修回日期: 2004-04-21
接受日期: 2004-05-13
在线出版日期: 2004-08-15
目的: 探讨CD95 L在肝癌细胞凋亡过程中的作用.
方法: ELISA法对慢性乙型肝炎、肝炎肝硬化与肝癌患者血清可溶性CD95 L(sCD95L)水平进行了初步检测, 构建了人CD95 L的重组真核表达体pcDNA3.1hisB-CD95 L, 将pcDNA3.1hisB-CD95 L转染至人肝癌细胞株HepG2细胞, 采用Annexin V/PI双染后双变量流式细胞仪检测细胞凋亡率.
结果: sCD95L在肝癌患者明显低于肝炎及肝硬患者, 构建的表达重组体pcDNA3.1hisB-CD95 L经菌落 PCR和限制性酶切消化有预期的目的片段出现, DNA序列分析证实CD95 L完整、正确插入, 转染后的HepG2细胞细胞凋亡率为36.30%; 未转染CD95L的对照组细胞凋亡率11.53%.
结论: CD95L可使肝癌细胞凋亡.
引文著录: 陈军, 苏先狮, 蒋永芳. CD95配体分子诱导人肝癌细胞凋亡的作用. 世界华人消化杂志 2004; 12(8): 1789-1792
Revised: April 21, 2004
Accepted: May 13, 2004
Published online: August 15, 2004
AIM: To investigate CD95 ligand and its physiological function in liver neoplasms.
METHODS: The levels of soluble Fas ligand (sFasL) were evaluated in a group of patients affected by hepatitis B virus (HBV)-induced chronic hepatitis, HBV-positive liver cirrhosis and hepatocellular carcinoma (HCC).To further study, we constructed recombinant eukaryotic expression vector pcDNA3.1 hisB-CD95L, which was then tranfected into human hepatoma cell line HepG2 by lipofection.After stained by annexin V and propidium iodine, HepG2 cells were detected by flow cytometer.
RESULTS: CD95L levels were significantly decreased in patients with HCC when compared to the patients with hepatitis or liver cirrhosis.The correct recombinant pcDNA3.1hisB-CD95L was selected by PCR and restriction endonuclease digestion and confirmed by DNA sequencing respectively.Subsequently a significant proportion of cells became apoptotic, as evidenced by positive annexin staining.
CONCLUSION: CD95-CD95 ligand system can induce apoptosis of hepatoma cells.
- Citation: Chen J, Su XS, Jiang YF. cDNA cloning and expression of human CD95 ligand and its role in apoptosis of HepG2 cell lines. Shijie Huaren Xiaohua Zazhi 2004; 12(8): 1789-1792
- URL: https://www.wjgnet.com/1009-3079/full/v12/i8/1789.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i8.1789
CD95分子及其配体CD95配体(CD95 ligand)在细胞凋亡的信号传导过程中有重要作用.CD95配体通过与靶细胞CD95分子结合, 传导凋亡信号, 诱导靶细胞凋亡.在肝癌细胞发生发展和转移过程中, CD95配体的作用尚不清楚. 拟比较可溶性CD95配体在慢性乙型肝炎、肝炎肝硬化、肝癌患者血清中的差异, 将CD95配体cDNA克隆, 构建pcDNA3.1hisB表达载体, 转染至人肝癌细胞HepG2, 观察CD95配体对该细胞株细胞凋亡的影响.
