修回日期: 2003-11-25
接受日期: 2003-12-08
在线出版日期: 2004-03-15
目的: 研究CTLA4Ig基因在移植小肠局部表达及其表达产物对急性排斥反应的治疗作用.
方法: 建立SD→Wistar的大鼠异位小肠移植模型, 并随机分为实验组(CTLA4Ig转基因组)和对照组(非转基因组). 实验组供肠移植前经肠系膜上动脉注入脂质体包裹的CTLA4Ig cDNA, 术后应用免疫组织学检查移植小肠中CTLA4Ig转基因产物的表达. 移植术后3, 7, 10 d分别获取各组的移植小肠进行组织学检查及细胞凋亡测定.
结果: 经CTLA4Ig cDNA处理的小肠在移植术后可见大量的CTLA4Ig表达. 对照组移植肠在术后7, 10 d分别出现Ⅰ, Ⅱ度急性排斥反应, 同时凋亡细胞数量显著增加. 实验组移植肠术后未见排斥反应的病理学证据, 凋亡细胞偶见.
结论: CTLA4Ig基因可在移植小肠局部转染表达, 其表达产物可防止移植术后急性排斥反应的发生.
引文著录: 王一芳, 许爱刚, 华一兵, 吴文溪. CTLA4Ig局部转基因对小肠移植急性排斥的治疗作用. 世界华人消化杂志 2004; 12(3): 685-688
Revised: November 25, 2003
Accepted: December 8, 2003
Published online: March 15, 2004
AIM: To evaluate the local expression of CTLA4Ig gene in small bowels and its action on preventing acute rejection of the small bowel allografts.
METHODS: Wistar rats underwent heterotopic small bowel transplantation from SD rats. The recipients were divided into experimental group (allografts were transfected with CTLA4Ig gene) and control group (CTLA4Ig gene not transfected) randomly. In the experimental group, the donor small bowels were perfused in vitro with CTLA4Ig cDNA packaged with lipofectin vector via intra-superior mesenteric artery before transplantation, and the CTLA4Ig expression in the small bowel grafts post-transplantation was assessed by immunohistology. On d 3, 7 and 10 post-transplantation, the allografts in each group were harvested for the examination of histology and assay of apoptosis.
RESULTS: Small bowel allografts treated with CTLA4Ig cDNA showed abundant CTLA4Ig expression after transplantation. Acute rejection grade Ⅰ on day 7 and grade Ⅱ on day 10 after transplantation was noticed in the control allografts, and a dramatically increased number of apoptotic enterocytes in parallel to the progressive rejection could be recognized. In contrast, the allografts treated with CTLA4Ig cDNA showed nonspecific histological changes and only a few of apoptotic enterocytes were found after transplantation.
CONCLUSION: Local CTLA4Ig gene transfection of small bowel allograft is feasible, and the local CTLA4Ig expression in the allograft can prevent acute rejection after transplantation.
- Citation: Wang YF, Xu AG, Hua YB, Wu WX. Effect of local CTLA4Ig gene transfection on acute rejection of small bowel allografts in rats. Shijie Huaren Xiaohua Zazhi 2004; 12(3): 685-688
- URL: https://www.wjgnet.com/1009-3079/full/v12/i3/685.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i3.685
CTLA4Ig为小鼠细胞毒性淋巴细胞抗原4的胞外部分与人IgG Fc段的重组融合蛋白, 可与B7-1/2分子结合, 阻断抗原递呈细胞(APC)和抗原特异性T淋巴细胞之间的共刺激信号传递. 应用CTLA4Ig基因转染治疗移植排斥, 可延长移植物的存活时间, 诱导出供者特异的耐受状态[1-7]. 将编码免疫抑制分子的基因转染移植器官, 旨在创造一个直接修饰免疫反应细胞的局部微环境[8], 移植器官内CTLA4Ig基因转染表达可使许多移植物长期存活[9-12]. 然而在移植小肠内进行CTLA4Ig基因转染尚无报道. 我们应用脂质体包裹的CTLA4Ig cDNA质粒经肠系膜上动脉注入冷冻保存的小肠, 观察CTLA4Ig基因在移植肠段的表达及其对急性排斥治疗的作用.
