修回日期: 2004-04-01
接受日期: 2004-04-13
在线出版日期: 2004-07-15
目的: 通过检测伴有幽门螺杆菌(Helicobacter pylori, H. pylori)感染的上消化道疾病患者血清对H. pylori OMP的反应性, 来探讨基因重组H. pylori OMP对H. pylori感染及其相关性疾病的诊断价值, 为进一步开发诊断试剂盒及其临床应用奠定基础.
方法: 分别将已鉴定、测序的含H. pylori Mr为18 000, 26 000 OMP重组质粒转化表达菌-大肠杆菌BL21中, 让其大量表达、纯化, 制备金标免疫检测试纸; 选择2002-01/2002-12因消化道症状来我院就诊的H. pylori阳性患者150例, 经胃镜证实: 慢性浅表性胃炎60例; 胃溃疡30例; 十二指肠球部溃疡30例; 胃癌30例, 以及33例H. pylori阴性的健康者作为对照, 分别采用以H. pylori Mr为18 000, 26 000 OMP为抗原制备金标免疫检测试纸, 对183例受试者血清进行特异性抗体检测.
结果: 重组蛋白经Ni2+-NTA琼脂糖树脂纯化后, 其纯度高达95%以上, 经检测其抗原性良好. 用金标免疫试纸对H. pylori感染的上消化道疾病患者150例, 以及H. pylori阴性的健康者33名进行了检测, 其结果为: 26 000 OMP对H. pylori感染的检出率为94.0%, 对慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡以及胃癌患者中H. pylori感染的检出率分别为95.0%, 96.7%, 96.7%和90.0%, 与常规ELISA方法检测结果相比, 二者无显著性差异(x2检验, P>0.05), 其敏感性、特异性和准确性分别为94.0%、97.0%、94.5%; 而18 000 OMP对H. pylori感染患者的检出率为52.0%, 对慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡和胃癌患者中H. pylori感染的检出率分别为40.0%、40.0%、53.3%和86.7%, 对胃癌患者中H. pylori感染检出率86.7%与慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡总检出率43.3%相比, 具有显著的差异性(P<0.05). 因此以26 000 OMP 的金标免疫检测试纸可以作为H. pylori感染的常规检测方法, 他与常规ELISA检测方法相比具有可靠的特异性以及敏感性, 同时与18 000 OMP金标免疫检测试纸一起, 对胃癌患者中H. pylori感染检出率高对胃肠道肿瘤特别是胃癌的诊断及预测方面具有独特之处, 这值得进一步的研究.
结论: H. pylori Mr 18 000, 26 000 OMP金标免疫检测试纸对H. pylori感染及其相关性疾病, 尤其对消化道恶性肿瘤的诊断及预测具有重要的价值, 可以作为常规检测手段对消化道溃疡及肿瘤高危人群进行检测.
引文著录: 姜政, 黄爱龙, 郑健, 陶小红, 蒲丹, 王丕龙. 幽门螺杆菌外膜蛋白对Helicobacter pylori感染的诊断. 世界华人消化杂志 2004; 12(7): 1588-1592
Revised: April 1, 2004
Accepted: April 13, 2004
Published online: July 15, 2004
AIM: To examine the serological response of patient with various upper gastrointestinal diseases and Helicobacter pylori (H. pylori ) infection to H. pylori outer membrane proteins (OMP) with Mr18 000 and 26 000 acquired by gene recombinant technique, and to determine its diagnostic significance.
METHODS: The recombinant vectors encoding OMP of H. pylori with Mr18 000 and 26 000 identified by restriction enzyme or PCR were used to transform and express in BL21 (DE3) E. coli respectively. After purification with Ni2+-NTA agarose resin, the colloid gold kits were prepared with purified recombinant proteins to detect H. pylori infection and disease associated with H. pylori by immunity-marker technology. We selected 150 patients with H. pylori infection and digestive symptoms without previous treatment, including chronic superficial gastritis (n = 60), duodenal ulcer (n = 30), gastric ulcer (n = 30), and gastric cancer (n = 30) during one year. Simultaneously, 33 cases without digestive symptoms and H. pylori infection were used as controls. All sera were collected and the antibody responded to specific proteins of H. pylori was tested with the colloid gold test kit. Taking as reference a combination of standard diagnostic methods (13C urea breath test, cultivate) and the classic enzyme-linked immunosorbent assay (ELISA) serological tests was compared with the results of this technique, and the sensitivity, specificity and accuracy of the colloid gold test were evaluated.