人CD95配体定量EIA检测试剂盒购自MBL公司, Trizol试剂、cDNA第一链合成试剂盒、Lifefectamine regent、RPMI1640培养基购自Gibco公司, Qiagen mini kit, Qia quick gel extration kit 购自Qiagen公司, plus SV minipreps DNA purification reagent system, EcoRI、BamH I内切酶、T4DNA连接酶、Taq酶、胎牛血清购自Promega公司, FITC-Annexin V试剂盒购自北京宝灵曼公司, 兔抗人CD95(即用型)及SP-超敏免疫组化检测试剂盒购自福建迈新公司, 其余试剂为本室自备.HepG2 人肝癌细胞株购自中南大学湘雅医学院细胞中心HepG2.2.15人肝癌细胞株由第一军医大学传染病研究所馈赠, PCR pGEM-T easy vector 为Promega公司产品, pcDNA3.1hisB为Invitrogen公司产品.选用2003-01/02中南大学湘雅二医院传染科住院患者22例, 其中慢性重度病毒性肝炎(乙型)12例, 肝炎肝硬化(活动期)6例, 诊断标准遵照2000-09西安中华医学会传染病与寄生虫病学分会、肝病学分会联合修订《病毒性肝炎防治方案》.选用2002-06/2003-01中南大学湘雅二医院肝胆外科住院患者16例, 经病检确诊为肝细胞癌.另选6名正常志愿者为对照.
采用双抗体夹心EIA检测慢性肝炎、肝硬化、肝癌患者血清的可溶性CD95配体水平, 酶标仪采用Lab Systems, Wellscan MK2全自动酶标仪.无菌采取一例慢性乙肝患者前臂静脉血10 mL, 进行PBMC分离, ConA活化后培养6 h, 采用Trizol RNA 提取细胞总RNA.将CD95配体基因编码区的cDNA克隆pGEM-T easy 克隆载体, 根据文献报道的人CD95L cDNA基因序列, 按照引物设计要求, 该对引物含有EcoRI和BamHI两个酶切位点设计引物序列如下: Primer1: 5'-GAC GGA TCC CCT CTA CAG GAC TGA GAA GAA G -3'; Primer2: 5'-GAC GAA TTC CAA CAT TCT CGG TGC CTG TAA C -3'.采用RT-PCR合成第一链cDNA, 然后采用标准PCR反应体系扩增其编码区, 采用Qia quick gel extration kit回收目的片段.按照PCR产物: pGEM-T easy vector(摩尔数)为3: 1的比例, 用T4连接酶于4 °C连接过夜, 将连接产物转化大肠杆菌TG1, 挑选白色转化菌落小量培养, 采用Qiagen mini kit提取质粒DNA, 酶切鉴定插入片段大小, PCR筛选阳性克隆后, ABI377自动测序仪测序证实.构建pcDNA3.1 hisB/CD95 配体表达载体 用适量EcoRI/BamH I双酶切闭环pcDNA3.1hisB和pGEM-T easy vector-CD95 配体重组质粒, 分别进行目的片段的再切胶回收, 将回收CD95 配体基因目的片段与线性pcDNA3.1hisB用T4连接酶于4 °C连接过夜, 将连接产物转化大肠杆菌TG1, 将连接产物转化大肠杆菌TG1, 铺于Am(+)平板中, 挑选白色转化菌落小量培养, 提取质粒DNA, EcoRI/BamH I双酶切鉴定插入片段大小, PCR筛选阳性克隆后, ABI377自动测序仪测序证实.pcDNA3.1 hisB/CD95 配体表达重组质粒转染HepG2细胞: 常规复苏HepG2细胞, 根据细胞生长情况传代, 收获生长良好细胞, 在6孔板中, 每孔接种3×105个细胞于完全培养液中, 待细胞生长至80%汇合期, 稀释pcDNA3.1hisB/CD95 配体表达重组质粒为2, 4, 6, 8 μg及pcDNA3.1hisB质粒6 μg, 分别加入5个无血清培养基的EP管; 另设第6管为阴性对照, 仅加入无血清培养基100 μL.将10 μL脂质体加入上述稀释液中, 轻轻摇匀, 室温放置20 min, 加无血清培养基800 μL于复合物中, 混匀后, 小心滴加细胞中, 37 °C, 50 mL/L CO2培养箱培养24 h, 取生长有细胞的盖玻片, PBS洗3次, 950 mL/L酒精固定后, 加入1抗兔抗人CD95, 然后采用S-P超敏试剂盒进行免疫组化检测, 收集细胞培养液上清采用EIA进行可溶性CD95 配体检测.将已转染CD95 配体的HepG2细胞与HepG2.2.15细胞共同培养24 h后收集细胞, 按FITC-Annexin V试剂盒及PI染色细胞, 进行流式细胞仪分析及激光共聚焦显微镜观察.
