肿瘤的生长和转移依赖于血管生成, 在缺乏血管供血的条件下, 肿瘤将趋向于休眠状态, 肿瘤细胞发生坏死和凋亡, 这为肿瘤治疗提供了一条新思路[1]. 目前已发现多种因子具有抑制肿瘤血管生成的活性, 其中内皮抑素(endostatin)效果最为理想[2-3]. 内皮抑素理化性质活泼, 易变性, 分离提纯过程复杂, 大量获取比较困难, 价格昂贵, 采用基因治疗方法, 在体内直接、持续表达内皮抑素, 可避免其体外失活快和需反复注射的缺点. 我们成功构建了分泌型人内皮抑素的真核表达质粒pCD-sEndo, 将其转染肝癌细胞系SMMC-7721, 为肝癌的基因治疗奠定实验基础.
携带人内皮抑素基因的质粒pGEM-sEndo由中山大学肿瘤防治中心黄文林教授赠送, 含人IL-2信号肽序列, IL-2信号肽序列加内皮抑素序列endostatin cDNA全长为666 bp, 位于克隆载体pGEM-T的2个XbaI酶切位点间; 真核表达载体pcDNA3.0, 购自Invitrogen公司, 其全长5.4 kb, 含有CMV启动子, 多克隆位点, 氨苄青霉素(Ampr)和新霉素(Neor)抗性基因选择标记;DNA大量制备纯化试剂盒、限制性内切酶EcoRI, HindIII, XbaI和小牛肠碱性磷酸酶(CIAP)及T4连接酶, TaqDNA聚合酶, Oligo(dT), 购自Promega公司; 脂质体Dosper Liposomal Transfection Reagent购自Boehringer Mannheim公司; 兔抗人endostatin多克隆抗体购自Chemicon International公司, 二抗及其他免疫组织化学试剂购自武汉博士德公司.
采用互补黏性末端连接构建质粒pCD-sEndo:pGEM-sEndo与pcDNA3.0经XbaI酶切、电泳鉴定后, 冻融法凝胶回收DNA片段; 线性化真核表达载体pcDNA3.0去磷酸化处理; 将pcDNA3.0 0.1 mg, sEndostatin片段0.1 mg, T4DNA连接酶2U混匀后, 置12-16 ℃保温瓶中温育16 h, 取连接物(pCD-sEndo)5 mL转化感受态大肠杆菌DH-5a. 应用Primer软件辅助设计endostatin基因PCR引物(上海博亚生物技术有限公司合成)为: Sense:5'-CGACTTCCAGTGCTTCCAG-3'(Endo90-108)Antisense: 5'-ACGATGTAGGCGTGATGGC-3'(Endo515-497), 扩增片段约426 bp, 挑取LB培养基中氨苄青霉素筛选的抗性克隆菌落, 加入500mL Amp+ LB培养液中, 培养3 h, 取出100 mL煮沸, 12 000 g离心10 min, 取上清作为PCR模板, 用pGEM-sEndo菌种作为阳性对照, 阴性对照为DH5a菌种, 反应体系:10×PCR buffer(含Mg2+)5.0 mL, 10 mmol/L dNTPs mix 1.0 mL, Sense(10mmol/L)2.0 mL, Antisense(10 mmol/L)2.0 mL, Taq DNA聚合酶(5 MU/L)0.5 mL, ddH2O2 38.5 mL, 模板1.0 mL(总共50.0 mL);PCR反应条件:94 ℃变性30 s, 54 ℃退火30 s, 72 ℃延伸1 min.35次循环后, 在72 ℃继续延伸10 min, 取PCR产物5mL于15 g/L琼脂糖凝胶电泳. 挑取经过PCR筛选的阳性克隆菌落, 接种培养扩增, 小量抽提重组质粒pCD-sEndo, 分别与EcoRI、XbaI内切酶混匀后, 置于37 ℃水浴3 h, 取各酶切样品5 mL在80V恒压下电泳2 h, 紫外灯下观察结果, 用Olympus电泳成像系统成像. 将构建的重组质粒pCD-sEndo送上海博亚生物技术有限公司, 检测载体pcDNA3.0上2个多克隆位点之间插入片段序列, 测序引物为T7和Sp6通用引物.
