修回日期: 2002-12-20
接受日期: 2002-12-26
在线出版日期: 2003-08-15
建立琼脂糖凝胶电泳法检测人血清醇脱氢酶(alcohol dehydrogenase isoenzymes ADH EC 1.1.1.1)同工酶.
采用自制琼脂糖凝胶板, 摸索实验条件, 在pH 8.6, 74 mmol/L巴比妥电泳缓冲系统中进行20 mA、20 min电泳, 可清晰地分离出ADH同工酶三条区带.
检测了67例患者血清ADH总活性0.013-0.021 Kat/L, 同工酶ADHⅠ占ADH总活性0.01-0.30, ADHⅡ占ADH总活性0.12 -0.31, ADH Ⅲ占ADH总活性0.39-0.80. 检测了10例人健康肝组织匀浆的 ADH 总活性为0.136-0.196 Kat/L, 同工酶ADHⅠ占总活性的0.07-0.25, 同工酶ADH Ⅱ 占总活性的0.19-0.27和同工酶ADH Ⅲ 占总活性的0.56-0.73.
正常人血清中ADH 酶活性很低, 为0-1.8×10-3 Kat/L, 其同工酶不易被检测出. 而肝脏组织中含有大量ADH酶活性, 当肝脏受到严重损伤时, ADH即从肝细胞内逸出, 进入血液, 使血清中ADH酶活性明显升高, 同时通过琼脂糖电泳对其同工酶的检测可测出三条区带, 帮助临床进一步了解肝细胞损伤程度, 对患者预后和病程的监测具有一定的临床意义.
引文著录: 宓庆梅, 曹鲁宁, 高春芳. 电泳法检测肝和血清中醇脱氢酶同工酶. 世界华人消化杂志 2003; 11(8): 1175-1177
Revised: December 20, 2002
Accepted: December 26, 2002
Published online: August 15, 2003
To establish an agarose gel electrophoretic method for detecting alcohol dehydrogenase isoenzymes in human serum or liver tissue.
The samples of human liver tissue or serum were electrophoresed with 74 mmol/L diethylbarbital buffer (pH 8.6) of 10 g/L agarose gel. Electrophoresis can separate the Alcohol Dehydrogenase (ADH) isoenzymes to three bands clearly at 20 mA for 20 min in serum.
The total ADH activity in serum of sixty-seven patients with liver diseases was ranged from 0.013 Kat/L to 0.021 Kat/L. ADHⅠ isoenzyme was ranged from 0.01 to 0.30 of the total activities . ADH Ⅲ isoenzyme was from 0.12 to 0.31 . ADH Ⅲ was from 0.39 to 0.80. Total ADH activity in liver tissues of ten healthy subjects was ranged from 0.136 Kat/L to 0.196 Kat/L. ADHⅠ isoenzyme was from 0.07 to 0.25 of the total activities. ADH Ⅲ isoenzyme was ranged from 0.19 to 0.27. ADH Ⅲ was from 0.56 to 0.73.
ADH activity is high in normal human liver. Three ADH isoenzymes can be separated with agarose gel electrophoresis . But serum ADH activity is low. High activities were obtained in serum from persons suffering from serious liver diseases.
- Citation: Mi QM, Cao LN, Gao CF. Detection of alcohol dehydrogenase isoenzymes in liver and serum by electrophoresis in liver disease. Shijie Huaren Xiaohua Zazhi 2003; 11(8): 1175-1177
- URL: https://www.wjgnet.com/1009-3079/full/v11/i8/1175.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i8.1175
人类醇脱氢酶(ADH, EC 1.1.1.1)在人体内, 主要催化醇与其相应的醛酮的互相变化. 在人类肝脏内, 约有20多种醇脱氢酶同工酶[1-7], 而基于其物理化学性质和电泳迁移率的不同, 大致可分为3种同工酶形式, ADH Ⅰ, ADH Ⅱ 和 ADH Ⅲ. ADH Ⅰ 由亚单位α, β, γ组成, ADH Ⅱ 由亚单位 π 组成, ADH Ⅲ 由亚单位χ组成. 这3种同工酶都是由两聚体组成, 是个含有四原子锌的金属酶. 亚单位Mr 40000, N-末端都有一组氨基酸, 整个酶的Mr 80000 [8].
