修回日期: 2003-05-20
接受日期: 2003-06-02
在线出版日期: 2004-01-15
目的: 利用AdEasy系统构建乙酰胆碱酯酶催化亚单位(AChET)重组腺病毒(AdAChET), 并观察其对平滑肌细胞细胞的影响.
方法: 将质粒pEFbos/AChET扩增、酶切获得AChET cDNA片段插入腺病毒穿梭载体质粒pAdTrack-CMV的巨细胞病毒(CMV)启动子下游, 构建重组穿梭载体pAdTrack-CMV- AChET, 线性化后与骨架载体AdEasy-1在细菌BJ5183内同源重组得到腺病毒质粒pAd AChET, 经293细胞包装后得到复制缺陷型重组腺病毒Ad AChET; 将Ad AChET体外感染猫平滑肌细胞, 以RT-PCR检测AChET在平滑肌细胞的表达; 同时测定胆碱酯酶活力.
结果: 连接、重组后通过酶切和测序法筛选出pAd AChET; 经293细胞包装, 3 d后观察到绿色荧光蛋白(GFP)明显表达, 氯化铯梯度离心纯化最终获得约4×1013efu/L滴度的重组病毒; Ad AChET体外感染平滑肌细胞3 d后, AChET表达明显增加, 乙酰胆碱酯酶活力较Ad GFP感染组和阴性对照组明显增加(0.546±0.048 vs 0.495±0.039, 0.546±0.048 vs 0.501±0.037) (P均<0.01).
结论: 利用新型腺病毒载体AdEasy系统可在短期内制备同时表达GFP和AChET 的重组腺病毒Ad AChET; Ad AChET体外感染平滑肌细胞可显著提高AChET的表达, 这将为基因治疗贲门失弛缓症提供新的手段.
引文著录: 徐恩斌, 张忠兵, 谢渭芬, 宁守斌, 林勇, 蔡洪培. 乙酰胆碱酯酶基因重组腺病毒的构建及其对猫食管平滑肌细胞的影响. 世界华人消化杂志 2004; 12(1): 117-120
Revised: May 20, 2003
Accepted: June 2, 2003
Published online: January 15, 2004
AIM: To construct the replication-deficient recombinant adenoviruses-AdAChET inserted both cat acetylcholinesterase (AChET) and green fluorescent protein (GFP) cDNA drived by CMV promoter using homologous recombination in bacteria provided by AdEasy system and to investigate the effect of AChET on cat smooth muscle cells.
METHODS: The AChET cDNA was obtained from the plasmids-pEFbos/AChET by digestion, and the shuttle plasmid- pAdTrack-CMV- AChET in which the AChET cDNA was inserted into the downstream of CMV promoter was established by ligation. Then the linearized shuttle plasmid was co-transformed into bacteria with backbone vector AdEasy-1 to obtain the recombinant adenoviral plasmids-pAd AChET by homologous recombination. After packed in 293 cells, the recombinant adenoviruses-Ad AChET were generated. The expression of AChET in cat smooth muscle cell was detected by RT-PCR and total AChE activity was determined.
RESULTS: The recombinant plasmid pAdAChET was established by homologous recombination and confirmed by restriction endonuclease digestion and sequencing. GFP expression could be observed on the third day after packing of the linearized pAdAChET in 293 cells and 4×1010 efu/mL titer of Ad AChET was obtained by CsCl gradient purification. When the cat smooth cells were infected by the viruses for 3 d, expression of AChET and AChE activity in smooth cells increased significantly.
CONCLUSION: AChET can be simply and rapidly generated by using the AdEasy system. The infection of cat smooth muscle cells by Ad AChET can result in the high expression of AChET. Ad AChET may serve as a new tool for gene therapy of achalasia.
