修回日期: 2004-06-15
接受日期: 2004-06-17
在线出版日期: 2004-09-15
目的: 研究脂肪酸合成酶(FAS)抑制剂cerulenin对人胃癌细胞株增生的影响及诱导凋亡的作用.
方法: MTT法观察终浓度2.5, 5, 10, 20和40 mg/L cerulenin分别作用于无血清培养及含血清培养MKN28, SGC7901和MKN45细胞株24 h后, 检测其对细胞增生的抑制作用. 40 mg/L cerulenin作用于无血清培养的三种细胞12 h后, 用流式细胞仪(FCM)、DNA凝胶电泳对其凋亡和细胞周期进行检测.
结果: Cerulenin对人MKN28, SGC7901, MKN45细胞株的增生有显著的抑制作用并呈剂量依赖性, 对无血清培养细胞株的抑制作用高于有血清组. 2.5, 5, 10, 20和40 mg/L cerulenin作用于有血清培养及无血清培养MKN28细胞24 h的抑制率分别为3.2±0.6%, 7.3±0.5%, 11.3±0.7%, 17.7±1.2%, 41.7±1.0%和5.1±1.3%, 11.1±1.7%, 20.9±1.3%, 31.3±2.3%, 60.2±3.9%, 分别与其对照组比较有显著差异(1.4±1.3%, 3.3±2.0%, 6.2±3.2%, 8.1±1.1%, 10.1±1.7%, P <0.05和2.8±1.1%, 6.1±0.8%, 8.5±1.3%, 11.5±0.9%, 17.2±2.2%, P <0.05). 2.5, 5, 10, 20和40 mg/L cerulenin作用于有血清培养及无血清培养SGC7901细胞24 h的抑制率分别为3.4±0.8%, 9.5±1.2%, 23.3±1.7%, 38.5±1.8%, 65.2±2.1%和6.6±0.9%, 14.3±2.1%, 33.0±1.8%, 56.9±2.2%, 78.2±1.4%, 与对照组比较有显著差异(2.3±1.0%, 5.0±1.2%, 7.3±1.0%, 9.7±1.9%, 13.4±1.3%, P <0.05和3.9±1.2%, 8.7±1.5%, 10.5±1.9%, 13.8±1.6%, 19.5±1.7%, P <0.05). 2.5, 5, 10, 20和40 mg/L cerulenin作用于有血清培养及无血清培养MKN45细胞24 h的抑制率分别为5.9±1.1%, 13.5±0.9%, 30.5±1.9%, 49.1±1.5%, 71.7±2.0% 和8.4±1.1%, 19.6±1.4%, 40.4±1.4%, 67.0±1.3%, 83.8±2.0%,与对照组比较有显著差异(2.7±1.4%, 7.4±1.1%, 9.6±1.3%, 12.6±1.1%, 16.7±2.2%, P <0.05和4.4±1.6%, 9.5±0.9%, 13.7±0.7%, 18.4±1.6%, 26.6±2.1%, P <0.05). Cerulenin作用无血清培养MKN28, SGC7901, MKN45细胞株24 h的IC50值分别为13.3 mg/L, 16.7 mg/L, 32.3 mg/L显著低于含血清组(20.1 mg/L, 26.6 mg/L, 46.3 mg/L, P <0.01). MKN28, SGC7901, MKN45细胞在Cerulenin(40 mg/L, 12 h)作用下, 凋亡率分别为10.1±0.7%, 12.5±0.4%, 14.9±0.8%, 与对照组比较有显著差异(1.0±0.2%, 0.9±0.5%, 0.9±0.7%, P <0.01), 并引起胃癌细胞发生G2/M期阻滞. DNA凝胶电泳出现梯状条带.
结论: Cerulenin显著地阻遏人胃癌细胞的增生并诱导人胃癌细胞凋亡, 是胃癌治疗的新靶点.