丹麦Amersham Pharmacia Biolich电泳仪、美国Helena扫描仪、 美国GS - 15R Centrifuge离心机、 日本 PRO Scientific Inc匀浆仪、日本7020 Automatic Analyzer; 电泳槽缓冲液: 巴比妥-巴比妥钠缓冲液pH 8.6. 琼脂糖凝胶板: 10 g/L 琼脂糖加热, 溶于甘氨酸-Tris pH 8.2 缓冲液中. 基质液: 乙醇 33.3 mmol/L, 戊醇10 mmol/L, 氧化型辅酶 Ⅰ(NAD+) 2.5 mmol/L, 溶于甘氨酸-Tris pH 8.2 缓冲液中. 显色液: 氯化亚硝基四氮唑蓝(NBT ) 2.8 mmol/L. 吩嗪二甲酯硫酸盐(PMS ) 3.3 mmol/L, 溶于甘氨酸- Tris pH 8.2 缓冲液中. 固定漂洗液: 50 ml/L 冰醋酸液. 标本: 人肝脏组织取自10例猝死男性, 生前身体健康, 年龄24-37岁. 肝脏组织各 0.8 g , 分别加入 KH2PO4 -Na2HPO4 缓冲液 pH 7.4 5 mL, 0 °C 条件下匀浆仪进行匀浆粉碎 5 min, 15 000 g×30 min离心, 取上清液, -70 °C 保存. 4例患者血清取自于4例住院患者, 第1例诊断为急性心肌梗死, 第二例为服用大量安眠药患者, 第3例诊断为急性肝炎, 第4例诊断为糖尿病.
测定ADH总活力基质液试剂300 μL, 肝匀浆(稀释)或血样本10 μL, 在日立7020全自动生化仪上, 37 °C, 340 nm波长, 测定其NADH的吸光度A值的变化值, 计算ADH总活力单位.
Kat/L = △A/min ÷6.3÷1.67×10-4×31×103 = △A/min×0.29
( 6.3×103 为340 nm处NADH 的摩尔吸光度)
加肝匀浆5 μL 琼脂糖凝胶板上后静置片刻, 用4层纱布搭桥, 进行电泳. 取出凝胶板, 加基质液 1 μL 于凝胶板上, 置于 37 °C 水浴箱 25 min. 取凝胶板, 加 50 ml/L 冰醋酸20 mL, 5 min, 漂洗凝胶板至底色透明. 用Helena 扫描仪 570 nm 波长直接扫描定量分析.
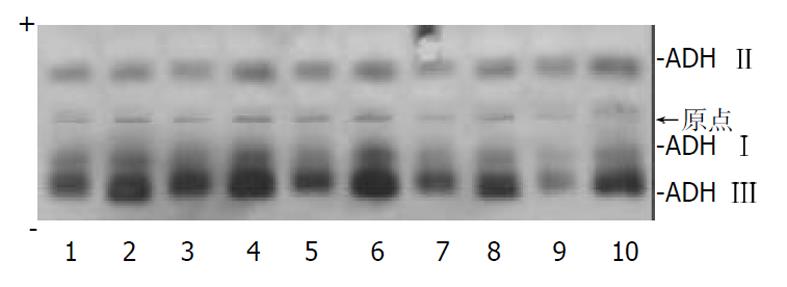
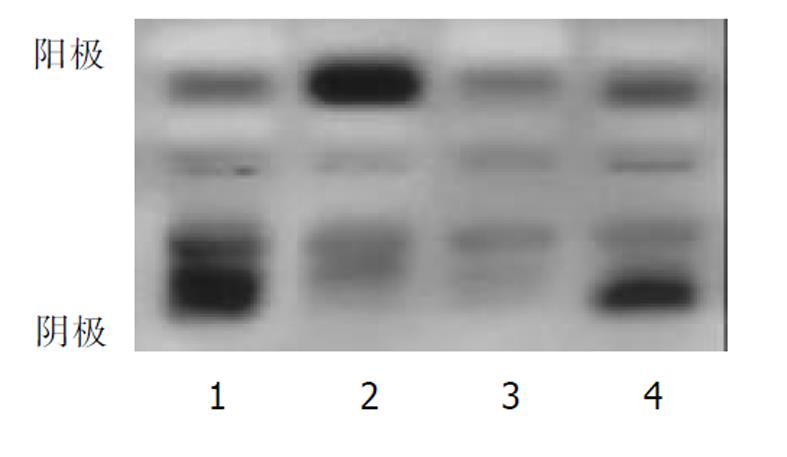
分别用 3, 5, 8 μL样品量加样后进行电泳, 结果显示样品量为 5 μL最适宜. 分别作基质液孵育 10, 20, 30 min测定, 其结果经Helena扫描仪扫描, 基本一致, 但是最佳条件为 20 min, 区带显色完全, 底板着色适宜. 10例正常人肝匀浆琼脂糖凝胶电泳结果见图1, 4例患者血清ADH 同工酶的检测结果见图2. 临床工作中检测了67例患者血样, 其同工酶的分布是ADHⅠ0.01-0.30, ADHⅡ 0.12 - 0.31, ADH Ⅲ 0.39 -0.80. 这67例患者都有严重肝脏组织损伤.
