修回日期: 2002-12-27
接受日期: 2003-01-03
在线出版日期: 2003-09-15
观察PKC β1和 PKC β2 在早期胃癌癌组织及其癌周不典型增生和肠上皮化生中的表达.
对42例早期胃癌胃全切标本的癌组织、癌旁不典型增生、肠上皮化生及正常胃黏膜进行PKC β1和 PKC β2免疫组织化学染色.
正常胃黏膜上皮颈部散在PKC β1阳性细胞, 黏膜表面上皮细胞及幽门腺PKC β1和 PKC β2阴性; 胃底腺壁细胞和主细胞PKC β1和 PKC β2呈强阳性. 胃黏膜内淋巴滤泡中心区PKC β1强阳性, PKC β2 阴性; 相反, 淋巴滤泡帽带区PKC β1阴性, 而PKC β2 强阳性. 胃癌、癌旁不典型增生、肠上皮化生PKC β1的表达明显增强, 而PKC β2的表达无明显增强. 肠型胃癌PKC β1的表达明显高于弥漫型. PKC β1的表达与早期胃癌的浸润深度无关.
PKC β1在胃黏膜腺上皮颈部和淋巴滤泡中心的强阳性表达提示PKC β1可能与细胞的增生活性有关; 胃癌及癌前病变中PKC β1的表达增强显示PKC β1参与了胃癌的癌变过程, 并可能在癌变早期发生改变.
引文著录: 冯瑞娥, 陈杰, 崔全才, 詹阳, 王振宇. PKC β1和PKC β2在早期胃癌中的表达. 世界华人消化杂志 2003; 11(9): 1286-1289
Revised: December 27, 2002
Accepted: January 3, 2003
Published online: September 15, 2003
To study the expression of PKC β1 and PKC β2 in carcinoma tissue, dysplasia and intestinal metaplasia of early gastric carcinoma.
Forty two cases of early gastric carcinoma were randomly selected from the file of PUMH during 1998-2001. The expression of PKC β1 and PKC β2 was investigated by immunohistochemistry method. The staining pattern of PKC β1 and PKC β2 was compared among carcinoma,dysplasia,intestinal metaplasia,and normal gastric tissue.
In normal gastric mucosa , PKC β1 and PKC β2 were strongly positive in fundic glandular epithelium, but negative in pyloric and entire surface of mucine-secretion epithelial cells. PKC β1 showed positive staining not only in the glandular cells at the neck region and but also in the follicular center cells of lymphoid tissue. However, PKC β2 was negative in those areas. Most importantly, PKC β1 demonstrated much stronger staining in carcinoma, dysplasia and intestinal metaplasia, especially in intestinal carcinoma, compared to normal gastric mucosa. There was no significant difference of PKC β1 positivity between intramucosa carcinoma and submucosa carcinoma.
The expression of PKC β1 may be related to the proliferation of gastric epithelium. Our findings strongly indicate that elevated expression of PKC β1 may be an early event in the pathway of gastric carcinogenesis.
- Citation: Feng RE, Chen J, Cui QC, Zhan Y, Wang ZY. Expression of PKC β1 and PKC β2 in early gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2003; 11(9): 1286-1289
- URL: https://www.wjgnet.com/1009-3079/full/v11/i9/1286.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i9.1286
蛋白激酶(protein kinase, PKC)是一组广泛分布的丝氨酸/苏氨酸蛋白激酶同功酶, 可催化多种底物蛋白丝氨酸/苏氨酸残基的磷酸化, 影响细胞生物信息的传递和细胞内环境的稳定. PKC β1和PKC β2为PKCβ 基因通过旁路剪接编码的2个不同的蛋白, 二者基因组成中只有羧基末端最后的50 个氨基酸成分不同. 近年来, 研究表明PKC在多种肿瘤细胞的癌变、转移、调亡及肿瘤对化疗药物的敏感性等过程中发挥重要作用[1-9]. 文献报道, PKC β 在早期前列腺癌中的表达明显下降[10]. 在致肠癌的小鼠中, PKC β2 在癌组织及癌前病变中的表达增强[11]. 因此, 推测他们是前列腺癌和肠癌癌变早期发生改变. PKC β1的过表达可以抑制消炎痛诱发的胃癌细胞株的调亡[12]. PKC β1和PKC β2 在早期胃癌及癌前病变中的表达国内外文献均未见报道.