- Citation: Xu EB, Zhang ZB, Xie WF, Ning SB, Lin Y, Cai HP. Construction of recombinant aden-oviruses carrying AChET and its effect on smooth muscle cells. Shijie Huaren Xiaohua Zazhi 2004; 12(1): 117-120
- URL: https://www.wjgnet.com/1009-3079/full/v12/i1/117.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i1.117
贲门失弛缓症是一种食管运动障碍性疾病, 以食管缺乏蠕动和食管下括约肌(lower esophageal sphincter, LES)松弛不良为特征. LES松弛是VIP和NO共同作用的结果[1-3] , LES收缩和张力升高是由外源性胆碱能神经控制的[4-6]; 而乙酰胆碱的酶解是主要由乙酰胆碱酯酶参与完成. 复制缺陷型重组腺病毒(replication-deficient recombinant adenovirus)是目前基因治疗最常用的载体之一, He TC[7-8]建立了AdEasy系统, 我们利用AdEasy系统构建了外源插入乙酰胆碱酯酶催化亚单位(acetylcholinesterase, AChET)AChET cDNA片段的复制缺陷性腺病毒Ad AChET, 并观察Ad AChET在猫食管平滑肌细胞中的表达, 为贲门失弛缓症基因治疗提供新的手段.
AdEasy系统: 穿梭质粒pAdTrack-CMV, 骨架质粒pAdEasy-1, 仅插入GFP的对照重组腺病毒质粒pAdGFP, 大肠杆菌BJ5183由He TC博士惠赠[8]; AChET表达质粒pEFbos/AChET由Legay教授(The Journal of Neuroscience, 1999; 19: 8252-8259)惠赠; 293细胞购自中科院细胞所, 传代数不超过50代; E. coli JM109, DH5α购自华舜公司; 5, 5-二巯基-3-硝基苯甲酸(DTNB), 由中科院生化所提供.
将E.coli JM109和BJ5183置于37 ℃生长至A550约为0.8时, 收集细菌, 分别用100 mL/L的冷甘油洗2次, 500倍浓缩后以20 L/管分装, 置-80 ℃冰箱保存. 取含AChET cDNA 的质粒pEFbos/AChET 2 L用氯化钙法转化感受态菌JM109, 选择阳性克隆, 用DNA抽提纯化试剂盒(Qiagen)抽提、纯化质粒, 并作酶切鉴定. 将扩增后的pEFbos/AChET 5 g用XbaI, Hind III (NEB)酶切后, 于10 g/L琼脂糖凝胶电泳. 将AChET cDNA片段割胶, 凝胶抽取试剂盒(Qiagen)纯化, 并委托上海联合基因科技集团有限公司测序. 将回收的AChET cDNA片段与质粒pAdTrack-CMV分别用限制性内切酶XbaI, Hind III(NEB)酶切后, 14 ℃连接过夜. 取5 L连接物氯化钙法转化感受态菌JM109. 小抽质粒并作酶切鉴定, 筛选已携带AChET cDNA片段的穿梭质粒pAdTrack-CMV- AChET, 并委托上海联合基因科技集团有限公司自动测序. 将测序正确的穿梭质粒pAdTrack-CMV-AChET抽提、纯化, 取1 g先后用限制性内切酶PmeI(NEB)酶切线性化、CIP(NEB)去磷酸化后用QIAquick PCR纯化试剂盒(Qiagen)回收. 取0.4 g线性化质粒与0.1 g超螺旋pAd Easy-1质粒在电压2 500V, 电容25 FD, 电阻200 Ohms条件下电穿孔共转化BJ5183感受态细菌. 37 ℃摇床孵育30 min, 卡那霉素LB培养基平板涂板, 于37 ℃培养24 h后挑选20个最小克隆, 小抽质粒并作酶切鉴定. 选择重组腺病毒质粒pAd AChET, 氯化钙法转化感受态菌JM109大量扩增.