人类肝匀浆的ADH同工酶研究国外报道很多[9-17], 在我国报道很少, 对血液的研究报道就更少. 我们主要建立一种新的琼脂糖凝胶电泳法, 在pH 8.6巴比妥-巴比妥钠电泳缓冲液中, 用10 g/L琼脂糖凝胶作支持物, 对人类血液或肝匀浆进行电泳. 条件为: 电压130 V, 时间20 min, 用乙醇和戊醇作为底物, 加入NAD+, PMS和NBT, 37 °C温育20 min, 能简便、灵敏、快速的将血液、肝匀浆ADH分离成清晰的3条蓝色区带. 通过扫描仪 570 nm波长扫描, 可对ADH同工酶定量分析.
肝匀浆ADH 总活性为0.136-0.196 Kat/L, 经10 g/L琼脂糖凝胶电泳后, 根据电泳迁移率和物理化学性质的不同, 从阳极到阴极分别为ADHⅠ, ADH Ⅱ和ADH Ⅲ. 其中ADHⅠ占0.07-0.25, ADH Ⅱ 占0.18-0.26, ADH Ⅲ占0.56-0.73. ADH Ⅰ∶Ⅱ∶Ⅲ 约1∶1.7∶5.4. ADH Ⅲ 最高, ADHⅠ最少, 且不耐热, 放置于室温10 h 就失活.
正常人血清中ADH 酶活性很低, 一般不易被检测出[4,5]. 当患者由于缺氧导致肝脏受到严重损伤时, ADH 大量从肝细胞内逸出入血, 血清中ADH 酶活性明显升高, ADH 在血清中出现的多少直接反映了肝脏的损害程度, 此时可通过检测血中其同工酶ADH Ⅰ, ADH Ⅱ, ADH Ⅲ 的活性, 对进一步了解患者肝细胞结构损伤程度, 估价其损伤的预后和对患者病程的监测具有一定的临床意义[5-9].
图2中例1为急性心肌梗死, 伴有严重肝脏组织损伤, 导致同工酶ADH Ⅲ 占0.75, 于发病后4 d死亡. 例2, 女, 23岁, 服用大量安眠药致昏迷, ADHⅠ为0.78, ADH Ⅱ0.12, ADH Ⅲ 0.10, 第2天死亡. 例4, 男, 46岁, 糖尿病患者, 其ADH活性明显升高, ADH Ⅲ 0.57, 也于第5天死亡. 例3, 急性肝炎患者, ADHⅠ0.47, ADH Ⅱ0.29, ADH Ⅲ 0.24, 该患者经过1 mo的治疗后出院.
国外有琼脂糖凝胶电泳测定乳酸脱氢酶同工酶的时候发现有同工酶"LDH6" 出现的报道[18,19]. 国内在1984年有也类似报道[20] . 我们在检测LDH同工酶时, 也发现了有"LDH6"的出现, 经过分离、提取, 再经SDS-聚丙烯酰胺凝胶电泳后, 一半凝胶膜测定"LDH6"的Mr为80 000, 另一半用以免疫固定(Western 印迹法)鉴定"LDH6", 结果在Mr 80 000 处有一条显色带, 鉴定该"LDH6" 区带即为ADH Ⅲ. 同时, 肝癌组织内均存有大量"LDH6". 我们在临床上测定了100例乳酸脱氢酶高的患者, 当"LDH6"活性占总乳酸脱氢酶活性0.08 以上时, 有91.3 % 的患者1 wk左右即死亡.