胃癌癌变是一个多种因素参与的连续性病变过程. 特别是肠上皮化生、不典型增生作为胃癌的癌前病变引起临床的重视. 我们将观察PKC β1和PKC β2在早期胃癌患者癌组织、癌旁肠上皮化生、不典型增生和正常胃黏膜的表达. 从而推测PKC β1和PKC β2在胃癌癌变过程中的作用.
北京协和医院1998/2001 年早期胃癌切除标本42例, 癌组织及其周围约2 cm范围组织全部取材, 并按1: 1比例画图, 标注每一组织块部位. 石蜡包埋, 常规制片. 显微镜下标注每张切片肿瘤部位, 再对应标注于图上, 按图所示测量肿瘤大小. 按Lanren分型把胃癌分为肠型、弥漫型和混合型.
选取每例癌组织、癌旁不典型增生、肠上皮化生所在组织块, 把无上述病变的部位视为相对正常胃黏膜. 对以上部位进行Envision法免疫组织化学染色. PKC β1和PKC β2均购自Santa Cruz, PKCβ1: Cat No. SC-209 cPKC (C-16) rabbit ; PKC β2: Cat No. SC-210-G cPKCII (C-18) Goat. 其他免疫组化所用试剂均购自 Dako. 免疫组化采用Envision二步法. 具体为: 切取5 mm厚切片, 常规脱蜡, 水化, 切片置于微波炉内处理15 min, PKC β1和PKC β2浓度均为1: 50, 孵浴30 min. Envision TM孵浴10 min. DAB 显色, 苏木素复染, 封片. 扁桃体组织作为阳性对照; 阴性对照中一抗改为兔及羊血清.
显微镜下观察PKC β1和PKC β2在每例癌组织、癌旁不典型增生、肠上皮化生及正常胃黏膜中的表达. 选取典型视野计数阳性细胞数, 阳性细胞数小于10 % 为阴性, 10-50 %为弱阳性(+), 大于50 %为强阳性(++). 数据经x2检验.