RESULTS: After purification with Ni2+-NTA agarose resin, the purities of recombinant fusion proteins were all about 95%. The ELISA results showed that recombinant fusion proteins could be recognized by monoclonal antibody of anti-H. pylori with Mr18 000 and 26 000 respectively. The results detected using colloid gold kits were as follows: all patients sera infected with H. pylori showed response to recombinant protein with Mr 26 000 were 94.0%, while 95.0%, 96.7%, 96.7% and 90.0% of patients with H. pylori-infected chronic superficial gastritis, duodenal ulcer, gastric ulcer, and gastric cancer respectively, showed responses. Compared with the serum-based ELISA results in detecting H. pylori-infection, there was no significant difference with the colloid gold kits (P > 0.05). The sensitivity, specificity, and accuracy of the rapid test kit with Mr 26 000 protein were 94.0%, 97.0%, and 94.5%, respectively; To recombinant protein with Mr18 000, 52.0%, 40.0%, 40.0%, 53.3% and 86.7%, of patients with H. pylori-infected chronic superficial gastritis, duodenal ulcer, gastric ulcer, and gastric cancer respectively, showed responses. There were a significant difference (P < 0.05) in the detecting rates of H. pylori infection between gastric cancer (86.7%) and the other diseases (43.3%). The results showed the colloid gold kits with Mr26 000 proteins of H. pylori could be used as a conventional examination method, with similar sensitivity and specificity as other conventional examination method, and simultaneously a significant association was found between the serologic response to Mr 18 000 OMP antigen and malignant outcome of H. pylori infection.
CONCLUSION: The two colloid gold kits with Mr18 000 and 26 000 proteins of H. pylori, can be a useful tool for detecting H. pylori infection, as well as predicting H. pylori related gastrointestinal diseases, such as gastric malignancy and peptic ulcer.
- Citation: Jiang Z, Huang AL, Zheng J, Tao XH, Pu D, Wang PL. Helicobacter pylori outer membrane proteins in diagnosis of Helicobacter pylori infection. Shijie Huaren Xiaohua Zazhi 2004; 12(7): 1588-1592
- URL: https://www.wjgnet.com/1009-3079/full/v12/i7/1588.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i7.1588
自1983年Marshall和Warren从慢性活动性胃炎患者的胃黏膜中分离出H. pylori之后, H. pylori与上胃肠道疾病之间的关系受到消化学界和微生物学界的极大关注. 我国普通人群的感染率为50-80%[1], 每年新增感染人数近千万. H. pylori感染不但与B型胃炎、消化性溃疡、MALT淋巴瘤、胃癌的发生有密切关系[2-13], 而且与肠外疾病的发生也有重要作用[14-16], 在动物实验中已证明H. pylori的致癌性[17], 世界卫生组织已明确指出H. pylori为第一类致癌因子. 因此对H. pylori感染的诊断及其治疗显得十分必要.
常规诊断方法有着各自的适应范围和特点[18-23]. 鉴于ELISA可同时处理大量标本, 适合流行病学调查、研究等特点, 应用较为广泛[24]. 目前H. pylori血清ELISA抗原主要采用H. pylori的培养所得, 由于H. pylori培养条件比较苛刻, 需要用特殊的厌氧培养设备, 不易获得大量的有效抗原成分; 而基因重组的纯化抗原, 目前H. pylori多种OMP基因已被克隆, 并在大肠杆菌中得到了成功表达[25-30], 但低分子OMP应用于H. pylori的诊断, 目前国内外很少报道. 为了获得大量的纯化H. pylori低分子的OMP抗原, 我们采用了基因重组技术, 分别构建了H. pylori Mr为18 000, 26 000 OMP编码基因重组载体, 并在大肠杆菌中得到了高效表达, 对其抗原性进行了鉴定[31-34]. 因此我们将纯化的重组融合OMP用于以抗原抗体反应及胶体金标记技术为基础的金标免疫检测试纸, 对H. pylori感染及其相关疾病的诊断价值进行了研究如下.
