修回日期: 2003-11-09
接受日期: 2003-12-08
在线出版日期: 2004-05-15
目的: 探讨幽门螺杆菌(H. pylori)相关性胃疾病胃蛋白酶原C的表达及其意义.
方法: 采用免疫组织化学染色法检测318例胃黏膜标本中胃蛋白酶原C的表达情况; 采用HE染色、ELISA及PCR方法检测H. pylori感染情况.
结果: H. pylori 阳性组浅表性胃炎胃蛋白酶原C抗原的过表达率高于H. pylori阴性组(P<0.05, χ2 = 0.032, 28/33 vs 15/25), H. pylori阳性组萎缩性胃炎胃蛋白酶原C的表达率低于H. pylori阴性组(P<0.01, χ2 = 0.003 4/61 vs 9/30), H. pylori阳性组的异型增生和胃癌胃蛋白酶原C的表达率与H. pylori 阴性组相比有下降趋势(P>0.05).
结论: H. pylori感染与胃蛋白酶原C的表达密切相关, 在胃黏膜炎症中胃蛋白酶原C的表达增加; 在萎缩性胃炎、异型增生和胃癌中胃蛋白酶原C的表达降低.
引文著录: 宁佩芳, 刘惠杰, 袁媛. 幽门螺杆菌相关性胃疾病胃蛋白酶原C的表达研究. 世界华人消化杂志 2004; 12(5): 1089-1091
Revised: November 9, 2003
Accepted: December 8, 2003
Published online: May 15, 2004
AIM: To investigate the expression of pepsinogen C and its relation with H. pylori infection in gastric cancer and precancerous lesions.
METHODS: The method of immunohistochemistry was used to examine the expression of pepsinogen C in 318 cases of stomach mucosa; the H. pylori infection was determined by H-E stain, PCR and ELISA.
RESULTS: The rate of PGC over-expression in group of superficial gastritis of H. pylori infection was higher than that of non-infection (P < 0.05, 28/33 vs 15/25). The positive rate of PGC in group of atrophic gastritis of H. pylori infection was lower than that of non-infection (P < 0.01, 4/61 vs 9/30) and so were in dysplasia and gastric cancer.
CONCLUSION: There is a relationship between the H. pylori infection and the expression of PGC in gastric mucosa. The expression of PGC increases in superficial gastritis and decreases in atrophic gastritis, dysplasia and gastric cancer with H. pylori infection.
- Citation: Ning PF, Liu HJ, Yuan Y. Expression of pepsinogen C in Helicobacter pylori-associated gastric lesions. Shijie Huaren Xiaohua Zazhi 2004; 12(5): 1089-1091
- URL: https://www.wjgnet.com/1009-3079/full/v12/i5/1089.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i5.1089
幽门螺杆菌(H. pylori)与胃癌的发生发展关系密切[1-3], 已被列为Ⅰ类致癌因子, 但H. pylori感染在胃癌发生中的作用机制目前还不甚明确. 胃蛋白酶原C是胃黏膜细胞分化成熟的终末产物, 他的变化可以反映胃黏膜的功能状态, 与胃癌及胃癌前疾病密切相关[4-8], 胃蛋白酶原与H. pylori感染的关系是近年来的研究热点. 我们利用从辽宁省庄河胃癌高发区普查中获得的不同类型胃黏膜活检标本观察胃蛋白酶原C的表达, 探讨H. pylori感染对胃蛋白酶原C表达的影响及其意义.
1997/2002年辽宁省庄河市胃癌高发区普查中获得的318例胃黏膜活检组织, 均由病理医师进行诊断. 包括浅表性胃炎58例, 萎缩性胃炎91例(均伴有肠上皮化生), 异型增生59例, 胃癌110例. 正常对照组与病例组在性别及年龄构成上无统计学差异. 抗胃蛋白酶原C抗体(anti-pepsinogen C antibody, 商品名2D5)由日本临床检验研究所惠赠; 免疫组化用SP二步法试剂盒为福建迈新公司产品(Lot No :Kit-9801D2); ELISA试剂盒购于华美公司; H. pylori基因PCR试剂盒购于上海复华公司.