42例早期胃癌, 男34例, 女7例, 年龄29-76岁; 肠型胃癌27例, 弥漫型12例, 混合型3例; 22例癌组织局限于黏膜内, 20例癌组织侵及黏膜下层. 1例有胃周淋巴结转移.
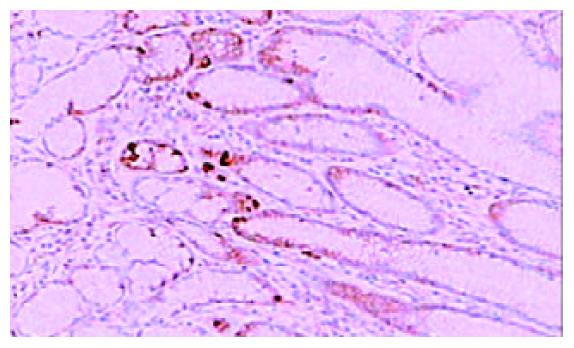
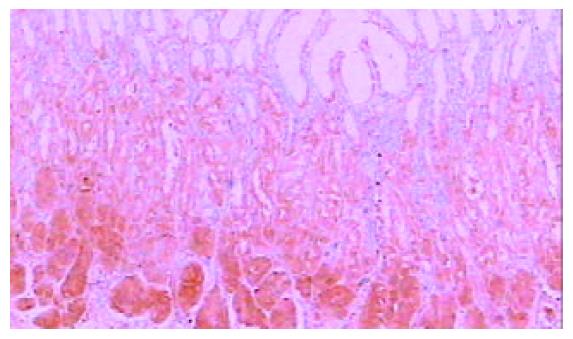
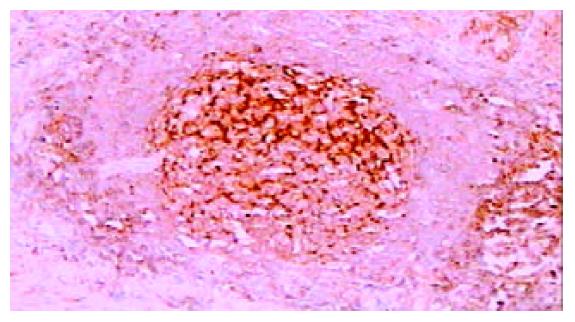
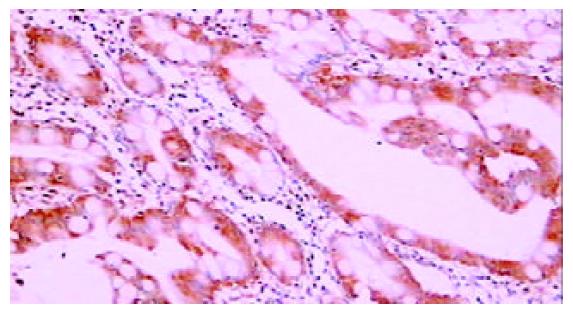
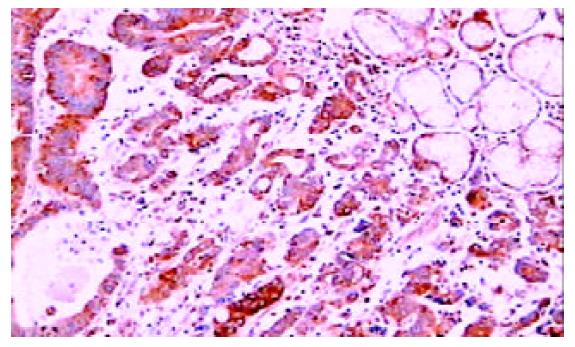
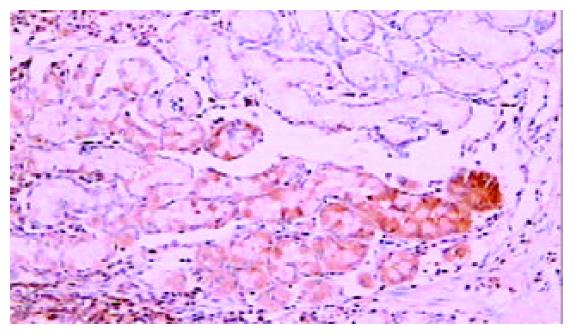
免疫组化染色PKC β1和PKC β2均位于细胞胞质内, 呈棕黄色颗粒状. 42例癌周正常胃黏膜中, 黏膜上皮颈部散在PKC β1阳性细胞 (图1), 幽门腺黏液上皮细胞及黏膜表面上皮细胞PKC β1阴性. 17例癌周正常胃黏膜中可见胃底腺, 胃底腺壁细胞和主细胞PKC β1呈强阳性(图2). 33例切片胃黏膜可见淋巴滤泡, 滤泡中心细胞PKC β1强阳性, 帽带区淋巴细胞呈阴性(图3). 胃黏膜间质中的淋巴细胞和浆细胞阳性.
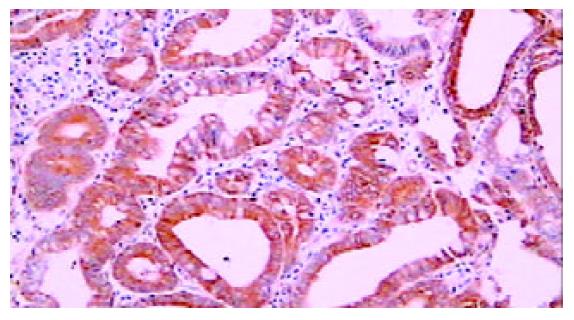
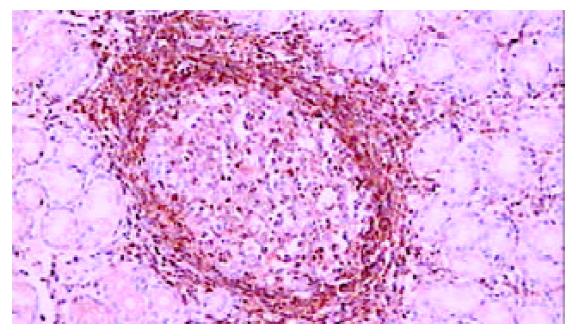
42例胃癌组织中, 29例癌细胞胞质PKCβ1强阳性, 4例弱阳性, 8例阴性. 29例癌周可见不典型增生上皮, PKC β1在22例不典型增生上皮呈强阳性(图4), 5例弱阳性, 2例阴性. 34例癌周可见肠上皮化生, 其中14例PKC β1强阳性(图5), 14例弱阳性, 6例阴性. 与正常胃黏膜相比, 癌组织、癌旁不典型增生及肠上皮化生中PKC β1的表达明显增强(P <0.001), 而三者之间相比无明显差异(P >0.05, 表1).