1.2.1 包装腺病毒Ad AChET和对照病毒AdGFP: 将293细胞以2×106/孔接种于60 mm培养皿, 24 h后细胞密度生长至60%-80%时, 将10 g pAd AChET用限制性内切酶PacI(NEB)酶切回收后, 取4 g线性化的pAd AChET以脂质体-DNA复合物的形式加入293细胞中, 24 h后更换含100 mL/L小牛血清(HighClone)的新鲜培液, 3 d后通过荧光显微镜观察GFP的表达. 7 d后收集293细胞, 于液氮和37 ℃水浴中反复冻融4次, 以3 mL/皿病毒上清再次感染293细胞进行扩增, 5 d后收集细胞, 1 500 r/min离心7 min, 小心弃上清, 以2 mL PBS/皿重悬, 反复冻融4次; 重复感染、收集步骤, 将最终收集的PBS重悬病毒上清用氯化铯(CsCl)梯度离心纯化. 以同样方法在293细胞内包装对照病毒AdGFP, 并作CsCl梯度离心. 将293细胞以5×105/孔接种于六孔板(Nunc), 于次日待细胞生长约90%后, 将病毒上清倍比稀释分别感染细胞, 72 h后荧光显微镜下计算表达GFP细胞个数, 测定Ad AChET和AdGFP滴度(表达形成单位/升, efu/L).
1.2.2 检测Ad AChET在猫平滑肌细胞中的表达: 原代猫平滑肌细胞的分离按Bitar et al (Am J Physiol, 1982; 242: G400-G407)报道的方法加以修改. 原代培养的平滑肌细胞以5×105/皿接种于60 mm培养皿, 将病毒以MOI 10-20感染细胞, 3 d后观察GFP表达; 以TRIzol试剂盒(Qibco)抽提总RNA后, 逆转录反应1 h, 取2 L逆转录产物为模板进行PCR扩增, 95 ℃ 30 s, 50 ℃ 30 s, 72 ℃ 2 min, 30个循环; 10 g/L琼脂糖凝胶电泳后测定AChET表达. 乙酰胆碱酯酶活力采用微量DTNB法测定. 应用UV-754型分光光度计于414 nm处测吸光度值(A值), 以实验管A值减去对照管A值来表示酶反应速度.
统计学处理 所有数据用mean±SD表示, 组间差异应用t检验.
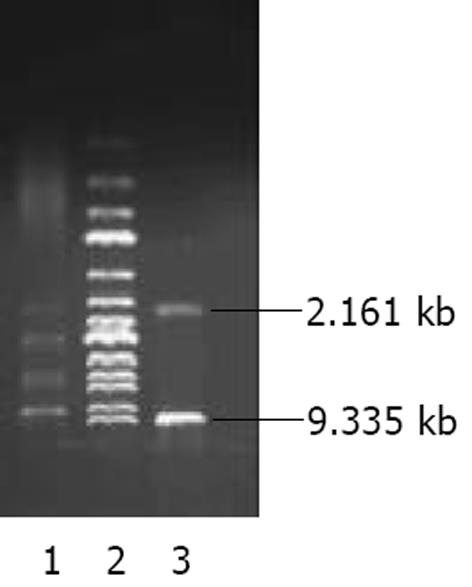
将pAdTrack-CMV经XbaⅠ、Hind III酶切后与割胶回收获得的AChET cDNA片段连接, 获得质粒pAdTrack-CMV- AChET, 酶切鉴定获得9.335 kb的载体片段和2.161 kb 大小AChET cDNA片段(图1), 测序并作同源分析.