人肝癌细胞株SMMC-7721由华中科技大学同济医学院免疫学教研室馈赠, 复苏后用100 mL/L新生牛血清RPMI1640培养基, 在37 ℃ 50 mL/L CO2条件下培养, 培养液中加入青霉素100 kU/L, 链霉素100 mg/L, 常规传代. 脂质体Dosper介导重组质粒pCD-sEndo转染SMMC-7721细胞: 参见Dosper转染试剂盒制备脂质体Dosper和质粒的混合液, 6孔培养板中每孔接种3×105个SMMC-7721细胞, 至细胞长满约50%左右, 更换细胞培养板中的培养液, 加入脂质体质粒混合液, 37 ℃ 50 mL/L CO2温育6 h, 去掉脂质体质粒混合液, 重新加入新鲜培养基2.5 mL. 抗性克隆细胞筛选: (1)SMMC-7721细胞培养于24孔培养板, 给予G418分别用100、200、300、400、500、600 mg/L加入, 各浓度3复孔, 设正常对照3复孔, 以10-14 d细胞全部死亡的浓度为G418筛选浓度, 结果为400 mg/L. (2)转染细胞继续培养12-36 h, 使转移基因得到表达, 用含400 mg/L G418的完全培养基进行加压筛选, 持续培养14 d后, 筛选出来的抗性克隆细胞命名为SMMC/sEndo. 挑取单个阳性克隆, 继续在G418抗性培养液内筛选2 wk, 将筛选转染成功的细胞扩大培养, 待细胞接近汇合时, 换无血清的培养基2 mL继续培养24 h, 收集培养液, 于4 ℃下, 10 000 g离心5 min, 除去死细胞和碎片, 取上清, BCA法测定蛋白浓度, 用离心式超滤浓缩装置(美国Millipore公司)浓缩, 将转染空质粒的SMMC-7721细胞培养上清同法处理作为对照, 进行SDS-PAGE电泳, 利用电转法将蛋白质转到硝酸纤维素膜上, 封闭液处理, 加入兔抗人endostatin多抗(用0.01 mol/L PBS按1:200比例稀释)温育, 用0.01 mol/L PBS分别洗膜, 加入辣根过氧化物酶偶联的二抗(用0.01 mol/L PBS按1:500比例稀释)温育, 充分洗膜后, 加入显色液, 避光显色至出现条带时放入双蒸水中终止反应, 以10 g/L BSA取代一抗作为阴性对照重复实验. 检测转染重组质粒的SMMC-7721细胞培养上清对鸡胚绒毛尿囊膜血管生成的影响: 将2 d龄的鸡胚用碘酒和酒精消毒后, 在超净工作台用眼科尖嘴镊小心敲开蛋壳, 用一次性注射器在壳膜下胎盘细胞周围的绒毛尿囊膜中分别注射0.005, 0.01, 0.02 mg浓缩后含内皮抑素蛋白的细胞分泌上清, 但不要损坏胚盘细胞, 然后用无菌的保鲜膜覆盖蛋壳缺口, 避免蛋清直接与空气接触, 放入37 ℃培养箱中培养4 d, 每4 h翻动一次, 5 d后打开保鲜膜, 扩大蛋壳缺口, 观察鸡胚绒毛膜血管生成情况, 并用彩色相机摄影保存.