| PKC β1 | |||
| ++ | + | - | |
| 胃癌 | 29 | 4 | 8 |
| 肠型 | 23 | 3 | 1 |
| 弥漫型 | 3 | 2 | 7 |
| 混合型 | 3 | 0 | 0 |
| 黏膜内癌 | 17 | 2 | 3 |
| 黏膜下癌 | 12 | 3 | 5 |
| 不典型增生 | 22 | 3 | 5 |
| 肠化 | 14 | 14 | 6 |
| 正常 | 0 | 0 | 42 |
27例肠型胃癌中, 23例癌细胞胞质PKC β1强阳性(图6), 3例弱阳性, 1例阴性; 12例弥漫型胃癌, 3例PKC β1强阳性, 2例弱阳性, 7例阴性. 二者比较PKC β1在肠型胃癌中的表达明显增强, (P <0.001, 表1).
22例癌组织局限于黏膜内, 其中, 17例呈强阳性, 2例弱阳性, 3例阴性. 20例癌组织侵及黏膜下层, 其中, 12例呈强阳性, 3例弱阳性, 5例阴性. 二者比较PKC β1的表达无明显差异, (P >0.05, 表1).
PKC β2在癌周正常胃黏膜表面上皮细胞阴性, 幽门腺黏液上皮细胞阴性. 17例癌周正常胃黏膜中可见胃底腺, 胃底腺壁细胞和主细胞PKC β2呈强阳性(图7). 33例切片胃黏膜可见淋巴滤泡, 滤泡中心细胞PKCβ2阴性, 帽带区淋巴细胞呈强阳性(图8). 胃黏膜间质中的淋巴细胞和浆细胞阳性.
42例胃癌组织中, 3例癌细胞胞质PKC β2弱阳性, 29例阴性. 37例癌周可见不典型增生上皮, PKC β2在2例不典型增生上皮呈弱阳性, 35例阴性. 34例癌周可见肠上皮化生, 均呈 PKC β2阴性. 与正常胃黏膜相比, 癌组织、癌旁不典型增生及肠上皮化生中PKC β2的表达无明显增强, 三者之间相比无明显差异.
关于PKC与胃癌的关系的研究, 文献中主要用体外胃癌细胞株或测定胃癌组织PKC的活性[13-18]. PKC各同功酶亚型在胃癌组织及胃正常黏膜中的表达和定位未见研究报道. 因此, 我们对早期胃癌组织及其周围癌前病变和正常黏膜进行免疫组织化学染色, 观察他们在以上组织中的表达和定位. 我们首次报道了PKC β1和PKC β2在正常胃黏膜组织的表达及分布. PKC β1和PKC β2在正常胃黏膜上皮细胞、幽门腺黏液细胞阴性, 胃底腺壁细胞和主细胞阳性. 胃黏膜淋巴滤泡中心区PKC β1阳性, PKC β2阴性. 帽带区PKC β2阳性, PKC β1阴性. PKC β1和PKC β2在胃底腺壁细胞和主细胞中的强阳性表达提示他们可能与胃底腺的分泌活动有关. 我们注意到有实验测定胃癌和胃正常组织PKC β的活性, 以比较二者的不同, 我们建议应用此方法时正常组织的选取应分清胃窦和胃体及胃底, 以避免胃底腺对结果的影响.