统计学处理 采用SPSS10.0统计软件处理数据, 计算采用t检验.
统计学分析表明, 肝癌患者血清的可溶性CD95L水平(2.8±0.4 pg/L) 与慢性肝炎(3.2±0.4 pg/L)、肝硬化患者(3.8±1.1 pg/L)及正常对照组(3.5±0.7 pg/L)比较, 均存在显著性差异(P<0.05).
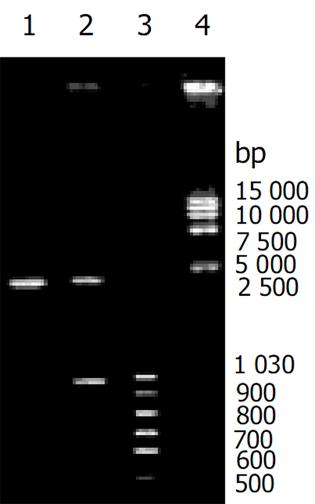
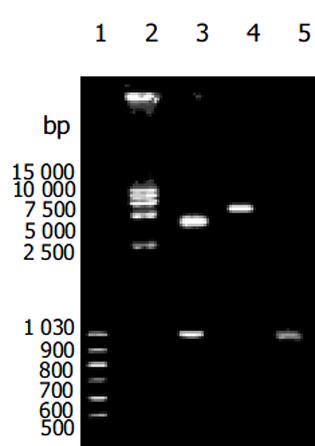
重组质粒pGEM-T easy vector-CD95 L重组质粒、pcDNA3.1 hisB-CD95 L表达重组质粒酶切及PCR扩增后, 鉴定含有预期目的片段(图1, 2), 测序后 GenBank Blast检测, 与人CD95序列一致.nBLAST FAQs nTaxonomy reports nDistribution of 150 Blast Hits on the Query Sequence nAlignments n>gi|601892|dbj|D38122.1|HUMHPC Human mRNA for Fas ligand, complete cds Length = 1890 Score = 499 bits (1083), Expect = e-139 Identities = 205/206 (99%), Positives = 205/206 (99%) Frame = -3/+1.转染后细胞CD95 L的表达(见图3).
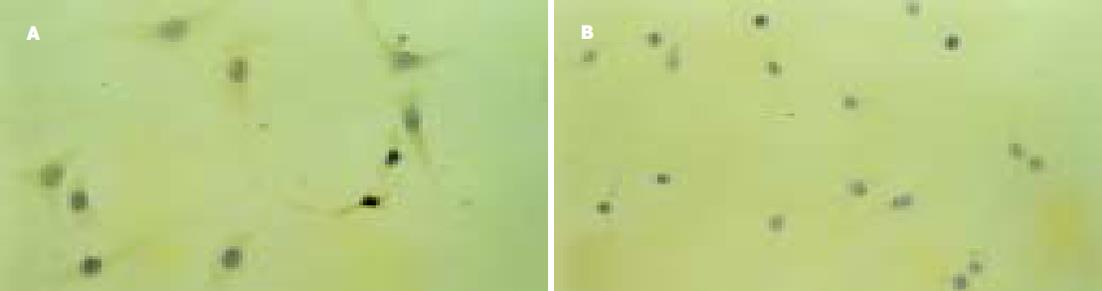
Annexin V/PI双染阴性为活细胞; 死细胞为PI染色阳性, Annexin V阴性; 早期凋亡细胞PI染色阴性, Annexin V阳性; 晚期凋亡细胞Annexin V/PI双染阳性.在本试验中, 有各种不同时期的细胞(图4). 流式细胞仪结果表明转染CD95 L的HepG2细胞与HepG2.2.15细胞凋亡率为36.3%, 未转染CD95 L的对照组细胞凋亡率为11.5%.