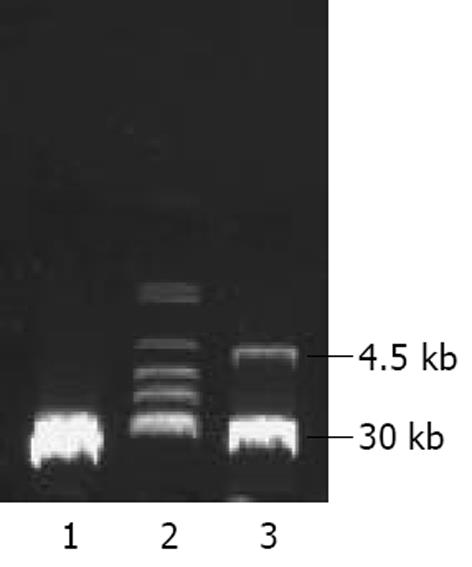
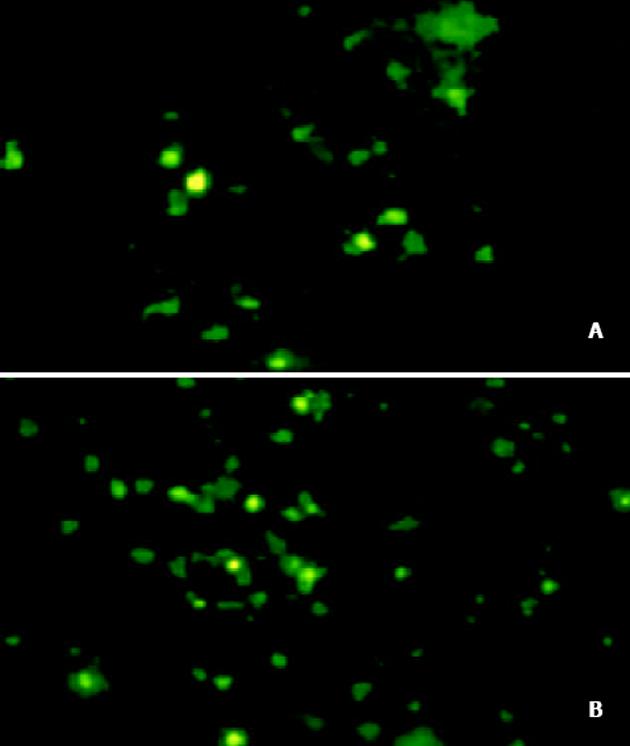
将PmeⅠ酶切线性化的pAdTrack-CMV- AChET与pAdEasy-1质粒共转化BJ5183感受态细菌, 筛选获得pAd AChET, PacⅠ酶切获得约30 kb大小的腺病毒基因组片段和3.0 kb 或 4.5 kb的ori及卡那霉素抗性编码基因片段(图2). PacⅠ酶切线性化的pAd AChET转染293细胞3d后, 荧光显微镜下见约10%的细胞表达GFP, 7 d后可见大量GFP表达(图3). 反复感染、冻融293细胞, CsCl梯度离心纯化后测定Ad AChET滴度约为4×1013efu/L. 以同样方法获得对照病毒AdGFP, 滴度约为3×1013efu/L.
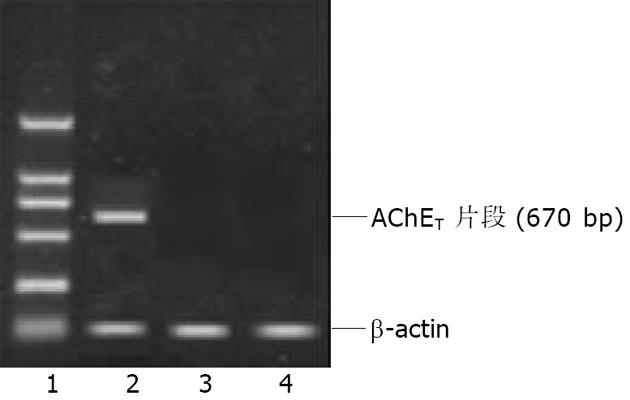
Ad AChET感染原代培养的猫平滑肌细胞3d后, 荧光显微镜下可见GFP表达. RT-PCR检测Ad AChET在平滑肌细胞中表达明显增强(图4). Ad AChET感染的平滑肌细胞的乙酰胆碱酯酶活力(A: 0.546±0.048)明显高于Ad GFP感染组(A: 0.495±0.039) (P<0.01)和阴性对照组(A: 0.501± 0.037) (P<0.01), 而Ad GFP感染组与阴性对照组比较无显著差异(P>0.05).