体质量250-300 g的♂SD和Wistar大鼠由南京医科大学动物实验中心提供; CTLA4Ig cDNA (AAVmCTLA4IgG质粒) 由法国Anegon教授赠送; 脂质体(DOTAP: chol, in vivo geneshuttle)为Qbiogene公司产品; 凋亡试剂盒购自Bochinger公司; 甲基绿为Vector Laboratories 公司产品; 仓鼠抗小鼠CTLA4单克隆抗体IgG和生物素标记的小鼠抗仓鼠Ig抗体购自BD Biosciences公司; HRP标记的抗生蛋白链霉素为Woburn公司产品.
参照Yang et al[13-17]的方法, 将SD鼠小肠(切取长度为20 cm)移植给Wistar鼠, 制作大鼠异位小肠移植模型. 所有动物在术后24 h内自由进水, 1 d后进食. 在室温下将AAVmCTLA4 IgG质粒和脂质体混合15 min制成脂质体/cDNA混悬液, 其中cDNA浓度为0.5 g/L. 移植肠段从供体取下经生理盐水冲洗后, 将50 L脂质体(对照组, n = 15)或脂质体/cDNA混悬液(实验组, n = 15)经肠系膜上动脉于5-10 min内缓慢注入. 经1.5 h冷冻保存后以4 ℃生理盐水5 mL冲洗肠系膜上动脉10 min, 随后植入受体鼠腹腔. 移植术后3, 7, 10 d, 各组分别处死5只动物获取移植小肠, 部分以多聚甲醛固定作组织学检查和凋亡测定, 部分以液氮冷冻保存作免疫组织学检查. (1)组织学检查: 石蜡包埋组织切片行HE染色, 以Kuusanmaki et al (Transplantation 1994; 58: 757-763)的标准判定排斥发生的等级; (2)凋亡细胞测定采用TUNEL技术[18-20], 操作步骤严格按照凋亡试剂盒说明书进行, 最后以甲基绿复染, 在光镜下凋亡细胞的核被染成棕色, 每例切片随机选取10个高倍视野读取凋亡细胞数量(Transplantation 1997; 63: 947-951); (3)免疫组织学检查: 组织冰冻切片5 m厚, 以仓鼠抗小鼠CTLA4单克隆抗体IgG (UC-4F10-11)为一抗, 以生物素标记的小鼠抗仓鼠Ig抗体(G70-204&G94-56)为二抗. 一抗与二抗的反应条件均为37 ℃, 60 min. 最后以HRP标记的抗生蛋白链霉素和DAB显色、苏木素复染. 在光镜下组织中表达的CTLA4IgG呈棕色颗粒.
统计学处理 数据以mean±SD表示, 采用t检验, P<0.05时差异具有显著性.