BL21/pET32a(+)/Omp26, BL21/pET32a(+)/Omp18和H. pylori 18 000, 26 000低Mr OMP mAb由本实验室构建、鉴定; 收集2002-01/2002-12上消化道症状就诊H. pylori阳性患者血清 150例, 以及H. pylori阴性健康者血清33名, 要求所有受试者在4 wk内未服用抗生素、糖皮质激素、H2受体拮抗剂以及质子泵抑制剂. 13C尿素呼吸实验由海德威公司提供; 金标免疫检测试纸(分别以H. pylori Mr为18000, 26 000 OMP制作金标免疫检测试纸, 由肝炎所提供); Ni2+-NTA多聚组氨酸蛋白纯化试剂盒为QIAGEN产品; 超声破碎液(50 mmoL/L NaH2PO4, 300 mmoL/L NaCl, pH 7.0); 洗涤液(50 mmoL/L NaH2PO4, 300 mmoL/L的NaCl, 20 mmoL/L imidazole, pH7.8); 洗脱液(50 mmoL/L NaH2PO4, imidazole 300 mmoL/L的NaCl, 250 mmoL/L Imidazole, pH7.8)由肝炎研究所提供.
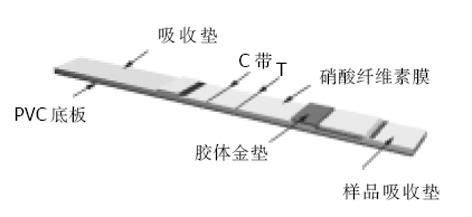
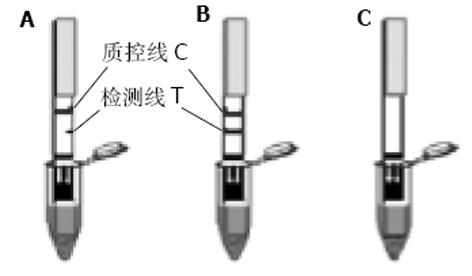
将酶切、PCR鉴定的重组载体转化大肠杆菌BL21, 随后分别挑选含重组载体pET32a (+)/Omp18和pET32a(+)/Omp26的单克隆菌落于盛有2 mL LB培养基的各试管中(含氨苄青霉素100 mg/L), 于37 ℃, 250 r/min培养过夜, 次日将过夜培养液加入盛有100 mL LB(含氨苄青霉素100 mg/L)、容积为500 mL的三角瓶中, 各培养500 mL细菌液, 于37 ℃培养至A600为0.4-0.6时, 加入终浓度为1.0 mmoL/L的IPTG, 诱导表达4 h, 以4 ℃, 10 000 r/min离心15 min, 收集菌体, 于-20 ℃冻存备用; 由于重组载体表达的融合蛋白质C端带有6聚组氨酸, 故表达产物用Ni2+-NTA树脂纯化. 分别制备500 mL细菌培养液, 离心, 菌体混悬于50 mL超声破碎液中, 在35%×600 W低温条件下, 超声破碎40 min, 以4 ℃ 10 000 r/min离心15 min, 将上清液按常规方法过滤柱子(Ni2+-NTA agarose), 以10 mL洗涤液洗脱2次, 然后以10 mL洗脱液洗脱并分3段收集过滤液, 分别取3段收集液10 L以等量2×蛋白上样缓冲液混匀, 煮沸5 min, 进行15% SDS-PAGE凝胶电泳. 同时采用相应的单克隆抗体以及免疫的动物血清, 以Western blot方法检测表达的目的蛋白的生物活性. 同时进行蛋白质含量的检测; H. pylori外膜蛋白金标免疫检测试验是采用高度特异性的抗原抗体反应及胶体金标记技术, 通过双抗原夹心法(抗原-抗体-抗原), 实现对H. pylori抗体特异检测. 即: 将重组H. pylori抗原包被在反应膜-硝酸纤维素膜上作为检测带, 同时将重组H. pylori抗原标记上胶体金作为显色剂, 利用双抗原夹心法原理, 一步检测血清样品中的H. pylori抗体. 关于金标免疫检测试纸组装示意图如图1所示. 其结果判断标准为: 阴性(-), 表示出现一条紫红色条带, 位于质控区(C)内. 如样本中不含特定的H. pylori抗体蛋白, 胶体金抗体在层析过程中不会被固定在膜上检测带内的单抗免疫, 因而测试区内(T)不会出现一条紫红色检测带. 质控区内(C)所显现的紫红色条带是判定是否有足够的样品液, 层析过程是否正常的标准, 同时也作为试剂的内控标准. 阴性结果表明, 待测样品中不含H. pylori抗体蛋白; 阳性(+)则表示质控区(C)出现一条紫红色条带, 同时在测试区(T)内也出现一条紫红色条带. 如果待测样品中H. pylori抗体蛋白浓度高于其检测阈值时, 抗原免疫金与检测带上的另一单抗结合, 因此在测试区内(T)出现紫红色检测带. 阳性结果表明, 特定H. pylori抗体蛋白含量在阈值以上; 无效则是在质控区(C)未出现紫红色条带, 表明不正确的操作过程(图2); 所有受试者经13C呼吸试验、细菌学培养上述检查方法中, 任意一种检测为阳性, 被确定为H. pylori感染; 而前二种检测手段中任意一种检测为阴性, 说明该患者无H. pylori的感染. 若H. pylori感染患者, H. pylori OMP金标免疫检测为阴性, 则视为假阴性; 若无H. pylori感染患者, H. pylori OMP金标免疫检测出现阳性, 则视为假阳性, 以此来判断H. pylori OMP金标免疫检测试纸在临床中的应用价值.
