修回日期: 2002-12-30
接受日期: 2003-01-02
在线出版日期: 2003-06-15
用携带分泌型抗肝癌单链免疫毒素基因(sFv-TNF-α)的重组逆转录病毒感染人外周血单个核细胞(peripheral blood mononuclear cells, PBMCs), 使其表达并分泌针对人肝癌细胞的sFv-TNF-α融合蛋白, 观察转导的PBMCs /PST静脉注射后对荷肝癌裸鼠的抑瘤作用.
分离正常人PBMCs并用PHA和IL-2进行体外刺激培养, 用感染性重组病毒产生细胞C22(PA317/PST)产生的病毒上清转导后, 经尾静脉输入荷肝癌裸鼠体内, 注射细胞数为每只1×106(0.2 mL), 每5 d注射1次, 共注射3次. 定期观察记录肿瘤生长情况, 注射3次后处死, 取出瘤组织称质量、记录, 进行统计学处理, 并制备石蜡切片进行常规HE染色及免疫组化染色观察.
实验组肿瘤生长速率为(20.8±4.9) mg/d , 对照组为(28.5±6.7) mg/d, 经t检验, P<0.05.
经重组逆转录病毒转导的PBMCs/PST在裸鼠体内对人肝癌细胞系SMMC-7721移植瘤具有一定的生长抑制作用.
引文著录: 程虹, 刘彦仿, 张惠中, 沈万安, 张菊, 张静. 抗肝癌单链免疫毒素基因修饰的PBMCs在动物体内的抑瘤作用. 世界华人消化杂志 2003; 11(6): 708-711
Revised: December 30, 2002
Accepted: January 2, 2003
Published online: June 15, 2003
To investigate In vivo antitumour activity of single-chain immunotoxin (sFv-TNF-α fusion protein).
HCC-specific killer cells were generated by transducing the recombinant retroviral virus in supernatant of the virus producing cells (C22) into human peripheral blood mononuclear cells (PBMCs). SMMC-7721 xenograft nude mice were given iv either 1×106 (0.2 mL) transduced or mock-transduced PBMCs once five days for three weeks and tumour growth was detected.
Tumour growth were (20.8±4.9) mg/d in PBMCs/PST group and (28.5±6.7)mg/d in PBMCs/ pLXSN group, with a significant difference (P<0.05).
Genetic modification of PBMCs by single-chain immunotoxin has antitumour activity In vivo.
- Citation: Cheng H, Liu YF, Zhang HZ, Shen WA, Zhang J, Zhang J. In vivo antitumour activity of PBMCs via genetic modification of single-chain immunotoxin. Shijie Huaren Xiaohua Zazhi 2003; 11(6): 708-711
- URL: https://www.wjgnet.com/1009-3079/full/v11/i6/708.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i6.708
肝细胞肝癌(hepatocellular carcinoma, HCC) 是人类常见的恶性肿瘤之一, 有较高的发病率和死亡率, 历有癌王之称. 其多发生于亚洲、非洲和地中海流域, 全世界每年约有100多万患者死于肝癌, 其中我国就有11万人[1-7], 且发病率有上升的趋势. 迄今为止, 肝癌的病因和发病机制仍不十分清楚, 更无理想的治疗手段[8-18]. 随着现代分子生物学和免疫学的发展, 自1990年代初迄今, 已有百余项肿瘤基因治疗方案获准进入临床试验, 但临床疗效与预期的目标之间还存在较大差距, 原因之一就是肿瘤细胞的特异性识别尚未解决. 肿瘤特异性单链抗体的问世为基因治疗靶向性问题的解决提供了新的手段, 近年来成为肿瘤免疫基因治疗中的热点, 目前已有多种单链免疫毒素基因治疗计划进入临床试验[19-27]. 我们在成功克隆抗肝癌单链抗体基础上, 将其与TNF-α基因相连构建成分泌型抗肝癌单链免疫毒素基因, 细胞学实验表明, 该重组基因转入人外周血单个核细胞(peripheral blood mononuclear cells, PBMCs)后可表达并分泌具有特异性结合活性的抗肝癌sFv-TNF-α融合蛋白, 后者对体外培养的肝癌细胞具有杀伤作用. 为进一步观察分泌型抗肝癌单链免疫毒素在体内的生物学作用, 我们用携带分泌型抗肝癌单链免疫毒素基因的逆转录病毒产生细胞C22的上清转导人PBMCs, 观察转导的PBMCs /PST静脉注射后对荷肝癌裸鼠的抑瘤作用.
