修回日期: 2002-12-30
接受日期: 2003-02-18
在线出版日期: 2003-08-15
肝细胞生成素(HPO)特异性刺激肝细胞增生的信号转导存在特异性核受体信号转导途径.
利用125I标记的HPO, 通过受体结合和特异性竞争抑制实验及Scatchard分析原代培养大鼠肝细胞和肝癌细胞核抽提物中核受体.
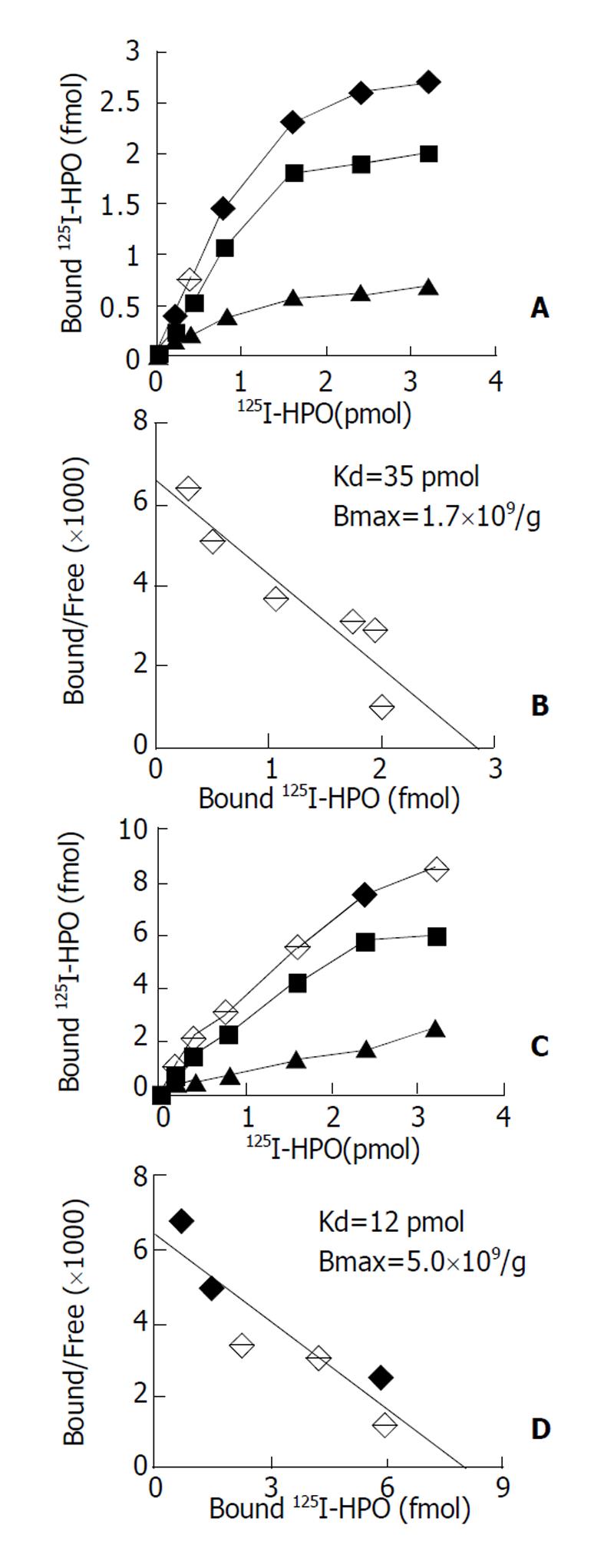
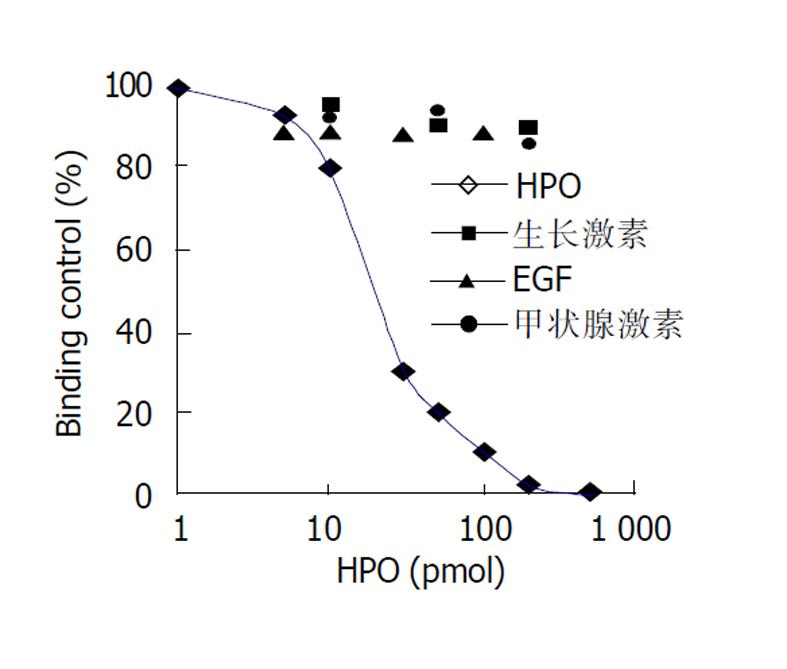
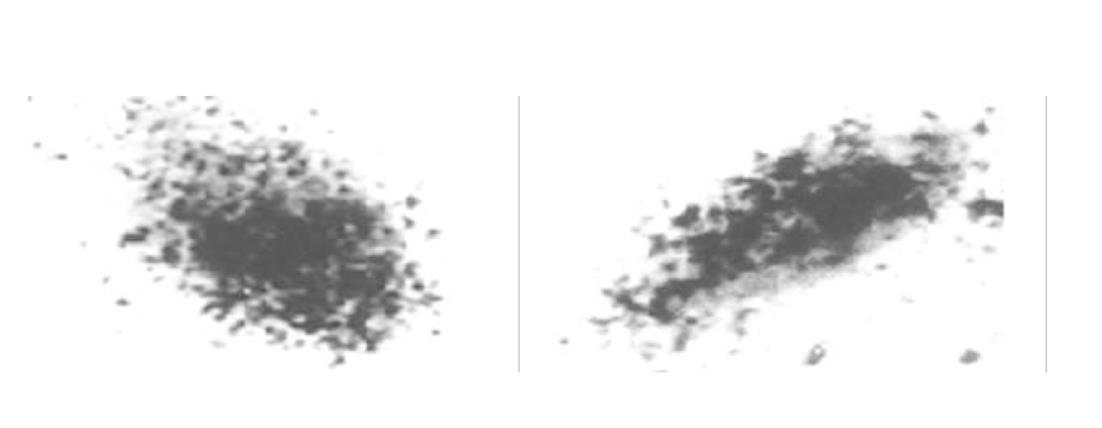
原代培养大鼠肝细胞和肝癌细胞核抽提物中存在核受体, 核受体数量分别为1.7×109/ g、和5.0×109/ g, 平衡解离常数(Kd)分别为35 pmol及12 pmol, 且存在数量上和亲和力的差异, 此结合不能被EGF、生长激素和甲状腺激素所竞争抑制. HepG2细胞放射自显影显示HPO核受体的存在.
HPO促肝细胞增生作用可以通过肝细胞核受体介导.
引文著录: 王阁, 陈东风, 胡辂, 王军, 樊丽琳, 张晓荣. 肝细胞生成素核受体的确定及特性. 世界华人消化杂志 2003; 11(8): 1178-1181
Revised: December 30, 2002
Accepted: February 18, 2003
Published online: August 15, 2003
To determine whether Hepatopoietin(HPO) acts via a novel nuclear receptor based signal transduction pathway.
125I-HPO was used to characterize its binding activity by specific displacement test and Scatchard analysis in nuclear extracts of primarily cultured rat hepatocytes and human hepatoma cells. 125I-HPO bound to its receptor complex by micro autoradiograph for the HPO receptor in HepG2 cells.
The binding was saturable and specific since it was replaceable by HPO but not by EGF, thyroid hormone or growth hormone. Scatchard analysis indicated the presence of a single class of high affinity receptor with dissociation constant (Kd) of 35 pmol and 12 pmol, and a receptor density of about 1.7×109 sites/g and 5.0×109 sites/g, in the rat hepatocytes and human hepatoma cells, respectively. Autoradiograph of the receptor showed that the receptor grains were well distributed around hepatocytes nuclei.