CD95 /CD95 L是研究较为广泛的细胞凋亡分子, 是传导细胞凋亡信号的重要途径之一[1-5].目前对CD95配体在肝癌的发生、增生和转移过程中的作用尚不明确, 方法仅限于一些肝组织原位检测方法如免疫组化、原位杂交等.初步证实肝癌患者CD95/CD95L及其mRNA表达在癌周正常组织明显高于癌组织, 且恶性程度愈高, 在分化很差的癌细胞, 难以发现CD95/CD95L及其mRNA表达表达量愈低[6-10].在肝癌细胞转移过程中, Kupffer细胞可释放TNFα, 诱导肝癌细胞表达CD95/CD95L导致肝癌细胞凋亡, 依此阻止肝癌细胞的转移[11].sCD95L以溶解状态存在于体液中, 可与靶细胞CD95结合, 启动凋亡信号的传导[12-17].我们通过对血清sCD95L检测表明, 肝癌患者血清sCD95L明显低于慢性肝炎及肝炎肝硬化患者, 提示在肝癌的发生中, sCD95L有一定的作用, 有进一步研究价值.
真核表达载体pcDNA3.1hisB在多克隆位点的上游和下游分别带有CMV的启动子和BGH的polyA尾, 这种强有力的巨细胞病毒的增强启动子序列, 能高效表达插入的目的基因, 并且可在范围广泛的宿主细胞中工作.该载体带有筛选标志Neo基因, 在Neo基因的上游和下游分别带有SV40的启动子和polyA尾, 保证了Neo基因的有效转录[18-24].为研究FasL的生物学效应, 我们选择了pcDNA3.1hisB作为表达载体, 将CD95LcDNA转染入功能接近正常肝细胞肝癌细胞系HepG2, 免疫组化显示CD95L已在细胞膜得以表达, 而且在培养液上清也检测到了sCD95L的表达, 表明CD95LcDNA转染后的HepG2细胞已具备表达CD95L的能力.在细胞凋亡检测手段上, 采用检测细胞膜成分变化的Annexin V 联合PI法, 其原理磷脂酰丝氨酸(phosphatidylserine, PS)正常位于细胞膜的内侧, 但在细胞凋亡期, PS可从细胞膜的内侧翻转到细胞膜的表面, 暴露在细胞外环境中.Annexin-V能与PS高亲和力特异性结合.碘化丙啶(propidine iodide, PI)是一种核酸染料, 他不能透过完整的细胞膜, 但在凋亡中晚期和坏死期的细胞, PI能够透过细胞膜而使细胞核红染.因此将Annexin-V与PI匹配使用, 就可以将凋亡早晚期的细胞以及坏死细胞区分开来.采用流式细胞仪测量细胞悬液中细胞荧光强度来区分正常细胞、坏死细胞和凋亡细胞[25-32].转染了CD95L的HepG2细胞与转染了HBV DNA的HepG2细胞(HepG2.2.15)的共同培养时, 可使HepG2细胞出现细胞凋亡.由此表明, 肝癌细胞可通过CD95/CD95L途径凋亡.