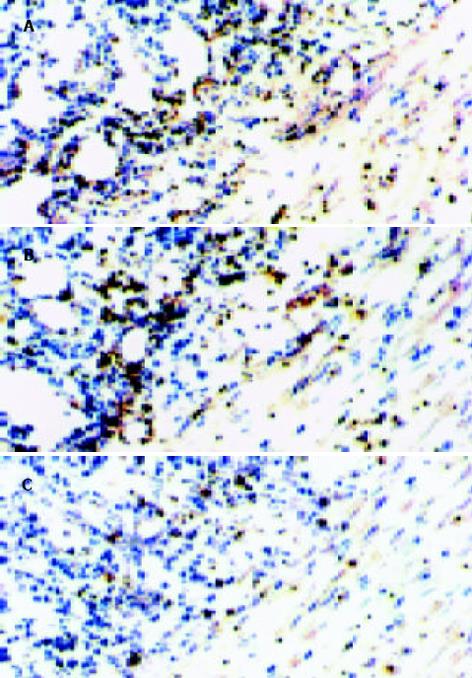
对照组移植肠于术后3 d未见特异性的排斥征象, 仅见局灶性肠系膜炎症、内皮细胞轻度空泡化、肠壁细胞轻度肿胀与脱落, 绒毛结构基本正常; 术后7 d, 肠系膜出现广泛的炎症性改变、肠壁中度炎性细胞浸润、绒毛变短、部分隐窝结构消失、肠系膜动脉内皮细胞肿胀增生、内膜增厚; 术后10 d, 肠系膜与肠壁的炎性浸润进一步加重、血管内皮增生与内膜增厚导致管腔堵塞、肠壁出现糜烂与局灶性溃疡等隐窝坏死征象. 然而, 经CTLA4 Ig转基因处理的移植肠于移植术后3, 7, 10 d, 绒毛始终保持正常的形态结构, 仅出现轻度的炎症细胞浸润与轻度的内皮细胞肿胀、脱落.
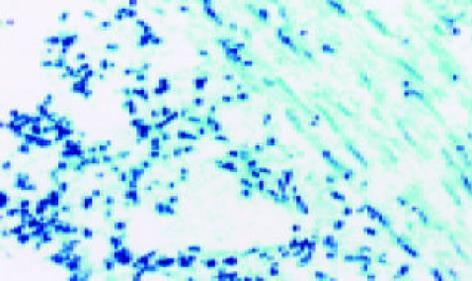
经CTLA4Ig基因转染的移植肠组织中可见大量的CTLA4Ig表达, CTLA4Ig表达广泛分布于肠系膜血管壁、肠壁肌层、黏膜下及小肠绒毛. 移植早期CTLA4Ig表达密度较高, 但移植后10d内均可见有CTLA4Ig的持续表达(图1). 对照组移植肠组织于术后未见CTLA4Ig表达(图2).
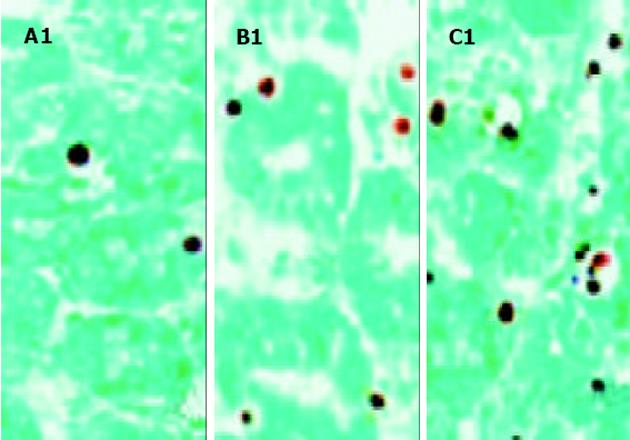
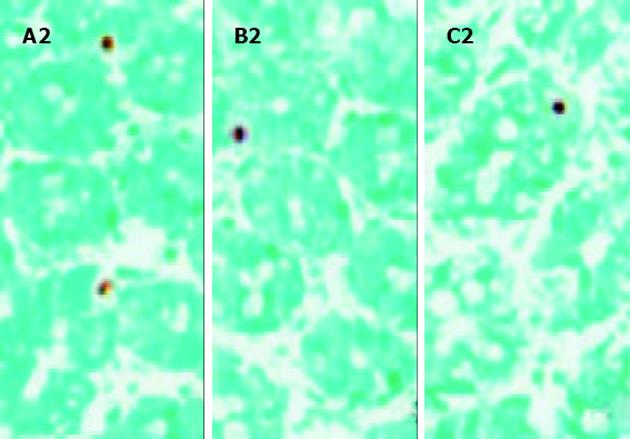
主要为隐窝细胞出现凋亡. 术后3 d, 各组移植小肠组织中均有少量细胞凋亡, 对照组为5.3 ± 1.6 (n = 5)、实验组为5.8±1.5 (n = 5), 两组间细胞凋亡数量差异无显著性. 对照组移植小肠组织中的凋亡细胞数量于术后7d显著增加(61.8±3.0, n = 5), 10 d增加更为明显(101±5.5, n = 5). (见图3). 但实验组移植小肠组织中的凋亡细胞数量于术后7, 10 d未见增加, 分别为3.4±1.1 (n = 5)和3.6±1.0 (n = 5). (见图4). 两组间细胞凋亡数量差异显著(7 d, aP<0.0 005, t =41.2 954; 10 d, bP<0.005, t =39.2 425).