分别收集BL21/pET32a (+)/Omp18和BL21/pET32a(+)/Omp26菌体, 进行15% SDS-PAGE, 结果发现, 经IPTG诱导后, 融合蛋白的表达量分别占菌体总蛋白的18.96%, 26.38%. 同时分别将LB培养基制备的大量细菌混悬于超声破碎液中, 在35%×600 W低温条件下, 超声破碎40 min, 以4 ℃ 10 000 r/min离心15 min, 将上清液按常规方法过柱(Ni2+-NTA agarose resin), 以洗涤液洗涤2次, 然后洗脱液洗脱, 并分3段收集, 分别取3段收集液20 L以等量2×蛋白上样缓冲液混匀, 煮沸5 min, 以150 g/L SDS-PAGE进行凝胶电泳, 通过染色、脱色, 可见大部分目的蛋白在第2段收集液中, 通过Image Master Totallab v1.11软件进行凝胶自动扫描分析, 分别获得了纯度达95%以上的1.25 g/L的重组融合蛋白, 其Mr为3 8000, 46 000的融合蛋白, 其中Mr 20 000的蛋白为pET32a(+)所表达. 目的蛋白能够被相应H. pylori Mr为18 000, 26 000的OMP mAb以及免疫的动物血清所识别, 说明表达的目的蛋白的生物活性良好.
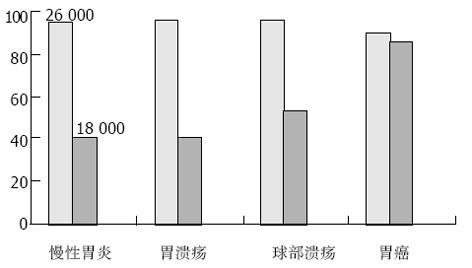
对H. pylori感染的上消化道疾病患者150例, 以及H. pylori阴性的健康者33名进行了金标免疫试纸检测, 其结果为: 26 000 OMP对H. pylori感染的检出率为94.0%, 对慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡以及胃癌患者中H. pylori感染的检出率分别为95.0%, 96.7%, 96.7%和90.0%, 与常规的ELISA检测方法相比, 二者无显著性差异(x2检验, P>0.05), 其敏感性、特异性和准确性分别为94.0%, 97.0%, 94.5%; 而18 000 OMP对H. pylori感染的检出率为52.0%, 对慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡和胃癌患者中H. pylori感染的检出率分别为40.0%, 40.0%, 53.3%和86.7%, 对胃癌患者中H. pylori感染检出率86.7%与慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡总检出率43.3%相比, 具有显著的差异性(P<0.05, 表1-2). 分别采用Mr 18 000, 26 000 OMP金标免疫检测试纸对H. pylori感染检测结果比较(见图3).