编辑: N/A
| 1. | Siegel RM, Muppidi J, Roberts M, Porter M, Wu Z. Death receptor signaling and autoimmunity. Immunol Res. 2003;27:499-512. [PubMed] [DOI] |
| 2. | Cui W, Li LY. [Functional modulating effect of CD40 ligand on CD40-transfected human lung carcinomas]. Zhonghua Zhong Liu Za Zhi. 2004;26:150-153. [PubMed] |
| 3. | De Freitas I, Fernández-Somoza M, Essenfeld-Sekler E, Cardier JE. Serum levels of the apoptosis-associated molecules, tumor necrosis factor-alpha/tumor necrosis factor type-I receptor and Fas/FasL, in sepsis. Chest. 2004;125:2238-2246. [PubMed] [DOI] |
| 4. | Li M, Liu GT. Inhibition of Fas/FasL mRNA expression and TNF-alpha release in concanavalin A-induced liver injury in mice by bicyclol. World J Gastroenterol. 2004;10:1775-1779. [PubMed] [DOI] |
| 5. | Oh SH, Yin HQ, Lee BH. Role of the Fas/Fas ligand death receptor pathway in ginseng saponin metabolite-induced apoptosis in HepG2 cells. Arch Pharm Res. 2004;27:402-406. [DOI] |
| 6. | Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, Michalopoulos GK, Zarnegar R. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell. 2002;9:411-421. [PubMed] [DOI] |
| 7. | Nakamoto Y, Kaneko S, Fan H, Momoi T, Tsutsui H, Nakanishi K, Kobayashi K, Suda T. Prevention of hepatocellular carcinoma development associated with chronic hepatitis by anti- Fas ligand antibody therapy. J Exp Med. 2002;196:1105-1111. [DOI] |
| 8. | Ito Y, Monden M, Takeda T, Eguchi H, Umeshita K, Nagano H, Nakamori S, Dono K, Sakon M, Nakamura M. The status of Fas and Fas ligand expression can predict recurrence of hepatocellular carcinoma. Br J Cancer. 2000;82:1211-1217. [PubMed] |
| 9. | Fukuzawa Y, Takahashi K, Furuta K, Tagaya T, Ishikawa T, Wada K, Omoto Y, Koji T, Kakumu S. Expression of fas/fas ligand (fasL) and its involvement in infiltrating lymphocytes in hepatocellular carcinoma (HCC). J Gastroenterol. 2001;36:681-688. [PubMed] [DOI] |
| 10. | Lau WY, Chen GG, Lai PB, Chun YS, Leung BC, Chak EC, Lee JF, Chui AK. Induction of Fas and Fas ligand expression on malignant glioma cells by Kupffer cells, a potential pathway of antiliver metastases. J Surg Res. 2001;101:44-51. [PubMed] [DOI] |
| 11. | Kubo K, Matsuzaki Y, Okazaki M, Kato A, Kobayashi N, Okita K. The Fas system is not significantly involved in apoptosis in human hepatocellular carcinoma. Liver. 1998;18:117-123. [PubMed] [DOI] |
| 12. | Mauz-Körholz C, Banning U, Körholz D. Regulation of interleukin-2 induced soluble Fas ligand release from human peripheral blood mononuclear cells. Immunol Invest. 2004;33:251-260. [PubMed] [DOI] |
| 13. | Kaur H, Jaso-Friedmann L, Evans DL. Single base oligodeoxyguanosine upregulates Fas ligand release by nonspecific cytotoxic cells. Dev Comp Immunol. 2004;28:571-579. [PubMed] [DOI] |
| 14. | Hu ZB, Zou P, Li AX, Zhang YS, Wang LL, Liu LB. Study on blocking the leukemia immune escape after BMT by Fas-Fas ligand pathway. Chin Med J (Engl). 2004;117:419-424. [PubMed] |
| 15. | Sakamoto N, Mukae H, Fujii T, Kakugawa T, Kaida H, Kadota J, Kohno S. Soluble form of Fas and Fas ligand in serum and bronchoalveolar lavage fluid of individuals infected with human T-lymphotropic virus type 1. Respir Med. 2004;98:213-219. [PubMed] [DOI] |
| 16. | Linghu H, Xu X, Luo J, Zhuang L. Changes of soluble fas and soluble fas ligand in serum and peritoneal fluid of infertile patients with endometriosis. Chin Med Sci J. 2004;19:56-59. |