重组腺病毒在一些疾病基因治疗中有着很好的治疗效果和应用前景[9-22]. 腺病毒不仅能够高效感染多种增生细胞, 而且也能感染静息哺乳类细胞, 且不与细胞的基因组发生整合, 不引起基因突变, 可以携带较大片段的外源基因, 是一种比较理想的用于基因治疗的表达载体[12,15,22-23]. 乙酰胆碱酯酶是胆碱能突触的必需成分, 他通过快速地水解乙酰胆碱精密地控制胆碱能神经递质[24-28]. 在电鳐和哺乳动物, 他可产生多种类型的亚型, AChET是其主要亚型之一, AChET是存在于脊椎动物的胆碱酯酶中的惟一的催化亚单位, 主要在大脑和肌肉中发挥作用[29-31]. 将AChET cDNA转入COS细胞, 可产生AChE的G1型单体和G4型四聚体, 其中G4分子在细胞表面表达[32]. 平滑肌细胞是组成胃肠道最重要的组织细胞之一, 是引起胃肠运动的效应细胞, 平滑肌膜上的毒蕈碱受体与胆碱能神经元释放的乙酰胆碱结合后引起平滑肌收缩. 抑制平滑肌收缩是治疗贲门失弛缓症、先天性巨结肠等疾病的方法之一, 其中构建携带AChET基因的重组腺病毒转染至平滑肌细胞表达, 进而提高乙酰胆碱的酶解效率, 是调控平滑肌收缩的主要方案之一.
为构建外源插入AChET cDNA片段的复制缺陷性腺病毒并应用于贲门失弛缓症的基因治疗, 我们利用细菌内同源重组机制构建腺病毒质粒的AdEasy系统. 首先通过体外连接构建插入AChET cDNA片段的穿梭质粒pAdTrack-CMV- AChET. 通过PmeⅠ酶切线性化后, 将线性的pAdTrack-CMV- AChET 质粒左臂和右臂的同源区分别与骨架载体pAdEasy-1上的腺病毒基因组在大肠杆菌内进行同源重组, 同时利用抗生素筛选, 2 wk内即可得到重组腺病毒质粒pAd AChET. 通过PacⅠ内切酶线性化, 去除质粒上的ori和卡那霉素抗性编码基因, 并暴露其ITR序列后, 直接转染至293细胞内包装便得到了所需的重组腺病毒Ad AChET. 由于在同源重组时整合了GFP, 可在包装和扩增的同时直接观察转染和感染的效率, 大大缩短了基因治疗的构建和鉴定过程, 克服了传统方法步骤周期长, 效率低的缺点[33-34].
为进一步将新构建的Ad AChET 应用于贲门失弛缓症的基因治疗, 我们将一定滴度的Ad AChET体外感染原代培养的猫平滑肌细胞. RT-PCR和乙酰胆碱酯酶活力检测结果提示, Ad AChET的表达不仅稳定高效, 同时大量瞬时表达AChET的平滑肌细胞乙酰胆碱酯酶活力增高, 从而可提高乙酰胆碱的分解, 抑制机体平滑肌的收缩.