小肠移植是治疗小肠功能衰竭的最终手段, 在一些器官移植中心小肠移植已作为常规疗法[22-23], 然而, 移植排斥仍是其最大的障碍[24-26]. 受体静脉内注入AdCTLA4Ig进行CTLA4Ig基因的系统转染, 可以延长移植小肠的存活时间[27]. 但小肠富含淋巴组织与细菌, 与心、肝、肾等脏器比较, 移植后更容易发生严重的排斥反应与感染, 对移植肠段进行局部CTLA4Ig cDNA转染, 可以抑制受体对移植肠段发生免疫排斥, 同时受体在其他部位的免疫功能(如抗感染功能)保持完好, 可能是解决小肠移植排斥更为理想的方法. 局部CTLA4Ig基因转染在心、肝、肾、胰腺和肺移植研究中已经获得成功[9-12], 但在移植小肠中尚未见有报道. 我们经肠系膜上动脉注入脂质体包裹的CTLA4Ig cDNA, 经免疫组织学检查发现, 在移植后3, 7, 10 d移植小肠组织内有大量CTLA4Ig表达, 表明在移植小肠局部进行CTLA4Ig cDNA转染是可行的. 未经CTLA4Ig基因转染的小肠移植物, 在移植术后3 d仅见少量凋亡细胞, 但在移植术后7, 10 d凋亡细胞数量显著增加. 同时, 组织学检查发现移植物存在进行性的急性排斥反应的病理学证据:虽然术后3 d未见特异性的排斥征象, 但术后7 d出现了Ⅰ度急性排斥反应, 10 d出现了Ⅱ度急性排斥反应. 然而, 经CTLA4Ig基因转染的小肠移植物于移植术后3, 7, 10 d均未见急性排斥反应的病理学证据, 凋亡细胞数量一直保持在很低的水平. 以上结果表明移植小肠局部CTLA4Ig基因转染可以防止移植术后急性排斥反应的发生. 局部CTLA4Ig基因转染防止小肠移植术后急性排斥反应发生的机制, 可能是由于同种异体抗原诱导下, T细胞的分裂增生反应受到抑制. CTLA4Ig基因局部基因转染可阻断移植小肠组织中抗原递呈细胞上B7分子的表达, 从而阻断B7/CD28介导的T细胞共刺激作用. 在缺乏B7/CD28共刺激信号下, T细胞识别抗原将诱导特异的无反应性[28-32].
细胞凋亡是细胞程序死亡的一种形式, 在生理或病理情况下均可发生. 在器官移植中, 凋亡是衡量移植排斥的一种重要的生化指标[18-21]. 在器官移植急性排斥反应中, 介导移植物损伤的效应细胞主要是细胞毒性T淋巴细胞, 其作用机制主要包括Fas/FasL系统和颗粒酶/穿孔素系统[33-35], 二者均可诱导靶细胞的凋亡. 研究证明, 在移植物排斥反应过程中, 穿孔素、颗粒酶B和FasL的表达水平明显上调[36-39]. 本结果显示, 经CTLA4Ig基因转染的小肠移植物于移植术后3, 7, 10 d, 特别是术后3 d, 可见少量的凋亡细胞. 这可能与冷保存和缺血再灌注损伤有关[40-42], 因为小肠移植后移植物内浸润的T淋巴细胞的毒性作用已经被移植物内表达的CTLA4Ig所中和或阻断.