人肝癌细胞系SMMC-7721为本室保存, RPMI1640常规传代培养. 转染的逆转录病毒产生细胞系C22由本室构建[28], 用DMEM常规传代培养. DMEM, G418, SuperscriptTM逆转录试剂盒和Trizol Reagent为Gibco公司产品, Polybrene为Sigma公司产品, MTT为Serva公司产品, PHA购自Sigma公司, 超级小牛血清购自杭州四季青生物工程材料研究所. 重组人IL-2为上海生物技术研究所产品. 淋巴细胞分离液购自上海试剂二厂.
消化对数生长期的SMMC-7721细胞, 无血清RPMI1640培养液洗涤2次, 计数活细胞数, 调整细胞数, 将细胞重悬于PBS中. 在无菌条件下, 将0.2 mL细胞悬液接种于裸鼠胸部皮下, 含活细胞数为每只5×106, 共10只. 接种后置无特殊病原体饲养室饲养, 定期观察记录肿瘤生长情况. 抽取正常人外周血50 mL, 肝素抗凝, 加入淋巴细胞分离液50 mL, 室温1400 g离心30 min, 吸取单个核细胞层悬液, 40 mL无血清RPMI1640洗涤细胞3次(1000 g离心10 min), 计数, 用含200 mL/L小牛血清的完全RPMI1640培养液稀释为活细胞1×109/L, 加入5×105/L IL-2及5×103 μg/L PHA, 于37 °C, 50 mL/L CO2培养3 d, 换含5×105/L IL-2, 200 mL/L小牛血清的完全RPMI1640培养液继续培养3 d后, 进行逆转录病毒的转导. 用C22细胞制备重组逆转录病毒上清, 取C22细胞上清(不含G418)5 mL及8 mg /L Polybrene感染1×106个PBMCs, 置37 °C, 50 mL/L CO2孵箱内培养6 h, 换普通完全RPMI1640培养液, 连续感染3 d, 转导的PBMCs命名为PBMCs/PST. 对照组PBMCs用PA317/pLXSN(空白载体)细胞上清感染, 转导的PBMCs命名为PBMCs/pLXSN. 接种7 d的荷肝癌裸鼠随机分为实验组5只和对照组5只. 分别离心收集连续感染3 d的PBMCs/PST和PBMCs/pLXSN细胞, 计数, 无血清RPMI1640培养液洗涤2次后重悬于生理盐水, 从尾静脉注射入裸鼠体内, 实验组注射PBMCs/PST, 对照组注射PBMCs/pLXSN, 注射细胞数为每只1×106(0.2 mL), 每5 d注射1次, 共注射3次. 注射后置无特殊病原体饲养室饲养, 定期观察记录肿瘤生长情况. 注射3次后处死各组荷瘤裸鼠, 取出瘤组织称重、记录, 进行统计学处理, 并固定, 制备石蜡切片, 常规HE染色、免疫组化染色观察.
接种人肝癌细胞系SMMC-7721的10只裸鼠7 d后均成瘤, 移植瘤直径约为0.58 cm.