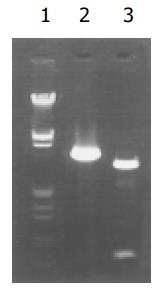
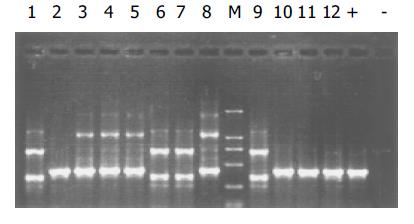
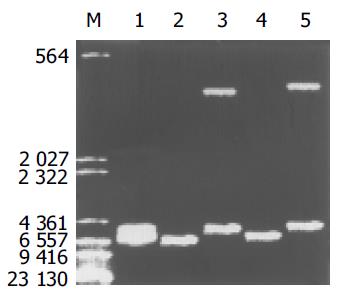
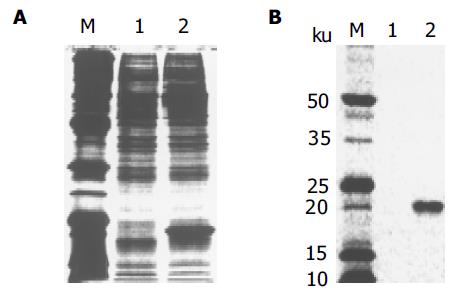
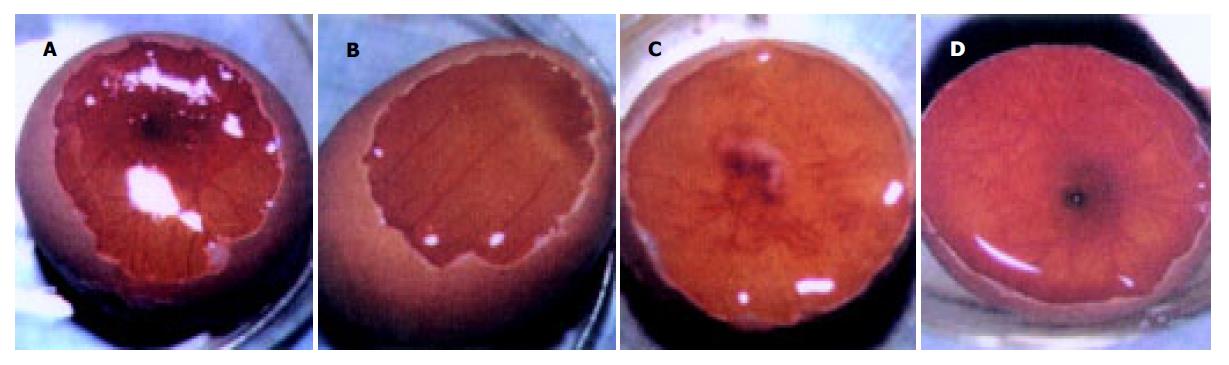
质粒pGEM-sEndo经EcoRI和XbaI酶切后, 电泳结果与其基因酶切图谱一致(图1). 将重组质粒pCD-sEndo转化大肠杆菌DH5a, 取培养细菌裂解上清为模板, 行PCR检测, 结果显示菌落2, 3, 4, 5, 10, 11, 12皆扩增出与阳性对照相同的约430 bp条带, 与实验设计的预期结果一致, 说明菌落2, 3, 4, 5, 10, 11, 12皆为转化了pCD-sEndo的阳性克隆(图2). 把PCR筛选的阳性克隆菌落, 继续接种培养, 重组质粒抽提纯化后经过HindIII, XbaI, EcoRI以及EcoRI和XbaI双酶切鉴定, HindIII仅切出一约6.1 kb片段, XbaI切出5.4 kb和670 bp 2个片段, 说明阳性克隆中已插入含IL-2信号肽序列的endostatin cDNA片段(sEndostatin), EcoRI酶切切出6.0 kb和130 bp 2个片段(130 bp片段在电泳时看不到), EcoRI和XbaI双酶切切出5.4 kb、580 bp、84 bp和45 bp 4个片段(84 bp和45 bp 2个片段在电泳时看不到), 说明sEndostatin插入方向正确(图3). 将纯化的重组质粒用T7和Sp6通用引物检测pcDNA3.0载体上的外源插入片段, 测得外源基因插入片段全长666 bp, 包含人内皮抑素的完整序列和人IL2信号肽序列, 无任何碱基错配, 证实重组质粒构建正确(图4). 转染内皮抑素基因的SMMC-7721细胞上清, 离心过滤后, BCA比色法检测培养液蛋白浓度为50 mg/L, 浓缩后行SDS-PAGE和Western-blot检测, 结果在转染endostatin基因的SMMC-7721细胞培养液上清中可以检测到能与endostatin抗体发生特异性抗原抗体反应的条带, Mr 20 000, 与人内皮抑素蛋白分子量一致, 说明转染endostatin基因的SMMC-7721细胞上清中有目的蛋白的表达(分泌型), 而转染空质粒对照组未检测到目的蛋白(图5). 鸡胚绒毛尿囊膜实验显示在给予内皮抑素蛋白的鸡胚绒毛膜血管稀疏, 血管密度明显减少, 并且随着蛋白量的增加, 血管减少程度加强(图6).