总之, 本结果提示经肠系膜上动脉灌注脂质体包裹的CTLA4Ig cDNA, 可达到移植小肠局部CTLA4Ig基因转染的目的, 并可防止小肠移植后急性排斥反应的发生.
南京医科大学实验研究中心郭锡荣教授提供实验指导.
编辑: N/A
| 1. | Laumonier T, Potiron N, Boeffard F, Chagneau C, Brouard S, Guillot C, Soulillou JP, Anegon I, Le Mauff B. CTLA4Ig adenoviral gene transfer induces long-term islet rat allograft survival, without tolerance, after systemic but not local intragraft expression. Hum Gene Ther. 2003;14:561-575. [PubMed] [DOI] |
| 2. | Kosuge H, Suzuki J, Gotoh R, Koga N, Ito H, Isobe M, Inobe M, Uede T. Induction of immunologic tolerance to cardiac allograft by simultaneous blockade of inducible co-stimulator and cytotoxic T-lymphocyte antigen 4 pathway. Transplantation. 2003;75:1374-1379. [PubMed] [DOI] |
| 3. | Shiraishi T, Yasunami Y, Takehara M, Uede T, Kawahara K, Shirakusa T. Prevention of acute lung allograft rejection in rat by CTLA4Ig. Am J Transplant. 2002;2:223-228. [PubMed] [DOI] |
| 4. | Kita Y, Nogimura H, Ida M, Kageyama Y, Ohi S, Ito Y, Matsushita K, Takahashi T, Suzuki K, Kazui T. Effects of adenoviral vectors containing CTLA4Ig-gene in rat heterotopic lung implants. Transplant Proc. 2002;34:1434-1436. [PubMed] [DOI] |
| 5. | Yanagida N, Nomura M, Yamashita K, Takehara M, Murakami M, Echizenya H, Konishi K, Kitagawa N, Furukawa H, Uede T. Tolerance induction by a single donor pretreatment with the adenovirus vector encoding CTLA4Ig gene in rat orthotopic liver transplantation. Transplant Proc. 2001;33:573-574. [PubMed] [DOI] |
| 6. | Shindo J. [Effect of CTLA4Ig gene transfer with adenovirus vector on allogeneic renal graft survival in the rat]. Hokkaido Igaku Zasshi. 2001;76:251-261. [PubMed] |
| 7. | Iwasaki N, Gohda T, Yoshioka C, Murakami M, Inobe M, Minami A, Uede T. Feasibility of immunosuppression in composite tissue allografts by systemic administration of CTLA4Ig. Transplantation. 2002;73:334-340. [PubMed] [DOI] |
| 8. | Guillot C, Ménoret S, Guillonneau C, Braudeau C, Castro MG, Lowenstein P, Anegon I. Active suppression of allogeneic proliferative responses by dendritic cells after induction of long-term allograft survival by CTLA4Ig. Blood. 2003;101:3325-3333. [PubMed] [DOI] |
| 9. | Kita Y, Li XK, Nogimura H, Ida M, Kageyama Y, Ohi S, Suzuki K, Kazui T, Suzuki S. Prolonged graft survival induced by CTLA4IG gene transfection in rat lung allografting. Transplant Proc. 2003;35:456-457. [PubMed] [DOI] |
| 10. | Umeda Y, Iwata H, Yoshikawa S, Matsuno Y, Marui T, Nitta T, Idia Y, Takagi H, Mori Y, Miyazaki J. Gene gun-mediated CTLA4Ig-gene transfer for modification of allogeneic cardiac grafts. Transplant Proc. 2002;34:2622-2623. [PubMed] [DOI] |
| 11. | Cheung ST, Tsui TY, Wang WL, Yang ZF, Wong SY, Ip YC, Luk J, Fan ST. Liver as an ideal target for gene therapy: expression of CTLA4Ig by retroviral gene transfer. J Gastroenterol Hepatol. 2002;17:1008-1014. [PubMed] [DOI] |
| 12. | Benigni A, Tomasoni S, Remuzzi G. Impediments to successful gene transfer to the kidney in the context of transplantation and how to overcome them. Kidney Int. 2002;61:S115-S119. [PubMed] [DOI] |