| 试验方法 | 受试者例数 | 阳性 | 阴性 | 假阳性 | 假阴性 |
| ELISA | 183 | 144 | 32 | 1 | 6 |
| 26 000胶体金 | 183 | 141 | 32 | 1 | 9 |
| 18 000胶体金 | 183 | 78 | 33 | 0 | 72 |
| 试验方法 | 受试者例数 | 年龄 | 阳性 | 阴性 |
| Mr 26 000 OMP | ||||
| 慢性浅表性胃炎 | 60 | 50.3±15.9 | 57 | 3 |
| 胃溃疡 | 30 | 57.3±13.2 | 29 | 1 |
| 十二指肠球部溃疡 | 30 | 46.5±14.2 | 29 | 1 |
| 胃癌 | 30 | 64.7±17.4 | 27 | 3 |
| Mr 18 000 OMP | ||||
| 慢性浅表性胃炎 | 60 | 50.3±15.9 | 24 | 36 |
| 胃溃疡 | 30 | 57.3±13.2 | 12 | 18 |
| 十二指肠球部溃疡 | 30 | 46.5±14.2 | 16 | 14 |
| 胃癌 | 30 | 64.7±17.4 | 26 | 4 |
所有H. pylori 都表达Mr18 000、26 000外膜蛋白, 而且与其他菌群的外膜蛋白无交叉反应[33]. 我们在大肠杆菌中表达了完整的H. pylori Mr18 000, 26 000 OMP, 经Western-blot鉴定, 有很强的抗原性. 分别将重组蛋白作为抗原, 采用高度特异性的抗原、抗体反应及胶体金标记技术, 通过双抗原夹心法(抗原-抗体-抗原), 一步检测血清样品中的H. pylori抗体, 建立检测H. pylori特异性IgG的金标免疫检测试纸, 从而实现了对H. pylori特异抗体的检测. 我们通过Ni2+-NTA agarose resin, 将重组蛋白进行纯化, 并且将蛋白浓度调整到1 g/L, 同时将胶体金的颗粒直径调整到40-60 nm, 并将抗体的浓度调至最佳, 获得稳定性的胶体金标记物以及满意的实验效果.
本实验我们选择了H. pylori感染的上消化道疾病患者150例, 以及H. pylori阴性的健康者33名作为对照, 金标免疫检测试验结果为: 26 000 OMP对H. pylori感染的检出率为94.0%, 对慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡以及胃癌患者中H. pylori感染的检出率分别为95.0%, 96.7%, 96.7%和90.0%, 与常规的ELISA检测方法相比, 二者无显著差异(x2检验, P>0.05), 其敏感性、特异性和准确率分别为94.0%, 97.0%, 94.5%; 而18 000 OMP对H. pylori感染患者的检出率为52.0%, 对慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡和胃癌患者中H. pylori感染的检出率分别为40.0%, 40.0%, 53.3%和86.7%, 对胃癌检出率86.7%与慢性浅表性胃炎、胃溃疡、十二指肠球部溃疡总检出率43.3%相比, 具有显著的差异性(P<0.05). 因此以26 000 OMP 的金标免疫检测试纸可以作为H. pylori感染的常规检测方法, 与常规的检测方法相比具有可靠的特异性以及敏感性, 同时与18 000 OMP 金标免疫检测试纸一起, 在胃肠道肿瘤的诊断和预测方面具有独特之处, 这值得进一步的研究, 上述结果与文献[35]报道一致. 据Raymond et al 报道, 以免疫印迹方法检测H. pylori血清抗体, 发现对H. pylori Mr 26 000 OMP抗原敏感性89.4%, 特异性87.9%, 正确率为88.7%; 同时Shiesh et al报道, H. pylori阳性患者的血清对H. pylori低分子量OMP116 000(CagA), 89 000(VacA), 60 000(Hsp), 45 000, 35 000, 30 000, 26 500和19 500的反应性分别为76.5%, 42.9%, 23.6%, 46.7%, 84.1%, 76.5%, 82.9%和32.4%, 同时提出H. pylori低分子量OMP 19 500, 26 500对上消化道溃疡、恶性肿瘤的高危人群可以作为有效的检测抗原.
研究发现表明, 虽然所有的H. pylori菌株都能表达Mr为18 000, 26 000的OMP, 刺激机体产生相应的抗体, 但抗体的产生与抗原分子大小、机体的免疫状态等诸多因素有关, 而且抗体滴度的衰减与相应抗体分子大小等密切相连, 本实验结果也表明, 26 000 OMP对H. pylori感染患者的阳性检出率(86.7%)明显高于18 000 OMP对H. pylori感染的检出率(52%); 而胃癌的发生是一个多因素、多阶段的过程, 在慢性胃炎-萎缩性胃炎-肠上皮化生-异型增生-胃癌这样一个漫长的过程中, 各种致病因子可能单独或协同作用于癌变的起始阶段, 实验结果也表明18 000 OMP对胃癌的H. pylori感染的检出率(86.7%)与26 000 OMP对胃癌的H. pylori感染的检出率(90.0%)几乎一致, 综上所述, Mr为18 000, 26 000的OMP不仅可作为检测H. pylori感染的一种标志性抗原, 同时对胃癌以及胃癌高发人群的筛选具有重要的价值.