引文著录: 陈凤鸣, 易粹琼, 陈涛, 叶建明, 钱伟. 脂肪酸合成酶抑制剂抑制人胃癌细胞增生并诱导凋亡. 世界华人消化杂志 2004; 12(9): 2024-2027
Revised: June 15, 2004
Accepted: June 17, 2004
Published online: September 15, 2004
AIM: To investigate the effect of fatty acid synthase inhibitor- cerulenin on the growth and apoptosis of human gastric cancer cells.
METHODS: The inhibitory effect of cerulenin was observed at 2.5, 5, 10, 20 and 40 mg/L on the growth of MKN28, SGC7901 and MKN45 cell lines in medium containing 100 mL/L fetal bovine serum and serum-free medium for 24 h by MTT assays. Flow cytometry (FCM) analysis and agarose gel electrophoresis were used to study the changes of cell apoptosis.
RESULTS: Cerulenin caused dose-dependent inhibition on the growth of human gastric cancer cells MKN28, SGC7901 and MKN45; Inhibition was much greater in serum-free medium than in medium with 100mL/L fetal bovine serum. The inhibitory rates of MKN28 after 24 h exposure to cerulenin at 2.5, 5, 10, 20 and 40 mg/L in medium with fetal bovine serum medium and serum-free were 3.2±0.6%, 7.3±0.5%, 11.3±0.7%, 17.7±1.2%, 41.7±1.0% and 5.1±1.3%, 11.1±1.7%, 20.9±1.3%, 31.3±2.3%, 60.2±3.9%, respectively (1.4±1.3%, 3.3±2.0%, 6.2±3.2%, 8.1±1.1%, 10.1±1.7% and 2.8±1.1%, 6.1±0.8%, 8.5±1.3%, 11.5±0.9%, 17.2±2.2%, P <0.05 vs controls, respectively). The inhibitory rates of SGC7901 after 24 h exposure to cerulenin at 2.5, 5, 10, 20 and 40 mg/L in medium with fetal bovine serum and serum-free medium were 3.4±0.8%, 9.5±1.2%, 23.3±1.7%, 38.5±1.8%, 65.2±2.1% and 6.6±0.9%, 14.3±2.1%, 33.0±1.8%, 56.9±2.2%, 78.2±1.4%, respectively (2.3±1.0%, 5.0±1.2%, 7.3±1.0%, 9.7±1.9%, 13.4±1.3% and 3.9±1.2%, 8.7±1.5%, 10.5±1.9%, 13.8±1.6%, 19.5±1.7%, P <0.05 vs controls, respectively).The inhibitory rates of MKN45 after 24 h exposure to cerulenin at 2.5, 5, 10, 20 and 40 mg/L in medium with fetal bovine serum medium and serum-free were 5.9±1.1%, 13.5±0.9%, 30.5±1.9%, 49.1±1.5%, 71.7±2.0% and 8.4±1.1%, 19.6±1.4%, 40.4±1.4%, 67.0±1.3%, 83.8±2.0%, respectively (2.7±1.4%, 7.4±1.1%, 9.6±1.3%, 12.6±1.1%, 16.7±2.2% and 4.4±1.6%, 9.5±0.9%, 13.7±0.7%, 18.4±1.6%, 26.6±2.1%, P <0.05 vs controls, respectively). After 24 h exposure to cerulenin, the IC50 of MKN28, SGC7901 and MKN45 cells in serum-free medium(13.3 mg/L, 16.7 mg/L, 32.3 mg/L, respectively) were lower than those of serum-medium group (20.1 mg/L, 26.6 mg/L, 46.3 mg/L, P <0.01). When MKN28, SGC7901 and MKN45 cells were treated for 12 h with 40 mg/L of cerulenin, the apoptotic rate revealed 10.1±0.7%, 12.5±0.4% and 14.9±0.8%, respectively (P <0.01 vs controls)and resulted in a block in G2/M phase of the cell cycle of gastric cancer cells. DNA agarose gel electrophoresis showed the typical DNA ladder of apoptosis.
CONCLUSION: Fatty acid synthase inhibitor- cerulenin inhibits the growth of human gastric cancer cells and induces apoptosis of human gastric cancer cells. Fatty acid synthase might be a potential target for anti-gastric cancer.