These data demonstrate the existence of HPO nuclear receptor in hepatocytes and hepatoma cells, which involves in hepatocytes proliferation signal transduction.
- Citation: Wang G, Chen DF, Hu L, Wang J, Fan LL, Zhang XR. Identification and characterization of nuclear receptor for hepatopoietin. Shijie Huaren Xiaohua Zazhi 2003; 11(8): 1178-1181
- URL: https://www.wjgnet.com/1009-3079/full/v11/i8/1178.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i8.1178
肝细胞生成素(HPO)是一类来源于肝脏自身、特异性刺激肝细胞增生的小分子物质. 前面实验结果证实HPO通过膜受体介导在体外不仅对原代培养的大鼠肝细胞、人胎肝细胞有刺激DNA合成作用, 而且对肝癌细胞也有直接刺激增生作用. HPO可促使肝细胞从G0/G1期进入S期[1-24], 进一步发现在HepG2肝癌细胞生长中, HPO以一种自分泌和旁分泌作用机制发挥生物学效应. 许多的生长因子具有核转位(nuclear translocation)现象, 并且在核内有相应的受体或结合蛋白的存在, 其中有与肝再生密切相关的细胞因子, 如EGF, TGF-α和aFGF等, 他们不仅有膜受体, 也有核受体[25,26]. HPO是否存在核受体, 国内外至今未见任何报道. 放射配基结合分析法是研究受体特性的经典方法, 因此我们利用前面具有高活性低失活已碘标记的HPO[4], 通过受体配基结合竞争实验和放射自显影方法, 首次证实HPO核受体的存在, 并对核HPO受体的特性进行初步的研究.
♂Wistar大鼠, 体质量200±20 g, 本院实验中心提供. HepG2细胞本科室保存. IV型核乳胶、Na125I购自Amersham公司. HPO为自行构建的PBV-hHPO表达菌株, 经诱导表达, 纯化, 获纯度>95%HPO[8]. 利用氯胺T法125I标记HPO. IV型胶原酶 为Sigma产品. 细胞培养耗材均为GIBCO产品.
1.2.1 大鼠原代肝细胞和细胞株的分离及培养 大鼠原代肝细胞分离参考Seglen方法[27,28], 将细胞悬液于含100 ml/L FCS、青链霉素的DMEM中, 计数后将细胞铺于10 cm培养皿, 在37 °C, 50 ml/L CO2的孵箱中培养, 细胞密度为5×105/ml. 分离及培养HepG2细胞株, 将细胞悬液置于100 ml/L FCS、DMEM培养液中, 铺于10 cm培养皿, 在37 °C, 50 ml/L CO2的孵箱中培养, 细胞密度为5×108/L.
1.2.2 125I-HPO受体结合实验[29-31] 原代大鼠肝细胞、HepG2、COS-7细胞、K562细胞和 Hep2细胞培养24 h后, 观察细胞贴壁生长良好, 利用蔗糖分度离心获得细胞核抽提物(50 g/L), 用结合缓冲液(20 mmol/L Hepes, 2 g/L BSA/Hanks, pH = 7.0)冲洗2遍, 一组用不同浓度的125I-HPO加入每孔反应, 另一组在加入不同浓度的125I-HPO的同时加入相应浓度为1 000倍的未标记HPO进行反应, 在4 °C下摇床内反应24 h, 再用4 °C的结合缓冲液冲洗5遍, 进行γ-计数仪计数, 完成受体结合曲线及Scatchard作图.
选择一组125I-HPO浓度为恒定反应浓度(1 pmol), 再分别加入500倍, 100倍, 50倍, 20倍, 10倍, 5倍, 2倍于125I-HPO反应浓度的未标记HPO, 另外一组加入不同浓度的EGF、甲状腺激素和生长激素, 反应条件同前, 最后收集细胞进行γ-计数仪计数, 制作特异性竞争抑制曲线.