采用HE染色、ELISA检测H. pylori-IgG抗体、PCR检测H. pylori DNA三种方法检测H. pylori, 同时具备三项中二项阳性者诊断为H. pylori感染阳性. 胃蛋白酶原C的免疫组织化学染色(SP二步法)按说明书进行操作. 结果判定采用综合评分法[GUT 1985;26:1319-1321]: 根据细胞着色强度及阳性细胞数积分, 积分数进行4级评分: 0分为阴性(-); 2-3分为(+); 4分为(++); 5-6分为(+++)大于或等于2分(+)为阳性表达; 大于或等于4分(++~+++)为过表达或强阳性.
统计学处理 利用SPSS 11.0统计软件包进行统计学处理, 采用χ2检验.
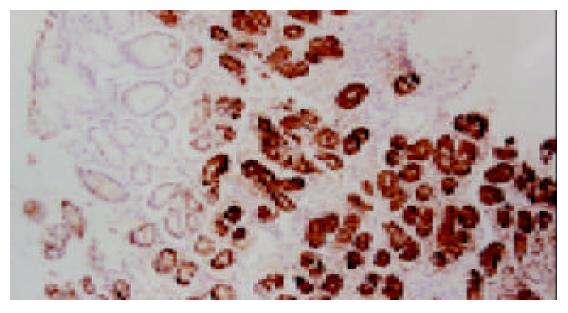
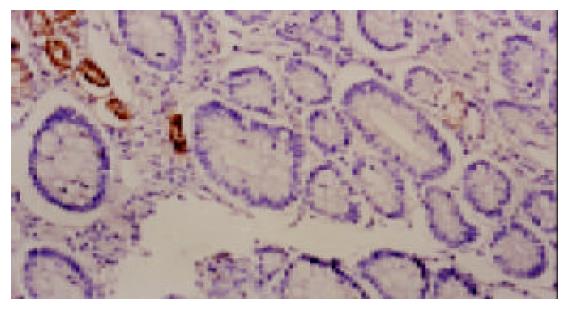
318例胃黏膜活检组织按有无H. pylori感染分组, H. pylori阳性组192例, H. pylori阴性组126例. 浅表性胃炎胃黏膜无论H. pylori感染与否PGC抗原均为阳性表达(100%), 但H. pylori阳性组PGC过表达率高于阴性组, 差异显著(P<0.05, 图1). 本组病例中萎缩性胃炎均伴有肠上皮化生, 肠化腺体的PGC均为阴性表达(0%); 而在其非肠化腺体中, H. pylori阳性组PGC表达率低于阴性组(图2), 差异非常显著(P<0.01). 同时H. pylori阳性组异型增生和胃癌PGC表达率也低于与其相应的阴性组, 差异不显著(P>0.05, 表1).
多数萎缩性胃炎都与H. pylori感染有关,H. pylori阳性患者胃癌的发生率是H. pylori阴性患者的2.8-6倍[8-10]. H. pylori感染导致慢性胃炎及消化性溃疡进一步发展为萎缩性胃炎、肠化、异型增生乃至癌变这一观点已被广泛认可. 大多数的研究认为H. pylori感染对胃癌的致病作用主要发生在病变早期[11-12], 对其在萎缩性胃炎以后的作用尚不清楚. 胃蛋白酶原(PG)是胃蛋白酶的前体, 可分为胃蛋白酶原A(PGA)和胃蛋白酶原C(PGC)[13-14]. 合成的PG大部分进入胃腔, 仅1%左右进入血循环. PGC最初出现于胚胎后期, 是胃黏膜细胞分化成熟的终末产物, 是消化功能逐渐成熟一种标志[4-5]. 近年来的研究表明, PGC的变化可以反映胃黏膜病变及分化程度, 与胃黏膜萎缩、肠上皮化生、异型增生有关, 慢性萎缩性胃炎和胃癌患者血清PGA的浓度和PGA/PGC的比值下降[6-7,15-16]. 那么, H. pylori感染以后PGC如何表达呢?