PKC β1和PKC β2是PKC β 基因通过旁路剪接编码的2个不同的蛋白, 二者基因组成中只有羧基末端最后的50 个氨基酸成分不同, 但二者具有相反的生物学作用. 本实验显示PKC β2与PKC β1在早期胃癌及胃正常黏膜的表达截然不同, 在早期胃癌、癌旁不典型增生及肠上皮化生中PKC β1的表达增强, PKC β2无明显改变. 在正常胃黏膜淋巴滤泡中心区PKCβ1强阳性, PKC β2阴性; 相反, 帽带区PKC β1阴性, PKC β2阳性.
PKC根据发生作用时协同因子的不同, 分为三组, 共12种同功酶亚型. 其中, 传统型PKC包括α、β1、β2和γ, 4个同功酶亚型. 各种PKC同功酶亚型在不同肿瘤中的作用不同[19-23], 文献报道, 在致肠癌的小鼠中, PKC β2 在癌组织及癌前病变中的表达增强[11] , 膀胱正常组织和低度恶性移行细胞癌中PKC β2的表达增强, 而高低恶性肿瘤中的表达减弱[24], 本结果显示, PKC β2在早期胃癌癌前病变和正常组织中的表达未见明显差异.
本实验显示PKC β1在早期胃癌、癌旁不典型增生及肠上皮化生中的表达明显增强. 表明PKC β1的表达在胃癌及其癌前病变中发生改变. PKC β1在不典型增生及肠上皮化生中的表达增强, 提示PKC β1在胃癌中的改变可能发生在癌变早期. 肠型胃癌与弥漫型比较, PKC β1在肠型胃癌中的表达明显增强.
PKC β1在胃黏膜上皮颈部和淋巴滤泡中心区呈强阳性表达, 以上部位均为细胞增生活性较强的部位, 因此PKC β1可能与细胞的增生活性有关. 增生活性是胃癌癌变中的一个重要因素. 与我们以上的推测相一致, 体外胃癌细胞株研究表明PKC的活性与胃癌细胞的调亡有关[25], PKC β1能抑制胃癌细胞的调亡, 其作用可能与p21(waf1/cip1)有关[12].
有文献报道, PKCb与胃癌细胞株的浸润性相关, PKC β抑制剂能降低胃癌细胞株的生长和血管的形成. 为了观察PKC β1和PKC β2的表达与胃癌浸润性的关系, 我们把早期胃癌分为癌组织局限于黏膜内和浸润到黏膜下两组, 结果表明, 此两组PKC β1和PKC β2的表达未见明显差异. PKC与胃癌浸润性的关系尤待大样本进一步的研究.
| 1. | Buchner K. The role of protein kinase C in the regulation of cell growth and in signalling to the cell nucleus. J Cancer Res Clin Oncol. 2000;126:1-11. [DOI] |
| 2. | Way D, Smith S, Sivendran S, Chie L, Kanovsky M, Brandt-Rauf PW, Chung DL, Michl J, Pincus MR. A protein kinase C inhibitor induces phenotypic reversion of ras-transformed pancreatic cancer cells and cooperatively blocks tumor cell proliferation with an anti-ras peptide. Cancer Chemother Pharmacol. 2002;49:429-437. [DOI] |
| 3. | Goekjian PG, Jirousek MR. Protein kinase C inhibitors as novel anticancer drugs. Expert Opin Investig Drugs. 2001;10:2117-2140. [DOI] |
| 4. | Carter CA. Protein kinase C as a drug target: implications for drug or diet prevention and treatment of cancer. Curr Drug Targets. 2000;1:163-183. [DOI] |
| 5. | Garcia-Fernandez LF, Losada A, Alcaide V, Alvarez AM, Cuadrado A, Gonzalez L, Nakayama K, Nakayama KI, Fernandez-Sousa JM, Munoz A. Aplidin induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta. Oncogene. 2002;21:7533-7544. [DOI] |
| 7. | Sagawa N, Fujita H, Banno Y, Nozawa Y, Katoh H, Kuzumaki N. Gelsolin suppresses tumorigenicity through inhibiting PKC activation in a human lung cancer cell line, PC10. Br J Cancer. 2003;88:606-612. [DOI] |
| 8. | Jarzabek K, Laudanski P, Dzieciol J, Dabrowska M, Wolczynski S. Protein kinase C involvement in proliferation and survival of breast cancer cells. Folia Histochem Cytobiol. 2002;40:193-194. |
| 9. | Masanek U, Stammler G, Volm M. Modulation of multidrug resistance in human ovarian cancer cell lines by inhibition of P-glycoprotein 170 and PKC isoenzymes with antisense oligonucleotides. J Exp Ther Oncol. 2002;2:37-41. [DOI] |