- Citation: Chen FM, Yi CQ, Chen T, Ye JM, Qian W. Effect of fatty acid synthase inhibitor on growth and apoptosis of human gastric cancer cells. Shijie Huaren Xiaohua Zazhi 2004; 12(9): 2024-2027
- URL: https://www.wjgnet.com/1009-3079/full/v12/i9/2024.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i9.2024
胃癌是威胁人类健康最常见的恶性肿瘤之一, 虽然手术治疗是目前惟一有可能根治胃癌的手段, 但其对中、晚期胃癌治疗效果多不佳, 因此研究开发有效的抗胃癌药物是科研工作者的使命[1-7]. 脂肪酸合成酶(FAS)是脂肪酸合成的关键酶, 而后者是肿瘤细胞增生的物质和能量来源. 研究发现FAS在肿瘤组织中高表达而在正常组织中不表达或低表达, 抑制FAS相关功能域, 可减少FAS表达, 抑制肿瘤生长. 因此选择性抑制FAS很可能成为肿瘤化学治疗的新靶点[8]. FAS抑制剂cerulenin对人胃癌细胞增生的影响, 尚未见报道.我们拟观察cerulenin对不同分化程度人胃癌细胞增生的影响及诱导凋亡作用, 以期为临床使用FAS抑制剂选择性杀伤胃癌细胞提供理论依据.
MKN28由华中科技大学同济医学院生物化学与分子生物学教研室惠赠, SGC7901、MKN45由华中科技大学同济医学院附属协和医院病毒实验室惠赠. 按常规传代于含100 mL/L小牛血清的RPMI1640培养液(Sigma公司)中, 37 ℃, 50 mL/L CO2条件下培养, 取对数生长期细胞进行实验. Cerulenin购自美国Sigma公司, 溶解于含1 kg/L二甲基亚砜(DMSO)的灭菌PBS中备用. 四甲基偶氮唑盐(MTT)为Fluka公司产品, 用PBS配制成5 g/L, 4 ℃避光保存. 碘化丙啶购于Clontech公司.
1.2.1 细胞增生抑制实验(MTT法): 选择对数生长期细胞, 调整细胞数为8×107/L, 接种于无菌96孔培养板, 每孔200 mL, 24 h后分别换入含100 mL/L血清及无血清RPMI1640. 有血清组和无血清组均给予终浓度2.5, 5, 10, 20和40 mg/L cerulenin, 两组均设立DMSO对照组. 每组的每个浓度下设3个复孔, 培养24 h后, 每孔加入20 mL 5 g/L MTT, 孵育4 h后, 弃上清, 加入DMSO, 终止反应.微振荡后, 用酶标仪(波长为490 nm)测吸光度(A). 计算抑制率(%)=(1-实验组A值/DMSO对照组A值)×100%. 平均抑制率<30%时, 无统计学意义.
1.2.2 DNA凝胶电泳: 细胞培养步骤同上, 24 h后换入无血清RPMI 1640及cerulenin 40 mg/L作用12 h, 并设DMSO对照组. 用胰酶消化收集每组约5×106个细胞, 离心, 去上清, 每份样品加入细胞裂解液500 mL, 20 g/L蛋白酶K 50 mL, 充分混合孵育4 h以上, 用酚-氯仿法抽提DNA, 经2 g/L琼脂糖凝胶电泳, 5 V/cm, 紫外线透射仪上显影拍照.
1.2.3 流式细胞仪检测细胞凋亡: 细胞培养步骤及给药处理同1.2.2. 收集细胞, 700 mL/L乙醇-4 ℃固定24 h, PBS缓冲液洗去固定液, 用缓冲液37 ℃孵育60 min, 然后加入碘化丙啶染色液, 4 ℃避光染色20 min, 检测DMSO对照组及cerulenin 40 mg/L作用12 h后胃癌细胞"亚G1"凋亡峰, 每组的每种细胞均设3个样本.