我们发现, 浅表性胃炎PGC均为阳性表达, 但H. pylori阳性组PGC的过表达率高于H. pylori阴性组, 这与血清学的研究结果相一致[7,17]. 文献报道, PGC的水平与H. pylori的定植密度成正比[18], H. pylori引起慢性胃炎及胃溃疡患者血清PG水平升高, 尤其使PGC升高更加明显; 根除H. pylori后血清PGC水平下降[19]. H. pylori引起胃黏膜分泌PGC增多可能由于[20-23]: 可诱导PG基因的表达, 脂多糖可刺激主细胞分泌胃蛋白酶原; 引起胃黏膜炎症, 参与炎症反应多种细胞因子如白三烯、TNF-α可刺激主细胞分泌PGC; 可引起胃酸和胃泌素分泌增加, 二者均能刺激PG分泌; 减少D细胞数量, 抑制生长抑素分泌, 减弱其对胃酸及胃蛋白酶原分泌的反馈性抑制, 从而增加PG水平. 受损的胃黏膜血管通透性增加, 血清PG水平增加. 大量PG进入胃腔, 遇酸活化形成胃蛋白酶, 对黏膜造成溶解和破坏, 促进胃黏膜炎症和溃疡形成.
H. pylori感染的重度胃黏膜病变中PGC表达减少, 肠化生黏膜PGC均为阴性表达, 表明肠化上皮不合成PGC. H. pylori阳性组的萎缩性胃炎PGC表达率低于H. pylori阴性组(P<0.01); 在异型增生和胃癌中, 也具有相同的趋势; PGC阳性的3例胃癌均为H. pylori阴性, 且均为高分化腺癌. 免疫组化研究表明[24-26], PGC在萎缩性胃炎和胃癌组织中的表达显著下降, PGC的表达下降与胃癌细胞的去分化程度和疾病预后密切相关. 张祥宏et al[27]通过对人群的随访研究发现, 血清PG异常伴有H. pylori感染者腺体萎缩、异型增生的发生率高于H. pylori 阴性者. H. pylori 感染的胃癌前疾病和胃癌黏膜PGC表达降低, 可能由于H. pylori引起的胃黏膜免疫-炎症反应和H. pylori细胞毒性因子等共同作用, 使自由基、超氧化物生成增加, 这些致癌因子使胚细胞中的胃蛋白酶原基因受损突变, 导致胃黏膜细胞的终末分化产物PGC-Ag表达减少, 细胞分化程度降低, 细胞增生、分化和凋亡失衡, 癌变危险性增加.
总之, H. pylori感染使胃黏膜炎症PGC表达增加, 而在萎缩性胃炎、异型增生和胃癌阶段PGC的表达降低, 对后者应密切随访, 有利于提高胃癌早诊率、监测复发及预后.
| 1. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [PubMed] [DOI] |
| 2. | Lu XL, Qian KD, Tang XQ, Zhu YL. Distribution of H.pylori antigens in gastric mucosa and its significance. J Zhejiang Univ Sci. 2004;5:242-245. [PubMed] [DOI] |
| 3. | International agency for research of cancer monographs on the evaluation of carcinogenic risks to humans. Infection with Helicobacter pylori. Vol 61. Lyon: IARC Scientific Publications 1994; 177-240. |
| 4. | Korstanje A, den Hartog G, Biemond I, Lamers CB. The serological gastric biopsy: a non-endoscopical diagnostic approach in management of the dyspeptic patient: significance for primary care based on a survey of the literature. Scand J Gastroenterol Suppl. 2002;22-26. [PubMed] [DOI] |
| 5. | Kageyama T, Ichinose M, Tsukada-Kato S, Omata M, Narita Y, Moriyama A, Yonezawa S. Molecular cloning of neonate/infant-specific pepsinogens from rat stomach mucosa and their expressional change during development. Biochem Biophys Res Commun. 2000;267:806-812. [PubMed] [DOI] |
| 6. | Broutet N, Plebani M, Sakarovitch C, Sipponen P, Mégraud F; Eurohepygast Study Group. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003;88:1239-1247. [PubMed] [DOI] |
| 7. | Miki K. [Serum pepsinogen test for the diagnosis of stomach cancer]. Nihon Rinsho. 2001;59 Suppl 4:204-207. [PubMed] |
| 8. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [PubMed] [DOI] |
| 9. | Filipe MI, Potet F, Bogomoletz WV, Dawson PA, Fabiani B, Chauveinc P, Fenzy A, Gazzard B, Goldfain D, Zeegen R. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut. 1985;26:1319-1326. [PubMed] [DOI] |
| 10. | Haruma K, Komoto K, Kamada T, Ito M, Kitadai Y, Yoshihara M, Sumii K, Kajiyama G. Helicobacter pylori infection is a major risk factor for gastric carcinoma in young patients. Scand J Gastroenterol. 2000;35:255-259. [PubMed] [DOI] |
| 11. | Ohkuma K, Okada M, Murayama H, Seo M, Maeda K, Kanda M, Okabe N. Association of Helicobacter pylori infection with atrophic gastritis and intestinal metaplasia. J Gastroenterol Hepatol. 2000;15:1105-1112. [PubMed] [DOI] |
| 12. | Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162-168. [PubMed] [DOI] |