总之, 用OMP作为抗原而建立的金标免疫检测试验用于检测抗H. pylori IgG抗体是可行的. 该方法体现了快速、简单以及无痛苦, 具有可靠的特异性, 而且与其他检测方法相比, 具有对胃癌患者H. pylori感染检出率高对胃肠道肿瘤特别是胃癌的诊断及预测方面具有独特之处.
编辑: N/A
| 1. | Chen M, Chen J, Liao W, Zhu S, Yu J, Leung WK, Hu P, Sung JJ. Immunization with attenuated Salmonella typhimurium producing catalase in protection against gastric Helicobacter pylori infection in mice. Helicobacter. 2003;8:613-625. [PubMed] [DOI] |
| 2. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. [PubMed] [DOI] |
| 3. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [PubMed] [DOI] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [DOI] |
| 5. | Kate V, Ananthakrishnan N, Badrinath S. Effect of Helicobacter pylori eradication on the ulcer recurrence rate after simple closure of perforated duodenal ulcer: retrospective and prospective randomized controlled studies. Br J Surg. 2001;88:1054-1058. [PubMed] [DOI] |
| 6. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] [DOI] |
| 7. | Meyer JM, Silliman NP, Dixon CA, Siepman NY, Sugg JE, Hopkins RJ. Helicobacter pylori and early duodenal ulcer status post-treatment: a review. Helicobacter. 2001;6:84-92. [PubMed] [DOI] |
| 8. | Hurenkamp GJ, Grundmeijer HG, Van Der Ende A, Tytgat GN, Assendelft WJ, Van Der Hulst RW. Arrest of chronic acid suppressant drug use after successful Helicobacter pylori eradication in patients with peptic ulcer disease: a six-month follow-up study. Aliment Pharmacol Ther. 2001;15:1047-1054. [PubMed] [DOI] |
| 9. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. |
| 10. | Morgner A, Miehlke S, Fischbach W, Schmitt W, Müller-Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer A, Stolte M. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol. 2001;19:2041-2048. [PubMed] [DOI] |
| 11. | Rieder G, Hofmann JA, Hatz RA, Stolte M, Enders GA. Up-regulation of inducible nitric oxide synthase in Helicobacter pylori-associated gastritis may represent an increased risk factor to develop gastric carcinoma of the intestinal type. Int J Med Microbiol. 2003;293:403-412. [PubMed] [DOI] |
| 12. | Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159:252-258. [PubMed] [DOI] |
| 13. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [PubMed] [DOI] |
| 14. | Ojetti V, Armuzzi A, De-Luca A, Nucera E, Franceschi F, Candelli M, Zannoni GF, Danese S, Di-Caro S, Vastola M. Helicobacter pylori infection affects eosinophilic cationic protein in the gastric juice of patients with idiopathic chronic urticaria. Int Arch Allergy Immunol. 2001;125:66-72. [DOI] |
| 15. | Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, Amarri S, Cagossi K, Torelli G. Helicobacter pylori eradication can induce platelet recovery in idiopathic thrombocytopenic purpura. Blood. 2001;97:812-814. [DOI] |
| 16. | Choe YH, Kwon YS, Jung MK, Kang SK, Hwang TS, Hong YC. Helicobacter pylori-associated iron-deficiency anemia in adolescent female athletes. J Pediatr. 2001;139:100-104. [PubMed] [DOI] |
| 17. | Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197-207. [PubMed] [DOI] |
| 18. | Chua TS, Fock KM, Teo EK, Ng TM. Validation of 13C-urea breathtest for the diagnosis of Helicobacter pylori infection in the Singapore population. Singapore Med J. 2002;43:408-411. [PubMed] |
| 19. | Kawai T, Kawakami K, Kudo T, Ogiahara S, Handa Y, Moriyasu F. A new serum antibody test kit (E plate) for evaluation of Helicobacter pylori eradication. Intern Med. 2002;41:780-783. [PubMed] [DOI] |
| 20. | Day AS, Sherman PM. Accuracy of office-based immunoassays for the diagnosis of Helicobacter pylori infection in children. Helicobacter. 2002;7:205-209. [PubMed] [DOI] |