统计学处理 应用SPSS10.0统计软件包统计, 结果以 mean±SD表示, cerulenin组与DMSO对照组之间、有血清组与无血清组之间的比较采用配对t检验. 采用概率单位及线性回归方法计算半数抑制剂量(IC50), MKN45、SGC7901和MKN28三组之间该值的比较采用One-Way ANOVA检验.
MTT法检测不同浓度cerulenin分别对有血清及无血清培养的MKN28、SGC7901和MKN45细胞增生的抑制率(表1)并计算半数抑制浓度IC50值[9](表2), 作用时间均为24 h. 结果显示实验组胃癌细胞增生抑制率与DMSO对照组相比差异有显著性并呈剂量依赖性; 对无血清培养细胞株的抑制作用高于有血清组; 胃癌细胞分化程度越差, 抑制作用越明显.
| 分组 | mg/L | MKN28 | SGC7901 | MKN45 | |||
| 有血清组 | 无血清组 | 有血清组 | 无血清组 | 有血清组 | 无血清组 | ||
| Cerulenin | 2.5 | 3.2±0.6 | 5.1±1.3 | 3.4±0.8 | 6.6±0.9ad | 5.9±1.1a | 8.4±1.1 |
| 5 | 7.3±0.5a | 11.1±1.7c | 9.5±1.2 | 14.3±2.1ac | 13.5±0.9a | 19.6±1.4bc | |
| 10 | 11.3±0.7 | 20.9±1.3bd | 23.3±1.7b | 33.0±1.8bc | 30.5±1.9b | 40.4±1.4bd | |
| 20 | 17.7±1.2b | 31.3±2.3bd | 38.5±1.8b | 56.9±2.2bd | 49.1±1.5b | 67.0±1.3bd | |
| 40 | 41.7±1.0b | 60.2±3.9bc | 65.2±2.1b | 78.2±1.4bd | 71.7±2.0b | 83.8±2.0bc | |
| DMSO | 2.5 | 1.4±1.3 | 2.8±1.1 | 2.3±1.0 | 3.9±1.2 | 2.7±1.4 | 4.4±1.6 |
| 5 | 3.3±2.0 | 6.1±0.8 | 5.0±1.2 | 8.7±1.5c | 7.4±1.1 | 9.5±0.9d | |
| 10 | 6.2±3.2 | 8.5±1.3 | 7.3±1.0 | 10.5±1.9 | 9.6±1.3 | 13.7±0.7c | |
| 20 | 8.1±1.1 | 11.5±0.9 | 9.7±1.9 | 13.8±1.6 | 12.6±1.1 | 18.4±1.6c | |
| 40 | 10.1±1.7 | 17.2±2.2c | 13.4±1.3 | 19.5±1.7c | 16.7±2.2 | 26.6±2.1c | |
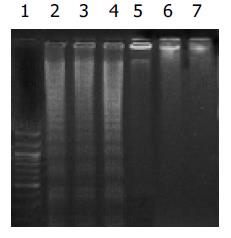
Cerulenin 40 mg/L 处理三种细胞12 h 后, DNA电泳出现典型的梯状条带, 而对照组则无DNA梯状条带, 呈基因组带(图1). 人胃癌细胞株MKN28, SGC7901和MKN45经40 mg/L cerulenin作用12 h后, FCM凋亡分析显示: 细胞凋亡的比例增加, 以MKN45组最为明显, 与DMSO对照组比较差异有显著性(P <0.05). 细胞周期分析显示: 实验组G0/G1期细胞减少, G2/M细胞增加, 提示G2/M期阻滞是cerulenin诱发人胃癌细胞发生凋亡的重要原因(表3).