研究显示, 在乳腺癌[4]、前列腺癌[5]、肺癌[6-8]、胃癌[9-10]、宫颈癌[11]、卵巢癌[12-13]及头颈部鳞状上皮癌[14]等患者中其肿瘤的转移率、预后及存活率和肿瘤的血管密度呈正相关. 因此, 抑制血管生成可能是治疗肿瘤的有效方法, 但是一般抗血管形成药物只能使肿瘤生长减缓, 而不能使之完全消除[15], 从肿瘤组织中发现的血管形成抑制因子, 包括angiostatin[16-17], endostatin[18], 他们对血管生成抑制作用更强[19-21]. 内皮抑素为胶原蛋白XVIII的C端球形末端部分片段或其类似物[2-3], 在体外和体内已证实能对多种移植的实体肿瘤及其转移物有完全抑制作用[22-25], Boehm et al[26]发现在经过6轮重复治疗后, 肿瘤对之仍然敏感, 而且未发现毒性和耐药性. 有报道用病毒载体在小鼠身上可明显抑制肿瘤的生长[27-30], 然而病毒载体的毒性问题限制了其基因治疗的推广, 用质粒载体可以显著降低毒性. 我们采用分子重组技术, 从质粒pGEM-sEndo上切下内皮抑素与人IL2信号肽序列, 定向插入真核表达载体pcDNA3.0, 构建人内皮抑素分泌型真核表达质粒pCD-sEndo, 通过酶切和测序显示, 其中的Endostatin cDNA序列与已知的人Endostatin cDNA序列完全相同, 全长552 bp, 无任何碱基错配, 融合基因全长666 bp, 读码框架和插入方向完全正确, 保证了表达产物的正确性. 重组质粒具有载体pcDNA 3.0的优点, 具有原核和真核细胞的筛选标志, 便于阳性细胞的筛选, 并且由于携带有人IL2信号肽序列, 因此转染细胞具有外分泌的功能, 可以成功分泌内皮抑素蛋白到组织中发挥其生物学活性, 插入的片段位于CMV启动子下游, 基因的转录是由CMV启动子启动, CMV启动子转录效率较其他的启动子高, 这也有助于内皮抑素高效的表达. 我们通过阳离子介导内皮抑素基因转染肝癌细胞后, 实验证明在稳定转染endostatin基因的SMMC-7721细胞培养液中Western-blot检测到Mr 20000的endostatin蛋白, 表明带有IL-2分泌信号肽的内皮抑素基因转染SMMC-7721细胞后, 可以诱导细胞分泌表达内皮抑素蛋白, 并且通过鸡胚绒毛尿囊膜血管生成实验证实了其具有抑制血管生成的活性, 其活性呈剂量依赖关系, 血管内皮抑素只特异性作用于处于增殖状态的新生血管内皮细胞, 而不影响静止的血管内皮细胞及其他正常细胞. 因此重组人血管内皮抑素可有效抑制供肿瘤生长的新生血管, 而对正常组织无作用, 更因血管内皮抑素并不直接作用于肿瘤细胞, 因此不存在形成肿瘤耐药的问题, 本研究为内皮抑素基因治疗实验打下了基础.
编辑: 潘伯荣 审读:张海宁
| 1. | Cherrington JM, Strawn LM, Shawver LK. New paradigms for the treatment of cancer: the role of anti-angiogenesis agents. Adv Cancer Res. 2000;79:1-38. [PubMed] [DOI] |
| 2. | Sasaki T, Fukai N, Mann K, Gohring W, Olsen BR, Timpl R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998;17:4249-4256. [PubMed] [DOI] |
| 3. | Zatterstrom UK, Felbor U, Fukai N, Olsen BR. Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct Funct. 2000;25:97-101. [PubMed] [DOI] |
| 4. | Cao Y, Paner GP, Kahn LB, Rajan PB. Noninvasive carcinoma of the breast: angiogenesis and cell proliferation. Arch Pathol Lab Med. 2004;128:893-896. [PubMed] |
| 5. | Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer:relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445-451. [PubMed] |
| 6. | Zou D, Shibuya M, Shinoda K, Hibino S, Matsuda K, Takenaka K, Gemma A, Kudoh S. The difference of angiogenesis in human lung adenocarcinoma cell lines with different metastatic potency. J Nippon Med Sch. 2004;71:181-189. [PubMed] [DOI] |
| 7. | Kim TE, Murren JR. Angiogenesis in non-small cell lung cancer: a new target for therapy. Am J Respir Med. 2002;1:325-38. [PubMed] [DOI] |
| 8. | Angeletti CA, Lucchi M, Fontanini G, Mussi A, Chella A, Ribechini A, Vignati S, Bevilacqua G. Prognostic significance of tumoral angiogenesis in completely resected late stage lung carcinoma (stage IIIA-N2). impact of adjuvant therapies in a subset of patients at high risk of recurrence. Cancer. 1996;78:409-415. [PubMed] [DOI] |
| 9. | Wada N, Otani Y, Kubota T, Kimata M, Minagawa A, Yoshimizu N, Kameyama K, Saikawa Y, Yoshida M, Furukawa T. Reduced angiogenesis in peritoneal dissemination of gastric cancer through gelatinase inhibition. Clin Exp Metastasis. 2003;20:431-435. [PubMed] [DOI] |
| 10. | Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604-1606. [PubMed] [DOI] |
| 11. | Terlikowski S, Lenczewski A, Sulkowska M, Famulski W, Sulkowski S, Kulikowski M. Tissue expression of VEGF as a prognostic factor in early cervical squamous cell carcinoma. Folia Histochem Cytobiol. 2001;2:112-113. [PubMed] |