| 21. | Oyedeji KS, Smith SI, Arigbabu AO, Coker AO, Ndububa DA, Agbakwuru EA, Atoyebi OA. Use of direct Gram stain of stomach biopsy as a rapid screening method for detection of Helicobacter pylori from peptic ulcer and gastritis patients. J Basic Microbiol. 2002;42:121-125. [PubMed] [DOI] |
| 22. | Wong WM, Wong BC, Tang VS, Lai KC, Yuen ST, Leung SY, Hu WH, Lam SK. An evaluation of the PyloriTek test for the diagnosis of Helicobacter pylori infection in Chinese patients before and after eradication therapy. J Gastroenterol Hepatol. 2001;16:976-980. [PubMed] [DOI] |
| 23. | Fujisawa T, Kaneko T, Kumagai T, Akamatsu T, Katsuyama T, Kiyosawa K, Tachikawa T, Kosaka O, Machikawa F. Evaluation of urinary rapid test for Helicobacter pylori in general practice. J Clin Lab Anal. 2001;15:154-159. [PubMed] [DOI] |
| 24. | Miwa H, Akamatsu S, Tachikawa T, Sogabe T, Ohtaka K, Nagahara A, Sugiyama Y, Sato N. On-site diagnosis of H. pylori infection by urine. Diagn Microbiol Infect Dis. 2001;39:95-97. [PubMed] [DOI] |
| 25. | Yan J, Liang SH, Mao YF, Li LW, Li SP. Construction of expression systems for flaA and flaB genes of Helicobacter pylori and determination of immunoreactivity and antigenicity of recombinant proteins. World J Gastroenterol. 2003;9:2240-2250. [PubMed] [DOI] |
| 26. | Liu X, Hu J, Zhang X, Fan D. Oral immunization of mice with attenuated Salmonella typhimurium expressing Helicobacter pylori urease B subunit. Chin Med J (Engl). 2002;115:1513-1516. [PubMed] |
| 27. | Bai Y, Li LR, Wang JD, Chen Y, Jin JF, Zhang ZS, Zhou DY, Zhang YL. Expression of Helicobacter pylori Hsp60 protein and its immunogenicity. World J Gastroenterol. 2003;9:2711-2714. [PubMed] [DOI] |
| 28. | Yan J, Mao YF. Construction of a prokaryotic expression system of vacA gene and detection of vacA gene, VacA protein in Helicobacter pylori isolates and ant-VacA antibody in patients' sera. World J Gastroenterol. 2004;10:985-990. [PubMed] |
| 29. | Mao YF, Yan J. Construction of prokaryotic expression system of ureB gene from a clinical Helicobacter pylori strain and identification of the recombinant protein immunity. World J Gastroenterol. 2004;10:977-984. [PubMed] |
| 30. | Jiang Z, Huang AL, Tao XH, Wang PL. Construction and characterization of bivalent vaccine candidate expressing HspA and M(r)18,000 OMP from Helicobacter pylori. World J Gastroenterol. 2003;9:1756-1761. [PubMed] [DOI] |
| 31. | Jiang Z, Huang A, Wang P. [Gene cloning and expression of outer membrane protein of Helicobacter pylori]. Zhonghua Yi Xue Za Zhi. 2001;81:1416-1419. [PubMed] |
| 32. | Jiang Z, Tao XH, Huang AL, Wang PL. A study of recombinant protective H.pylori antigens. World J Gastroenterol. 2002;8:308-311. [PubMed] |
| 33. | Jiang Z, Pu D, Huang AL, Tao XH, Wang PL. [Construction, expression and antigenic study of bivalent vaccine candidate with 26,000 OMP and heat short protein A of human Helicobacter pylori]. Zhonghua Yi Xue Za Zhi. 2003;83:862-867. [PubMed] |
| 34. | Bode G, Piechotowski I, Rothenbacher D, Brenner H. Helicobacter pylori-specific immune responses of children: implications for future vaccination strategy. Clin Diagn Lab Immunol. 2002;9:1126-1128. [PubMed] [DOI] |
| 35. | Chua TS, Fock KM, Chan YH, Dhamodaran S, Sim CS, Ng TM, Teo EK. Seroreactivity to 19.5-kDa antigen in combination with absence of seroreactivity to 35-kDa antigen is associated with an increased risk of gastric adenocarcinoma. Helicobacter. 2002;7:257-264. [PubMed] [DOI] |