编辑: N/A
| 1. | Vittoria A, Costagliola A, Carrese E, Mayer B, Cecio A. Nitric oxide-containing neurons in the bovine gut, with special reference to their relationship with VIP and galanin. Arch Histol Cytol. 2000;63:357-368. [PubMed] [DOI] |
| 2. | Rumessen JJ, de Kerchove d'Exaerde A, Mignon S, Bernex F, Timmermans JP, Schiffmann SN, Panthier JJ, Vanderwinden JM. Interstitial cells of Cajal in the striated musculature of the mouse esophagus. Cell Tissue Res. 2001;306:1-14. [PubMed] [DOI] |
| 3. | Kurjak M, Fritsch R, Saur D, Schusdziarra V, Allescher HD. Functional coupling between nitric oxide synthesis and VIP release within enteric nerve terminals of the rat: involvement of protein kinase G and phosphodiesterase 5. J Physiol. 2001;534:827-36. [PubMed] [DOI] |
| 4. | Cao Y, Xie P, Xing Y. Role of endogenous cholinergic nerve in esophageal dysmotility with reflux esophagitis. Zhonghua Neike Zazhi. 2001;40:670-672. [PubMed] |
| 5. | Xie P, Medda B, Ren J, Mustin E, Shaker R, Koch TR. Choline acetyltransferase activity parallels the pressure gradient in the feline pharyngo-esophageal region. Auton Neurosci. 2001;89:125-127. [PubMed] [DOI] |
| 6. | Nguyen VT, Hall LL, Gallacher G, Ndoye A, Jolkovsky DL, Webber RJ, Buchli R, Grando SA. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res. 2000;79:939-949. [PubMed] [DOI] |
| 7. | Breyer B, Jiang W, Cheng H, Zhou L, Paul R, Feng T, He TC. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1:149-162. [PubMed] [DOI] |
| 8. | Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P. Potential use of sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28:755-763. [PubMed] [DOI] |
| 9. | Takeda T, Inaba H, Yamazaki M, Kyo S, Miyamoto T, Suzuki S, Ehara T, Kakizawa T, Hara M, DeGroot LJ. Tumor-specific gene therapy for undifferentiated thyroid carcinoma utilizing the telomerase reverse transcriptase promoter. J Clin Endocrinol Metab. 2003;88:3531-3538. [PubMed] [DOI] |
| 10. | Gao H, Wang J, Zhang W, Jiang Y, Niu D, Wang X. Recombinant adenovirus carrying glial cell line-derived neurotrophic factor gene protect midbrain dopaminergic neurons in mice. Beijing Daxue Xuebao. 2003;35:256-260. [PubMed] |
| 11. | Siemens DR, Crist S, Austin JC, Tartaglia J, Ratliff TL. Comparison of viral vectors: gene transfer efficiency and tissue specificity in a bladder cancer model. J Urol. 2003;170:979-984. [PubMed] [DOI] |
| 12. | Appleby CE, Kingston PA, David A, Gerdes CA, Umana P, Castro MG, Lowenstein PR, Heagerty AM. A novel combination of promoter and enhancers increases transgene expression in vascular smooth muscle cells in vitro and coronary arteries in vivo after adenovirus-mediated gene transfer. Gene Ther. 2003;10:1616-1622. [PubMed] [DOI] |
| 13. | Gao JQ, Tsuda Y, Katayama K, Nakayama T, Hatanaka Y, Tani Y, Mizuguchi H, Hayakawa T, Yoshie O, Tsutsumi Y. Antitumor effect by interleukin-11 receptor alpha-locus chemokine/CCL27, introduced into tumor cells through a recombinant adenovirus vector. Cancer Res. 2003;63:4420-4425. [PubMed] |
| 14. | Li H, Tang QY, Zhang Y, Wang SH, Guo CY. Development of a canine adenovirus type 1 vaccine strain E3-deleted based expression vector. Zhongguo Yixue Kexueyuan Xuebao. 2001;23:40-44. [PubMed] |
| 15. | Dong X, Hu JY, Xie TH, Sun MS, Dai CB, Ma YB. Construction of a recombinant human adenovirus expressing the ORF2 antigen of HEV and immunization of mice by mucosal system. Zhongguo Yixue Kexueyuan Xuebao. 2003;25:324-328. [PubMed] |