| 10. | Cornford P, Evans J, Dodson A, Parsons K, Woolfenden A, Neoptolemos J, Foster CS. Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am J Pathol. 1999;154:137-144. [DOI] |
| 11. | Gokmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP. Elevated protein kinase C betaII is an early promotive event in colon carcinogenesis. Cancer Res. 2001;61:1375-1381. |
| 12. | Zhu GH, Wong BC, Slosberg ED, Eggo MC, Ching CK, Yuen ST, Lai KC, Soh JW, Weinstein IB, Lam SK. Overexpression of protein kinase C-beta1 isoenzyme suppresses indomethacin杋nduced apoptosis in gastric epithelial cells. Gastroenterology. 2000;118:507-514. [DOI] |
| 13. | Atten MJ, Attar BM, Milson T, Holian O. Resveratrol-induced inactivation of human gastric adenocarcinoma cells through a protein kinase C: mediated mechanism. Biochem Pharmacol. 2001;62:1423-1432. [DOI] |
| 14. | Teicher BA, Menon K, Alvarez E, Galbreath E, Shih C, Faul MM. Antiangiogenic and antitumor effects of a protein kinase Cbeta inhibitor in human HT-29 colon carcinoma and human CaKi1 renal cell carcinoma xenografts. Anticancer Res. 2001;21:3175-3184. |
| 15. | Han Y, Han ZY, Zhou XM, Shi R, Zheng Y, Shi YQ, Miao JY, Pan BR, Fan DM. Expression and function of classical protein kinase C isoenzymes in gastric cancer cell line and its drug-resistant sublines. World J Gastroenterol. 2002;8:441-445. [DOI] |
| 18. | Uchida N, Okamura S, Kuwano H. Protein kinase C activity in human gastric carcinoma. Oncol Rep. 2000;7:793-796. [DOI] |
| 19. | Wu D, Foreman TL, Gregory CW, McJilton MA, Wescott GG, Ford OH, Alvey RF, Mohler JL, Terrian DM. Protein kinase cepsilon has the potential to advance the recurrence of human prostate cancer. Cancer Res. 2002;62:2423-2429. |
| 20. | Schondorf T, Kurbacher CM, Becker M, Warm M, Kolhagen H, Gohring UJ. Heterogeneity of proteinkinase C activity and PKC-zeta expression in clinical breast carcinomas. Clin Exp Med. 2001;1:1-8. [DOI] |
| 21. | Masso-Welch PA, Winston JS, Edge S, Darcy KM, Asch H, Vaughan MM, Ip MM. Altered expression and localization of PKC eta in human breast tumors. Breast Cancer Res Treat. 2001;68:211-223. [DOI] |
| 22. | Clark AS, West KA, Blumberg PM, Dennis PA. Altered protein kinase C (PKC) isoforms in non-small cell lung cancer cells: PKCdelta promotes cellular survival and chemotherapeutic resistance. Cancer Res. 2003;63:780-786. |
| 23. | Ding L, Wang H, Lang W, Xiao L. Protein kinase C-epsilon promotes survival of lung cancer cells by suppressing apoptosis through dysregulation of the mitochondrial caspase pathway. J Biol Chem. 2002;277:35305-35313. [DOI] |
| 24. | Langzam L, Koren R, Gal R, Kugel V, Paz A, Farkas A, Sampson SR. Patterns of protein kinase C isoenzyme expression in transitional cell carcinoma of bladder. Relation to degree of malignancy. Am J Clin Pathol. 2001;116:377-385. [DOI] |