| 12. | Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190-2196. [PubMed] |
| 13. | Guenther U, Herbst H, Bauer M, Isbert C, Buhr HJ, Riecken EO, Schuppan D. Collagen type XVIII/endostatin is differentially expressed in primary and metastatic colorectal cancers and ovarian carcinomas. Br J Cancer. 2001;85:1540-1545. [PubMed] [DOI] |
| 14. | Shemirani B, Crowe DL. Head and neck squamous cell carcinoma lines produce biologically active angiogenic factors. Oral Oncol. 2000;36:61-66. [PubMed] [DOI] |
| 15. | Cao Y, Chen C, Weatherbee JA, Tsang M, Folkman J. Gro-beta, a-C-X-C-chemokine, is an angiogenesis inhibitor that suppresses the growth of Lewis lung carcinoma in mice. J Exp Med. 1995;182:2069-2077. [PubMed] [DOI] |
| 16. | Stack MS, Gately S, Bafetti LM, Enghild JJ, Soff GA. Angiostatin inhibits endothelial and melanoma cellular invasion by blocking matrix-enhanced plasminogen activation. Biochem J. 1999;340:77-84. [PubMed] [DOI] |
| 17. | O'Reilly MS. Angiostatin: an endogenous inhibitor of angiogenesis and of tumor growth. EXS. 1997;79:273-294. [PubMed] [DOI] |
| 18. | O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285. [PubMed] [DOI] |
| 19. | Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605-4610. [PubMed] [DOI] |
| 20. | Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA. 1999;96:2811-2816. [PubMed] [DOI] |
| 21. | Dhanabal M, Volk R, Ramchandran R, Simons M, Sukhatme VP. Cloning, expression, and in vitro activity of human endostatin. Biochem Biophys Res Commun. 1999;258:345-352. [PubMed] [DOI] |
| 22. | Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J Gene Med. 2004;6:1382-1393. [PubMed] [DOI] |
| 23. | Kruger EA, Duray PH, Tsokos MG, Venzon DJ, Libutti SK, Dixon SC, Rudek MA, Pluda J, Allegra C, Figg WD. Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem Biophys Res Commun. 2000;268:183-191. [PubMed] [DOI] |
| 24. | Blezinger P, Wang J, Gondo M, Quezada A, Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the Endostatin gene. Nat Biote chnol. 1999;17:343-348. [PubMed] [DOI] |
| 25. | Dhanabal M, Ramchandran R, Waterman M, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721-11726. [PubMed] [DOI] |
| 26. | Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404-407. [PubMed] [DOI] |
| 27. | Calvo A, Feldman AL, Libutti SK, Green JE. Adenovirus-mediated endostatin delivery results in inhibition of mammary gland tumor growth in C3 (1)/SV40 T-antigen transgenic mice. Cancer Res. 2002;62:3934-3938. [PubMed] |
| 28. | Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci USA. 2000;97:4802-4807. [PubMed] [DOI] |
| 29. | Heideman DA, van Beusechem VW, Bloemena E, Snijders PJ, Craanen ME, Offerhaus GJ, Derksen PW, de Bruin M, Witlox MA, Molenaar B. Suppression of tumor growth, invasion and angiogenesis of human gastric cancer by adenovirus-mediated expression of NK4. J Gene Med. 2004;6:317-327. [PubMed] [DOI] |
| 30. | Feldman AL, Restifo NP, Alexander HR, Bartlett DL, Hwu P, Seth P, Libutti SK. Antiangiogenic gene therapy of cancer utilizing a recombinant adenovirus to elevate systemic endostatin levels in mice. Cancer Res. 2000;60:1503-1506. [PubMed] |