| 13. | Miki K, Morita M, Sasajima M, Hoshina R, Kanda E, Urita Y. Usefulness of gastric cancer screening using the serum pepsinogen test method. Am J Gastroenterol. 2003;98:735-739. [PubMed] [DOI] |
| 14. | Foster C, Aktar A, Kopf D, Zhang P, Guttentag S. Pepsinogen C: a type 2 cell-specific protease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L382-L387. [PubMed] [DOI] |
| 15. | Fahey MT, Hamada GS, Nishimoto IN, Kowalski LP, Iriya K, Gama-Rodrigues JJ, Tsugane S. Ethnic differences in serum pepsinogen levels among Japanese and non-Japanese Brazilian gastric cancer patients and controls. Cancer Detect Prev. 2000;24:564-571. [PubMed] |
| 16. | Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, Guilherme M, Barbosa J, Lomba-Viana H, Silva R, Moreira-Dias L. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol. 2004;57:177-182. [PubMed] [DOI] |
| 17. | Basso D, Gallo N, Zambon CF, Navaglia F, Stockreiter E, Di Mario F, Rugge M, Plebani M. Different effects of H. pylori water extracts on cytokines, pepsinogen C and gastrin mucosal release in patients with or without duodenal ulcer. J Med. 2001;32:97-112. [PubMed] |
| 18. | Bodger K, Wyatt JI, Heatley RV. Variation in serum pepsinogens with severity and topography of Helicobacter pylori-associated chronic gastritis in dyspeptic patients referred for endoscopy. Helicobacter. 2001;6:216-224. [PubMed] [DOI] |
| 19. | Kikuchi S, Kurosawa M, Sakiyama T, Tenjin H, Miki K, Wada O, Inaba Y. Long-term effect of Helicobacter pylori infection on serum pepsinogens. Jpn J Cancer Res. 2000;91:471-476. [PubMed] [DOI] |
| 20. | Kishi K, Kinoshita Y, Matsushima Y, Okada A, Maekawa T, Kawanami C, Watanabe N, Chiba T. Pepsinogen C gene product is a possible growth factor during gastric mucosal healing. Biochem Biophys Res Commun. 1997;238:17-20. [PubMed] [DOI] |
| 21. | Sato Y, Iwafuchi M, Ueki J, Yoshimura A, Mochizuki T, Motoyama H, Sugimura K, Honma T, Narisawa R, Ichida T. Gastric carcinoid tumors without autoimmune gastritis in Japan: a relationship with Helicobacter pylori infection. Dig Dis Sci. 2002;47:579-585. [PubMed] [DOI] |
| 22. | Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92-105. [PubMed] [DOI] |
| 23. | Milutinovic AS, Todorovic V, Milosavljevic T, Micev M, Spuran M, Drndarevic N. Somatostatin and D cells in patients with gastritis in the course of Helicobacter pylori eradication: a six-month, follow-up study. Eur J Gastroenterol Hepatol. 2003;15:755-766. [PubMed] |
| 24. | Testino G, Cornaggia M, De Iaco F, Gada D. Is it possible with an immunophenotypic study to foresee the oncologic risk of epithelial gastric dysplasia? Hepatogastroenterology. 2002;49:601-603. [PubMed] |
| 25. | Karita M, Noriyasu A, Kosako E, Teramukai S, Matsumoto S. Relationship between pepsinogen I&II and H. pylori infection considered with grade of atrophy and gastroduodenal diseases. Dig Dis Sci. 2003;48:1839-1845. [PubMed] [DOI] |
| 26. | Fernández R, Vizoso F, Rodríguez JC, Merino AM, González LO, Quintela I, Andicoechea A, Truan N, Díez MC. Expression and prognostic significance of pepsinogen C in gastric carcinoma. Ann Surg Oncol. 2000;7:508-514. [PubMed] [DOI] |