既往人们只知道糖酵解途径通过戊糖磷酸途径与脂肪酸合成相连, 很少人注意到内源性脂肪酸合成与人类肿瘤的关系. 直到1980年代中期Ookhtens et al[10]用体内14C标记的葡萄糖处理Ehrlich腹水癌模型以探测肿瘤中的脂肪酸是否由体内自身合成, 结果表明肿瘤细胞中合成的脂肪酸占所有甘油三酯中脂肪酸的93%, 又根据游离脂肪酸和血浆甘油三酯从宿主细胞到肿瘤细胞的转运速度, 证实内源性脂肪酸是肿瘤细胞生长所需脂肪酸的重要来源. 因此选择性抑制内源性脂肪酸合成有可能成为肿瘤治疗的新途径. Cerulenin是真菌cephalosporium的天然代谢产物, 他与FAS关键组分b-酮酯酰聚合域的半胱氨酸巯醇共价结合从而抑制FAS表达[11]. Pizer et al[12]发现cerulenin仅对肿瘤细胞有抑制作用, 对正常细胞则无影响. 我们用MTT法证实cerulenin对胃癌细胞有显著的抑制作用, 并呈剂量依赖性, 推测内源性脂肪酸合成是恶性肿瘤增生所必需, cerulenin 通过抑制细胞内源性脂肪酸合成, 从而阻遏人胃癌细胞生长.该实验还显示cerulenin对无血清培养细胞的抑制作用高于含血清培养细胞, 二者相比有统计学意义, 这可能与无血清培养基缺乏内源性脂质迫使细胞加强内源性脂肪酸合成, 以满足细胞生长所需的膜磷脂有关[13].
在细胞凋亡过程中, Ca2+, Mg2+依赖性核酸内切酶被激活, 降解细胞核内的DNA, 产生大约180-200 bp整数倍的DNA片段, 在琼脂糖凝胶电泳图上呈现"梯状DNA"条带. 我们用DNA电泳证实cerulenin可诱导不同分化程度胃癌细胞产生凋亡. 进一步FCM分析结果揭示cerulenin作用12 h后, 不同分化程度的胃癌细胞在DNA直方图上均出现明显的凋亡峰. 而且分化程度越低, cerulenin的抑制作用就越明显, 凋亡率也越高, 其具体机制仍不清楚. Pizer et al[14]认为丙二酰辅酶A在cerulenin或C75处理后发生积聚, 表明前者部分介导FAS抑制导致的细胞毒性. 其细胞毒性最早可通过抑制脂肪酸b-氧化产生作用, 但最终需激活凋亡通路促使细胞损伤, 猜测FAS高表达可阻止肿瘤细胞发生凋亡[15]. 但Heiligtag et al[16]研究显示cerulenin介导细胞凋亡程度与FAS的表达无直接关系, 而且抑制FAS和诱导凋亡效应所需的cerulenin浓度不一致, 低浓度cerulenin可抑制FAS活性, 但不能诱导肿瘤细胞凋亡, 表明抑制FAS活性不是引起细胞凋亡的惟一因素.
细胞周期分析显示cerulenin可使胃癌细胞发生G2/M期阻滞. G0/G1和G2/M期是细胞周期的两个关键调控点, 如果不能顺利通过这两个调控点, 则细胞停止分裂增生, 走向死亡. Furuya et al[17]观察到的现象与本研究一致, 他同时发现细胞周期调节蛋白D1表达减少, 其抑制剂p21, p27表达增加, 认为这些细胞周期调节因子在cerulenin诱导的G2/M期阻滞中起一定作用. 我们猜测cerulenin使肿瘤细胞的磷脂生长减少, 导致细胞生物膜合成受阻是触发G2/M期阻滞的重要始动因素[18-19]. 事实上, 磷脂代谢的主要调控因子载脂蛋白E在高FAS蛋白的肿瘤组织中高表达. 因而高FAS表达肿瘤中大量脂肪酸不仅可通过提供细胞分裂所需的膜脂质, 也可作用于细胞复制有关的第二信使分子, 从而影响细胞增生[20-22].