| 13. | Yang YL, Li JP, Dou KF, Li KZ. Influence of liver nonparenchymal cell infusion combined with cyclosporin A on rejection of rat small bowel transplantation. World J Gastroenterol. 2003;9:2859-2862. [PubMed] [DOI] |
| 14. | Zhu M, Wei MF, Liu F, Shi HF, Wang G. Interleukin-10 modified dendritic cells induce allo-hyporesponsiveness and prolong small intestine allograft survival. World J Gastroenterol. 2003;9:2509-2512. [PubMed] |
| 15. | Wu XT, Li JS, Zhao XF, Li N, Ma YK, Zhuang W, Zhou Y, Yang G. Effects of n-3 fatty acid, fructose-1,6-diphosphate and glutamine on mucosal cell proliferation and apoptosis of small bowel graft after transplantation in rats. World J Gastroenterol. 2003;9:1323-1326. [PubMed] [DOI] |
| 16. | Wu XT, Li JS, Zhao XF, Zhuang W, Feng XL. Modified techniques of heterotopic total small intestinal transplantation in rats. World J Gastroenterol. 2002;8:758-762. [PubMed] [DOI] |
| 17. | Li YX, Li JS, Li N. Improved technique of vascular anastomosis for small intestinal transplantation in rats. World J Gastroenterol. 2000;6:259-262. [PubMed] |
| 18. | Wu MY, Liang YR, Wu XY, Zhuang CX. Relationship between Egr-1 gene expression and apoptosis in esophageal carcinoma and precancerous lesions. World J Gastroenterol. 2002;8:971-975. [PubMed] [DOI] |
| 19. | Sun P, Ren XD, Zhang HW, Li XH, Cai SH, Ye KH, Li XK. Serum from rabbit orally administered cobra venom inhibits growth of implanted hepatocellular carcinoma cells in mice. World J Gastroenterol. 2003;9:2441-2444. [PubMed] |
| 20. | Huang ZH, Fan YF, Xia H, Feng HM, Tang FX. Effects of TNP-470 on proliferation and apoptosis in human colon cancer xenografts in nude mice. World J Gastroenterol. 2003;9:281-283. [PubMed] [DOI] |
| 21. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] [DOI] |
| 22. | Platell CF, Coster J, McCauley RD, Hall JC. The management of patients with the short bowel syndrome. World J Gastroenterol. 2002;8:13-20. [PubMed] [DOI] |
| 23. | Kato T, Ruiz P, Thompson JF, Eskind LB, Weppler D, Khan FA, Pinna AD, Nery JR, Tzakis AG. Intestinal and multivisceral transplantation. World J Surg. 2002;26:226-237. [PubMed] [DOI] |
| 24. | Ding J, Guo CC, Li CN, Sun AH, Guo XG, Miao JY, Pan BR. Postoperative endoscopic surveillance of human living-donor small bowel transplantations. World J Gastroenterol. 2003;9:595-598. [PubMed] [DOI] |
| 25. | Zhang WJ, Liu DG, Ye QF, Sha B, Zhen FJ, Guo H, Xia SS. Combined small bowel and reduced auxiliary liver transplantation: case report. World J Gastroenterol. 2002;8:956-960. [PubMed] [DOI] |
| 26. | Ghanekar A, Grant D. Small bowel transplantation. Curr Opin Crit Care. 2001;7:133-137. [PubMed] [DOI] |
| 27. | Echizenya H, Yamashita K, Takehara M, Konishi K, Nomura M, Yanagida N, Kitagawa N, Kobayashi T, Furukawa H, Inobe M. Adenovirus-mediated CTLA4-IgG gene therapy in orthotopic small intestinal transplantation in rats. Transplant Proc. 2001;33:183-184. [PubMed] [DOI] |