肝脏受到严重损伤, ADH即从肝脏内释放入血, 使血清内ADH明显升高[21-23], Chrostek et al [24]经研究统计认为肝损伤患者ADH Ⅰ明显升高, 其活性升高同转氨酶相关. Chao et al [29]认为ADH同工酶与肝硬化有一定关系[22-32]. 但肝脏损伤后ADH 一旦释放入血, 会导致LDH 测定时受到ADH -"LDH6"的干扰, 并且其活性占总LDH活性0.08 以上时, 患者1 wk左右即死亡, 其致死机制与ADH的从肝内细胞大量释放的相关性有多少, ADHⅠ、Ⅱ、Ⅲ 哪一条带的明显升高对临床更有意义, 目前还不知道, 还有待继续的研究和观察.
| 1. | Duester G, Hatfield GW, Buhler R, Hempel J, Jornvall H, Smith M. Molecular cloning and characterization of a cDNA for the beta subunit of human alcohol dehydrogenase. Proc Natl Acad Sci USA. 1984;81:4055-4059. [DOI] |
| 2. | Fong WP, Keung WM. Substrate specificity of human class Ⅰ alcohol dehydrogenase homo- and heterodimers containing the bate 2 (Oriental) subunit. Biochemistry. 1987;26:5726-5732. [PubMed] [DOI] |
| 3. | Montavon P, Felber JP, Holmquist B, Vallee BL. A human liver alcohol dehyd-rogenase enzyme-linked immunosorbent assay method specific for classⅠ, Ⅱ and Ⅲ isozymes. Anal Biochem. 1989;176:48-56. [DOI] |
| 4. | Moulis JM, Holmquist B, Vallee BL. Hydrophobic anion activation of human liver chi chi alcohol dehydrogenase. Biochemistry. 1991;30:5743-5749. [DOI] |
| 5. | Tsui HT, Mock WY, Lau KK, Fong WP. Proteolytic activation of grass carp (Ctenopharygodon idellus) liver alcohol dehydro-genase. Biochimica et Biophysica Acta. 1996;1296:41-46. [DOI] |
| 6. | Watabiki T, Tokiyasu T, Yoshida M, Okii Y, Yoshimura S, Akane A. Intralobular distribution of class I alcohol dehydrogenase and aldehyde dehydrogenase 2 activities in the hamster liver. Alcohol Clin Exp Res. 1999;23:52S-55S. [PubMed] [DOI] |
| 7. | Connally HE, Hamar DW, Thrall MA. Inhibition of canine and feline alcohol dehydrogenase activity by fomepizole. Am J Vet Res. 2000;61:450-455. [DOI] |
| 8. | Wang Y, Schubert M, Ingendoh A, Franzen J. Analysis of non-covalent protein complexes up to 290 kDa using electrospray ionization and ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:12-17. [DOI] |
| 9. | Jensen DE, Belka GK, DuBois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J. 1998;331:659-68. [PubMed] [DOI] |
| 11. | Van ophem PW, Van Beeumen J, Duine JA. Nicotinoprotein [NAD(P)-containing] alcohol /aldehyde oxidoreductases purification and characterization of a novel type from amycolatopsis methanolica. Eur J Biochem. 1993;212:819-826. [DOI] |
| 12. | Keung WM, Ditlow CC, Vallee BL. Identification of human alcohol dehydrogenase isozymes by disc polyacrylamide gel electrophoresis in 7 M urea. Anal Biochem. 1985;151:92-96. [DOI] |
| 13. | O'Carra P, Griffin T, O'Flaherty M, Kelly N, Mulcahy P. Further studies on the bioaffinity chromatography of NAD(+)-dependent dehydrogenases using the locking-on effect. Biochimica Biophysica Acta. 1996;1297:235-243. [DOI] |
| 14. | Daussmann T, Aivasidis A, Wandrey C. Purification and characterization of an alcohol: N, N-dimethyl-4-nitrosoaniline oxidoreductase from the methanogen methanosarcina barkeri DSM 804 strain fusaro. Eur J Biochem. 1997;248:889-896. [DOI] |
| 15. | Bosron WF, Li TK, Bosron WF, Li TK, Vallee BL. Heterogeneity and new molecular forms of human liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1979;91:1549-1555. [DOI] |
| 16. | Onorato VA, Manly KF, Vladutiu AO. Association of an oxygen-sensitive lactate dehydrogenase isoenzyme, LDk, with LD-6 in serum of critically yill patients. Clin Chem. 1984;30:1603-1606. [PubMed] |