| 16. | Xu ZQ, Su CB, Chen SS, Ren ZY, Di X, Ma WB. Gene therapy of rat prolactinomas mediated by adenoviral vectors with rat tyrosine hydroxylase gene. Zhongguo Yixue Kexueyuan Xuebao. 2003;25:185-189. [PubMed] |
| 17. | Mukogawa T, Koyama F, Tachibana M, Takayanagi A, Shimizu N, Fujii H, Ueno M, Matsumoto H, Takeuchi T, Nakajima Y. Adenovirus-mediated gene transduction of truncated IkappaBalpha enhances radiosensitivity in human colon cancer cells. Cancer Sci. 2003;94:745-750. [PubMed] [DOI] |
| 18. | Sun M, Zan Y, Ma Y, Zhang G, Du Q, Dai C. Expression and glycosylation of rotavirus strain SA11 VP4 protein in a recombinant adenovirus. Chin Med Sci J. 2001;16:129-134. [PubMed] |
| 19. | Tsutsui T, Koide H, Fukahori H, Isoda K, Higashiyama S, Maeda I, Tashiro F, Yamato E, Miyazaki J, Yodoi J. Adenoviral transfection of hepatocytes with the thioredoxin gene confers protection against apoptosis and necrosis. Biochem Biophys Res Commun. 2003;307:765-770. [PubMed] [DOI] |
| 20. | Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000;28:382-390. [PubMed] [DOI] |
| 21. | Fujino M, Adachi K, Kawasaki M, Kitazawa Y, Funeshima N, Okuyama T, Kimura H, Li XK. Prolonged survival of rat liver allograft with adenoviral gene transfection of human immunodeficiency virus type 1 nef. Liver Transpl. 2003;9:805-813. [PubMed] [DOI] |
| 22. | Chen SL, Zhang BR, Mei J, Xu ZY, Zhu JL, Cai KH, Huang SD, Liu YL. Induction of angiogenesis in ischemic myocardium by adenovirus mediated angiopoietin-1 gene transfer, an experimental study. Zhonghua Yixue Zazhi. 2003;83:637-640. [PubMed] |
| 23. | Davis AR, Wivel NA, Palladino JL, Tao L, Wilson JM. Construction of adenoviral vectors. Mol Biotechnol. 2001;18:63-70. [PubMed] [DOI] |
| 24. | Breyer B, Jiang W, Cheng H, Zhou L, Paul R, Feng T, He TC. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1:149-162. [PubMed] [DOI] |
| 25. | Minic J, Chatonnet A, Krejci E, Molgo J. Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions. Br J Pharmacol. 2003;138:177-187. [PubMed] [DOI] |
| 26. | Mense S, Simons DG, Hoheisel U, Quenzer B. Lesions of rat skeletal muscle after local block of acetylcholinesterase and neuromuscular stimulation. J Appl Physiol. 2003;94:2494-2501. [PubMed] [DOI] |
| 27. | Deprez P, Inestrosa NC, Krejci E. Two different heparin-binding domains in the triple-helical domain of ColQ, the collagen tail subunit of synaptic acetylcholinesterase. J Biol Chem. 2003;278:23233-23242. [PubMed] [DOI] |
| 28. | Steen MS, Froehner SC. Perle Can fix your muscle AChEs. Trends Neurosci. 2003;26:241-242. [PubMed] [DOI] |
| 29. | Morel N, Leroy J, Ayon A, Massouli J, Bon S. Acetylcholinesterase H and T dimers are associated through the same contact. J Biol Chem. 2001;276:37379-37389. [PubMed] [DOI] |
| 30. | Massoulie J. The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals. 2002;11:130-143. [PubMed] [DOI] |
| 31. | Perrier AL, Massoulie J, Krejci E. PRiMA: the membrane anchor of acetylcholinesterase in the brain. Neuron. 2002;33:275-285. [PubMed] [DOI] |
| 32. | Ohno K, Engel AG, Brengman JM, Shen XM, Heidenreich F, Vincent A, Milone M, Tan E, Demirci M, Walsh P. The spectrum of mutations causing end-plate acetylcholinesterase deficiency. Ann Neurol. 2000;47:162-170. [PubMed] [DOI] |