| 17. | Xiao J, Zou P, Liu Z, Liu L, Hu Z. Selective depletion of the allo-antigen specific T cells by Fas/FasL pathway by cytokine IFN-gamma and IL-2. J Huazhong Univ Sci Technolog Med Sci. 2003;23:344-347. [PubMed] [DOI] |
| 18. | Ma F, Zhang SR, Ning L, Sun WX, Liang X, Zhang XY, Fu M, Lin C. [Construction of eukaryotic expression vector of murine SLC gene and characterization of its chemotactic function]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:528-530. [PubMed] |
| 19. | Feng RH, Zhu ZG, Li JF, Liu BY, Yan M, Yin HR, Lin YZ. Inhibition of human telomerase in MKN-45 cell line by antisense hTR expression vector induces cell apoptosis and growth arrest. World J Gastroenterol. 2002;8:436-440. [PubMed] [DOI] |
| 20. | Jiang W, Yang CQ, Liu WB, Wang YQ, He BM, Wang JY. Blockage of transforming growth factor beta receptors prevents progression of pig serum-induced rat liver fibrosis. World J Gastroenterol. 2004;10:1634-1638. [PubMed] [DOI] |
| 21. | Jin Z, Guan T, Li S. [Effects of wild-type p53 gene on the chemotherapy sensitivity of ovarian cancer SKOV-3 cells to cisplatin]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2002;19:218-220. [PubMed] |
| 22. | Guo C, Ding J, Yu Z, Han Q, Meng F, Liu N, Fan D. [Development of oral DNA vaccine based on MG(7)-Ag mimotope of gastric cancer]. Zhonghua Zhong Liu Za Zhi. 2002;24:110-113. [PubMed] |
| 23. | Gu ZP, Wang YJ, Li JG, Zhou YA. VEGF165 antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol. 2002;8:44-48. [PubMed] [DOI] |
| 24. | Tao KS, Dou KF, Wu XA. Expression of angiostatin cDNA in human hepatocellular carcinoma cell line SMMC-7721 and its effect on implanted carcinoma in nude mice. World J Gastroenterol. 2004;10:1421-1424. [PubMed] [DOI] |
| 25. | Murakami M, Sasaki T, Miyata H, Yamasaki S, Kuwahara K, Chayama K. Fas and Fas ligand: Expression and soluble circulating levels in bile duct carcinoma. Oncol Rep. 2004;11:1183-1186. [PubMed] [DOI] |
| 26. | Linghu H, Xu X, Luo J, Zhuang L. Changes of soluble fas and soluble fas ligand in serum and peritoneal fluid of infertile patients with endometriosis. Chin Med Sci J. 2004;19:56-59. [PubMed] |
| 27. | Reutelingsperger C, Hofstra L, Narula J. Cooking annexin V: A Simple 1-Pot procedure to destroy its phosphatidylserine-binding activity. J Nucl Med. 2004;45:1098-1099. |
| 28. | Van de Wiele C, Vermeersch H, Loose D, Signore A, Mertens N, Dierckx R. Radiolabeled annexin-V for monitoring treatment response in oncology. Cancer Biother Radiopharm. 2004;19:189-194. [PubMed] [DOI] |
| 29. | Wang QH, Xie Y, Fan HH, Gao L, Liu Y, Xie YH. [Effect of HMBA on differentiation and apoptosis of HL-60 and U937 cells and its mechanism]. Zhonghua Xue Ye Xue Za Zhi. 2004;25:154-157. [PubMed] |
| 30. | Reutelingsperger C, Hofstra L, Narula J. Cooking annexin V: a simple 1-pot procedure to destroy its phosphatidylserine-binding activity. J Nucl Med. 2004;45:1098; author reply 1098-1098; author reply 1099. [PubMed] |
| 31. | Li HM, Zhang HG, Wang HC, Qian XP, Kong XT, Chen WF. [Effect of CD28 costimulator on T cell receptor(TCR)-induced apoptosis of thymocytes]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:14-16. [PubMed] |
| 32. | Olgun S, Gogal RM Jr, Adeshina F, Choudhury H, Misra HP. Pesticide mixtures potentiate the toxicity in murine thymocytes. Toxicology. 2004;196:181-195. [PubMed] [DOI] |