1.2.3 HepG2上HPO受体的放射自显影 接种HepG2细胞悬液于加有盖玻片的35 mm平皿中, 细胞密度为5×102 /孔, 每孔加入含100 ml/L FCS的DMEM 1ml, 37 °C, 50 ml/L CO2孵箱培养24 h后, 用Hanks液冲洗细胞1次, 加入预先在37 °C预热20 min的含125I-HPO, 50 ml/L FCS的DMEM培养液, 浓度为37 k Bq/L, 37 °C孵育24 h, 用Hanks液冲洗3次, 再用70%酒精固定30 min, 暗室中涂抹核乳胶, -20 °C暗盒曝光3-5 d, 显影定影后用Giemsa染色5 min, 酒精逐级脱水, 二甲苯透明, 中性树脂封片, 光镜观察.
通过受体结合实验和结合竞争抑制实验发现, 肝源性细胞包括正常肝细胞(原代培养大鼠肝细胞)与非正常肝细胞(HepG2肝癌细胞等) 核抽提物与125I-HPO反应具有剂量效应关系, 以原代培养大鼠肝细胞和HepG2细胞核抽提物为代表进行分析. 图1A示125I-HPO原代培养大鼠肝细胞核抽提物结合实验的典型饱和结合曲线, 图1B为Scatchard作图分析, 其最大结合位点数量为1.7×109/g, Kd值为35 pmol; 图1C代表125I-HPO与HepG2肝癌细胞受体结合实验的典型饱和曲线, 图1D为Scatchard作图分析其最大结合位点5.0×109/ g, Kd值为12 pmol; 来源于其他组织的非肝源性细胞, 如COS-7细胞、K562细胞、Hep2细胞和CHO细胞核抽提物与125I-HPO均无明显的剂量效应关系.
只有未标记的HPO可竞争抑制125I-HPO与核受体的结合(图2), 并呈剂量效应关系, 在浓度为反应浓度的100倍左右时可发生完全竞争抑制, 而EGF、生长激素和甲状腺激素却不能竞争抑制125I-HPO与核受体的结合.
HPO刺激肝细胞增生通过与靶细胞膜上特异性受体结合而发挥生物学效应的, HPO受体具有高度特异性, 可逆性和饱和性, 其组织分布和细胞定位具有特异性, 原代大鼠肝细胞、肝癌细胞和人胎肝细胞上均有HPO的受体, 但在数量和亲和力上存在明显的差异. 通过调节受体的数量和亲和力发挥着HPO在肝细胞生长发育中的重要作用. HPO可能通过自分泌和旁分泌方式调控肝细胞的生长发育, 受体数目与亲和力的变化或HPO产生的异常可能是肝癌发生的机制之一. 通过125I标记的HPO, 观察125I-HPO与大鼠肝细胞和肝癌细胞核抽提物的结合, 发现125I-HPO与核抽提物有剂量效应关系, 且有饱和趋向及可逆性, 即结合后若再加入大量未标记HPO, 则125I-HPO与细胞核结合位点的结合下降; 另外, 其结合具有特异性, 即加入EGF、生长激素和甲状腺激素, 125I-HPO与肝细胞结合位点不受影响, 与核抽提物均无明显的剂量效应关系, 结果证明肝细胞核抽提物中的特异性位点可能就是HPO的核受体, 具有特异性, 可逆性和饱和性.
核受体具有转录活性, 结构中有与特异配体结合的功能域, 在配体存在或不存在的情况下与不同的蛋白分子作用, 调控相关基因的转录[32-34]. 肝细胞可自分泌HPO, HPO的核受体的存在提示, 在肝细胞处于增生静息(G0)期时, HPO核受体可能以同源或异源二聚体(辅助因子)的形式与DNA相应作用区段结合, 抑制DNA的转录; 在外源性肝再生信号或HPO配体转运到达肝细胞核内, HPO核受体同源或异源二聚体发生构象的变化, DNA转录抑制的解除, 发生肝细胞DNA的转录, 产生肝细胞增生效应. 在虽然原代大鼠肝细胞和肝癌细胞上核抽提物中均有HPO的受体, 但在数量和亲和力上存在明显的差异. 原代大鼠肝细胞上核抽提物中受体的数量少, 亲和力低; 肝癌细胞上受体的数量多, 亲和力高; 肝细胞HPO核受体在不同生理病理状态下所表现的不同状况, 提示肝细胞中HPO核受体数目与亲和力的变化可能是肝癌发生有关.