| 17. | Aasmoe L, Winberg JO, Aarbakke J. The role of liver alcohol dehydrogenase isoenzymes in the oxidation of glycolethers in male and female rats. Toxicol Appl Pharmacol. 1998;150:86-90. [PubMed] [DOI] |
| 18. | Ketchum CH, Robinson CA, Hall LM, Grizzle WE, Maclaren NK, Riley WJ, Trost C. Clinical significance and partial biochemical characterization of lactate dehydrogenase iso enzyme 6. Clin Chem. 1984;30:46-49. [PubMed] |
| 19. | Kato S, Ishii H, Kano S, Horii K, Tsuchiya M. Evidence that "lactate" dehydrogenase isoenzyme 6 is in fact alcohol dehydrogenase. Clin Chem. 1984;30:1585-1586. [PubMed] |
| 21. | Ditlow CC, Holmquist B, Morelock MM, Vallee BL. Physical and enzymatic properties of a class Ⅱ alcohol dehydrogenase isozyme of human liver: pi-ADH. Biochemistry. 1984;23:6363-6368. [DOI] |
| 22. | Wagner FW, Pares X, Holmquist B, Vallee BL. Physical and enzymatic properties of a class Ⅲ isozyme of human liver alcohol dehydrogenase : chi-ADH. Biochemistry. 1984;23:2193-2199. [DOI] |
| 23. | Strydom DJ, Vallee BL. Characterization of human Alcohol dehydrogenase isoenzymes by high-performance liquid chromatographic peptide mapping. Anal Bio. 1982;123:422-429. [DOI] |
| 24. | Chrostek L, Szmitkowski M. Isoenzymes of class I and II alcohol dehydrogenase in chronic hepatitis. Clin Chem Lab Med. 1999;37:145-147. [PubMed] [DOI] |
| 25. | Bello AT, Bora NS, Lange LG, Bora PS. Cardioprotective effects of alcohol : mediation by human vascular alcohol dehydrogenase. Biochem Biophys Res Commun. 1994;203:1858-1864. [PubMed] [DOI] |
| 26. | Chrostek L, Szmitkowski M. Activity of class Ⅰ and Ⅱ isoenzymes of alcohol dehydrogenase measured by a fluorometric method in the sera of patients with obstructive jaundice. Clinica Chimica Acta. 1997;263:117-122. [DOI] |
| 27. | Borras E, Coutelle C, Rosell A, Fernandez-Muixi F, Broch M, Crosas B, Hjelmqvist L, Lorenzo A, Gutierrez C, Santos M. Genetic polymorphism of alcohol dehydrogenase in europeans: the ADH2*2 allele decreases the risk for alcoholism and is associated with ADH3*1. Hepatology. 2000;31:984-989. [PubMed] [DOI] |
| 28. | Marschall HU, Oppermann UC, Svensson S, Nordling E, Persson B, Hoog JO, Jornvall H. Human liver class I alcohol dehydrogenase gammagamma isozyme: the sole cytosolic 3beta-hydroxysteroid dehydrogenase of iso bile acids. Hepatology. 2000;31:990-996. [PubMed] [DOI] |
| 29. | Chao YC, Wang LS, Hsieh TY, Chu CW, Chang FY, Chu HC. Chinese alcoholic patients with esophageal cancer are genetically different from alcoholics with acute pancreatitis and liver cirrhosis. Am J Gastroenterol. 2000;95:2958-2964. [PubMed] [DOI] |
| 30. | Lieber CS. Alcohol and the liver: metabolism of alcohol and its role in hepatic and extrahepatic diseases. Mt Sinai J Med. 2000;67:84-94. [PubMed] |
| 31. | Pastino GM, Flynn EJ, Sultatos LG. Genetic polymorphisms in ethanol metabolism: issues and goals for physiologically based pharmacokinetic modeling. Am J Gastroenterol. 2000;95:2958-2964. [DOI] |
| 32. | Cheung B, Holmes RS, Easteal S, Beacham IR. Evolution of class I alcohol dehydrogenase genes in catarrhine primates: gene conversion, substitution rates, and gene regulation. Drug Chem Toxicol. 2000;23:179-201. |