目前有关FAS与胃癌关系的研究较少, 本实验结果表明脂肪酸合成酶抑制剂能够阻遏胃癌细胞生长并诱导其凋亡, 为实体肿瘤的化学治疗提供了一条新思路.
| 1. | Chen BQ, Yang YM, Wang Q, Gao YH, Liu JR, Zhang JS, Wang XL, Liu RH. Effects of c9,t11-conjugated linoleic acid on adhesion of human gastric carcinoma cell line SGC-7901. World J Gastroenterol. 2004;10:1392-1396. [PubMed] [DOI] |
| 2. | Gulmann C, Hegarty H, Grace A, Leader M, Patchett S, Kay E. Differences in proximal (cardia) versus distal (antral) gastric carcinogenesis via the retinoblastoma pathway. World J Gastroenterol. 2004;10:17-21. [PubMed] |
| 3. | Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L, Liu YX. Correlation of tumor-positive ratio and number of perigastric lymph nodes with prognosis of patients with surgically-removed gastric carcinoma. World J Gastroenterol. 2004;10:182-185. [PubMed] |
| 4. | Yu Y, Zhang YC, Zhang WZ, Shen LS, Hertzog P, Wilson TJ, Xu DK. Ets1 as a marker of malignant potential in gastric carcinoma. World J Gastroenterol. 2003;9:2154-2159. [PubMed] [DOI] |
| 5. | Zhang J, Su XQ, Wu XJ, Liu YH, Wang H, Zong XN, Wang Y, Ji JF. Effect of body mass index on adenocarcinoma of gastric cardia. World J Gastroenterol. 2003;9:2658-2661. [PubMed] [DOI] |
| 6. | Guo HQ, Guan P, Shi HL, Zhang X, Zhou BS, Yuan Y. Prospective cohort study of comprehensive prevention to gastric cancer. World J Gastroenterol. 2003;9:432-436. [PubMed] [DOI] |
| 7. | Shi XY, Zhao FZ, Dai X, Ma LS, Dong XY, Fang J. Effect of jianpiyiwei capsule on gastric precancerous lesions in rats. World J Gastroenterol. 2002;8:608-612. [PubMed] [DOI] |
| 8. | Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202-208. [PubMed] [DOI] |
| 9. | Vermeirssen V, Van Camp J, Verstraete W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J Biochem Biophys Methods. 2002;51:75-87. [PubMed] [DOI] |
| 10. | Ookhtens M, Kannan R, Lyon I, Baker N. Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol. 1984;247:R146-R153. [PubMed] |
| 11. | Funabashi H, Kawaguchi A, Tomoda H, Omura S, Okuda S, Iwasaki S. Binding site of cerulenin in fatty acid synthetase. J Biochem. 1989;105:751-755. [PubMed] [DOI] |
| 12. | Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56:2745-2747. [PubMed] |
| 13. | Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58:4611-4615. [PubMed] |
| 14. | Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213-218. [PubMed] |
| 15. | Thupari JN, Pinn ML, Kuhajda FP. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem Biophys Res Commun. 2001;285:217-223. [PubMed] [DOI] |
| 16. | Heiligtag SJ, Bredehorst R, David KA. Key role of mitochondria in cerulenin-mediated apoptosis. Cell Death Differ. 2002;9:1017-1025. [PubMed] [DOI] |
| 17. | Furuya Y, Akimoto S, Yasuda K, Ito H. Apoptosis of androgen-independent prostate cell line induced by inhibition of fatty acid synthesis. Anticancer Res. 1997;17:4589-4593. [PubMed] |
| 18. | Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van de Sande T, Heemers H, Heyns W, Verhoeven G. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898-903. [PubMed] [DOI] |
| 19. | Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci USA. 2003;100:6358-6363. [PubMed] [DOI] |
| 20. | Lomnitski L, Oron L, Sklan D, Michaelson DM. Distinct alterations in phospholipid metabolism in brains of apolipoprotein E-deficient mice. J Neurosci Res. 1999;58:586-592. [PubMed] [DOI] |
| 21. | Igbavboa U, Hamilton J, Kim HY, Sun GY, Wood WG. A new role for apolipoprotein E: modulating transport of polyunsaturated phospholipid molecular species in synaptic plasma membranes. J Neurochem. 2002;80:255-261. [PubMed] [DOI] |
| 22. | Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, Bubley G, Balk S, Loda M. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707-715. [PubMed] |