| 1. | Li Y, Xing G, Wang Q, Li M, Wei H, Fan G, Chen J, Yang X, Wu C, Chen H. Hepatopoietin acts as an autocrine growth factor in hepatoma cells. DNA Cell Biol. 2001;20:791-795. [PubMed] [DOI] |
| 2. | Wang G, Zhang XR, Hu L, Wang J, Leng ER, Fang DC, Yang XM, Zhang Y, He FC. Rapid induction of mRNAs for liver regeneration genes by hepatopoietin and partial hepatectomy. Zhonghua Ganzangbing Zazhi. 2002;10:256-259. [PubMed] |
| 3. | An W, Du HJ, Chen L. Increased cellular proliferation in BEL-7402 hepatoma cells transfected by human hepatic stimulator substance gene. Shengli Xuebao. 2001;53:473-477. [PubMed] |
| 4. | Wang G, Yang X, Zhang Y, Wang Q, Chen H, Wei H, Xing G, Xie L, Hu Z, Zhang C. Identification and characterization of receptor for mammalian hepatopoietin that is homologous to yeast ERV1. J Biol Chem. 1999;174:11469-11472. [DOI] |
| 5. | Cheng J, Zhong Y. Cloning and sequence analysis of human genomic DNA of augmenter of liver regeneration hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:12-14. |
| 6. | Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. J Gastroenterol. 2000;35:112-119. [DOI] |
| 7. | Li Y, Li M, Xing G, Hu Z, Wang Q, Dong C, Wei H, Fan G, Chen J, Yang X. Stimulation of the mitogen-activated protein kinase cascade and tyrosine phosphorylation of the epidermal growth factor receptor by hepatopoietin. J Biol Chem. 2000;275:37443-37447. [PubMed] [DOI] |
| 8. | Adams GA, Maestri M, Squiers EC, Alfrey EJ, Starzl TE, Dafoe DC. Augmenter of liver regeneration enhances the success rate of fetal pancreas transplantation in rodents. Transplantation. 1998;65:32-36. [DOI] |
| 9. | Stein G, Lisowsky T. Functional comparison of the yeast scERV1 and scERV2 genes. Yeast. 1998;14:171-180. [DOI] |
| 10. | Li Y, Wang HY, Cho CH. Effects of heparin on hepatic regeneration and function after partial hepatectomy in rats. World J Gastroenterol. 1999;5:305-307. [PubMed] |
| 11. | Gandhi CR, Kuddus R, Subbotin VM, Prelich J, Murase N, Rao AS, Nalesnik MA, Watkins SC, DeLeo A, Trucco M. A fresh look at augmenter of liver regeneration in rats. Hepatology. 1999;29:1435-1445. [PubMed] [DOI] |
| 12. | Hofhaus G, Stein G, Polimeno L, Francavilla A, Lisowsky T. Highly divergent amino termini of the homologous human ALR and yeast scERV1 gene products define species specific differences in cellular localization. Eur J Cell Biol. 1999;78:349-356. [DOI] |
| 13. | Becher D, Kricke J, Stein G, Lisowsky T. A mutant for the yeast scERV1 gene displays a new defect in mitochondrial morphology and distribution. Yeast. 1999;15:1171-1181. [DOI] |
| 14. | Liu Q, Wang Z, Luo Y. The cDNA clone and sequence analysis of the coding region of human augmenter of liver regeneration (hALR) gene. Zhonghua Ganzangbing Zazhi. 1999;7:156-158. [PubMed] |
| 15. | Yang X, Wang A, Zhou P, Wang Q, Wei H, Wu Z, He F. Protective effect of recombinant human augmenter of liver regeneration on CCl4-induced hepatitis in mice. Chin Med J. 1998;111:625-629. |
| 16. | Dong J, Cheng J, Liu Y, Wang Q, Wang G, Shi S, Si C. Cloning and sequence analysis of a pseudogene of liver regeneration augmenter in rats. Zhonghua Ganzangbing Zazhi. 2001;9:105-107. [PubMed] |
| 17. | Lu C, Li Y, Zhao Y, Xing G, Tang F, Wang Q, Sun Y, Wei H, Yang X, Wu C. Intracrine hepatopoietin potentiates AP-1 activity through JAB1 independent of MAPK pathway. FASEB J. 2002;16:90-92. [PubMed] [DOI] |