| 28. | Vasilevko V, Ghochikyan A, Sadzikava N, Petrushina I, Tran M, Cohen EP, Kesslak PJ, Cribbs DH, Nicolson GL, Agadjanyan MG. Immunization with a vaccine that combines the expression of MUC1 and B7 co-stimulatory molecules prolongs the survival of mice and delays the appearance of mouse mammary tumors. Clin Exp Metastasis. 2003;20:489-498. [PubMed] [DOI] |
| 29. | Chung JB, Wells AD, Adler S, Jacob A, Turka LA, Monroe JG. Incomplete activation of CD4 T cells by antigen- presenting transitional immature B cells: implications for peripheral B and T cell responsiveness. J Immunol. 2003;171:1758-1767. [DOI] |
| 30. | Elhalel MD, Huang JH, Schmidt W, Rachmilewitz J, Tykocinski ML. CTLA-4. FasL induces alloantigen-specific hyporesponsiveness. J Immunol. 2003;170:5842-5850. [PubMed] [DOI] |
| 31. | Arpinati M, Terragna C, Chirumbolo G, Rizzi S, Urbini B, Re F, Tura S, Baccarani M, Rondelli D. Human CD34(+) blood cells induce T-cell unresponsiveness to specific alloantigens only under costimulatory blockade. Exp Hematol. 2003;31:31-38. [DOI] |
| 32. | Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192:161-180. [PubMed] [DOI] |
| 33. | Mandrup-Poulsen T. Beta cell death and protection. Ann N Y Acad Sci. 2003;1005:32-42. [PubMed] [DOI] |
| 34. | Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55-63. [PubMed] [DOI] |
| 35. | Catalfamo M, Henkart PA. Perforin and the granule exocytosis cytotoxicity pathway. Curr Opin Immunol. 2003;15:522-527. [PubMed] [DOI] |
| 36. | D'Errico A, Corti B, Pinna AD, Altimari A, Gruppioni E, Gabusi E, Fiorentino M, Bagni A, Grigioni WF. Granzyme B and perforin as predictive markers for acute rejection in human intestinal transplantation. Transplant Proc. 2003;35:3061-3065. [PubMed] [DOI] |
| 37. | Liang LW, Zhang Q, Gjertson D, Gritsch HA, Reed EF. Non-invasive immune monitoring of perforin/granzyme B in peripheral blood may predict renal allograft rejection. Hum Immunol. 2003;64:S32. [PubMed] [DOI] |
| 38. | Simon T, Opelz G, Wiesel M, Ott RC, Süsal C. Serial peripheral blood perforin and granzyme B gene expression measurements for prediction of acute rejection in kidney graft recipients. Am J Transplant. 2003;3:1121-1127. [PubMed] [DOI] |
| 39. | Zhang SG, Wu MC, Tan JW, Chen H, Yang JM, Qian QJ. Expression of perforin and granzyme B mRNA in judgement of immunosuppressive effect in rat liver transplantation. World J Gastroenterol. 1999;5:217-220. [PubMed] [DOI] |
| 40. | Wang SF, Li GW. Early protective effect of ischemic preconditioning on small intestinal graft in rats. World J Gastroenterol. 2003;9:1866-1870. [PubMed] [DOI] |
| 41. | Ma K, Yu Y, Bu XM, Li YJ, Dai XW, Wang L, Dai Y, Zhao HY, Yang XH. Prevention of grafted liver from reperfusive injury. World J Gastroenterol. 2001;7:572-574. [PubMed] [DOI] |
| 42. | Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112-1116. [PubMed] [DOI] |