| 18. | Theocharis SE, Margeli AP, Agapitos EV, Mykoniatis MG, Kittas CN, Davaris PS. Effect of hepatic stimulator substance administration on tissue regeneration due to thioacetamide-induced liver injury in rats. Scand J Gastroenterol. 1998;33:656-663. [DOI] |
| 19. | Liakos AA, Mykoniatis MG, Kokala ME, Papadimitriou DG, Liatsos GD. Levels of hepatic stimulator substance in liver regenerating process of partially hepatectomized rats pretreated with a single dose of carbon tetrachloride. Dig Dis Sci. 1999;44:1046-1053. [DOI] |
| 20. | Zhang BH, Gong DZ, Mei MH. Protection of regenerating liver after partial hepatectomy from carbon tetrachloride hepatotoxicity in rats: role of hepatic stimulator substance. J Gastroenterol Hepatol. 1999;14:1010-1017. [DOI] |
| 21. | Margeli AP, Skaltsas SD, Spiliopoulou CA, Mykoniatis MG, Theocharis SE. Hepatic stimulator substance activity in the liver of thioacetamide-intoxicated rats. Liver. 1999;19:519-525. [DOI] |
| 22. | Margeli AP, Manolis E, Skaltsas SN, Tsarpalis KS, Mykoniatis MG, Theocharis SE. Hepatic stimulator substance activity in animal model of fulminant hepatic failure and encephalopathy. Dig Dis Sci. 2002;47:2170-2178. [DOI] |
| 23. | Zhang Y, Yang XM, Wang G, He FC. Biological activity of recombinant human hepatopoietin. Shengli Xuebao. 1999;51:347-350. [PubMed] |
| 24. | Brenner DA. Signal transduction during liver regeneration. J Gastroenterol Hepatol. 1998;13:S93-95. [DOI] |
| 25. | Tomassoni ML, Albi E, Magni MV. Changes of nuclear membrane fluidity during rat liver regeneration. Biochem Mol Biol Int. 1999;47:1049-1059. [DOI] |
| 26. | Grasl-Kraupp B, Schausberger E, Hufnagl K, Gerner C, Low-Baselli A, Rossmanith W, Parzefall W, Schulte-Hermann R. A novel mechanism for mitogenic signaling via pro-transforming growth factor alpha within hepatocyte nuclei. Hepatology. 2002;35:1372-1380. [PubMed] [DOI] |
| 27. | Yan JP, Jia JB, Ma XH, Wu XR, Zhao YC, Han DW. Immunohistochemical study on expression of epidermal growth factor receptor at hepatocyte nuclei in experimental rat liver cirrhosis. World J Gastroenterol. 1998;4:143. |
| 28. | Jia JB, Han DW, Xu RL, Gao F, Zhao LF, Zhao YC, Yan JP, Ma XH. Effect of endotox in on fibronectin synthesis of rat primary cultured hepatocytes. World J Gastroenterol. 1998;4:329-331. [DOI] |
| 29. | Huang ZS, Wang ZW, Liu MP, Zhong SQ, Li QM, Rong XL. Protective effects of polydatin against CCl4 induced injury to primarily cultured rat hepatocytes. World J Gastroenterol. 1999;5:41-44. [DOI] |
| 30. | Higuchi O, Nakamura T. Identification and change in the receptor for hepatocyte growth factor in rat liver after partial hepatectomy or induced hepatitis. Biochem Biophys Res Commun. 1991;176:599-607. [DOI] |
| 31. | Zarnegar R, DeFrances MC, Oliver L, Michalopoulos G. Identification and partial characterization of receptor binding sites for HGF on rat hepatocytes. Biochem Biophy Res Commun. 1990;176:1179-1185. [DOI] |
| 32. | Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677-684. [PubMed] [DOI] |
| 33. | Lobie PE, Wood TJ, Chen CM, Waters MJ, Norstedt G. Nuclear translocation and anchorage of the growth hormone receptor. J Biol Chem. 1994;269:31735-31746. [PubMed] |
| 34. | Lobie PE, Mertani H, Morel G, Morales-Bustos O, Norstedt G, Waters MJ. Receptor-mediated nuclear translocation of growth hormone. J Biol Chem. 1994;269:21330-21339. [PubMed] |