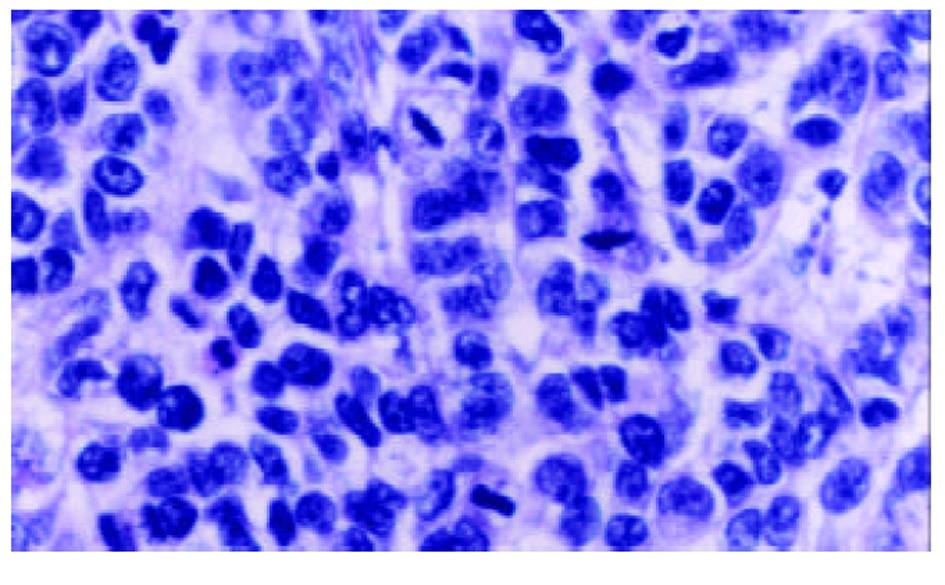
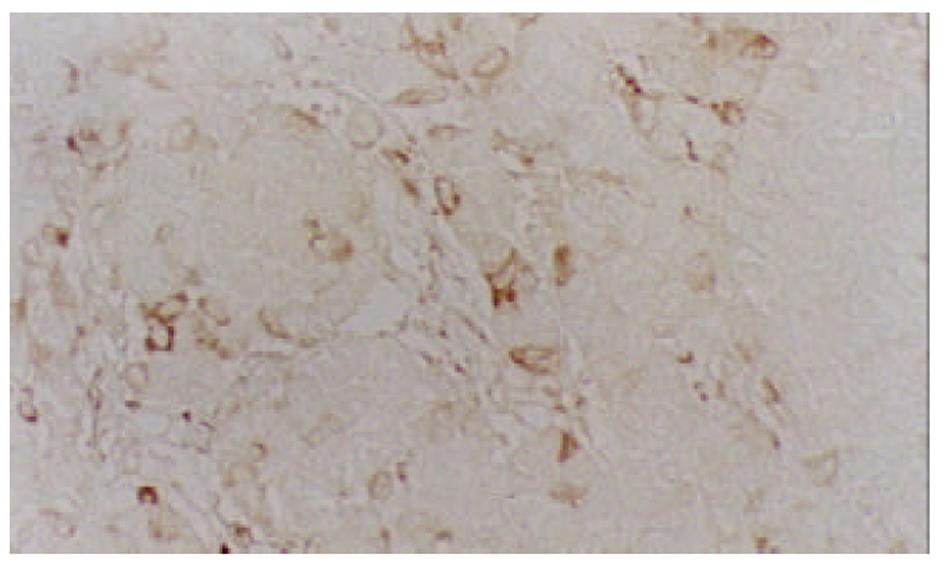
经尾静脉注射PBMCs/PST 3次后, 肉眼观察接种SMMC-7721细胞的实验组裸鼠移植瘤体积较小, 处死后取出瘤组织称质量并计算肿瘤生长速率, 实验组为(20.8±4.9) mg/d , 对照组为(28.5±6.7) mg/d, 经t检验, P<0.05. HE染色可见肿瘤细胞呈巢状分布, 纤维组织分隔, 瘤细胞大小不等, 核大且深染, 核分裂相多见(图1), 符合肝细胞肝癌的组织学形态. 以鼠抗人TNF-α多克隆抗体进行免疫组化染色, 可见瘤组织中少数细胞有强、弱不等的阳性信号, 分布于细胞膜上(图2), 对照组肿瘤组织免疫组化染色未见阳性信号.
要实现基因治疗的目的, 最终必须将目的基因转入靶细胞. 实现人类实体瘤基因治疗的途径主要有2种, (1)在体靶细胞的转染(In vivo), 即直接基因治疗途径, 例如将细胞因子或肿瘤抗原的基因表达载体直接体内注射或将MHC-I基因直接注射入瘤体内等治疗肿瘤; (2)离体靶细胞的转染(ex vivo), 即间接基因治疗途径, 必须分离并培养离体的靶细胞, 并在接受基因转移后移植回人体以治疗疾病, 例如应用体外基因修饰的TIL、瘤苗、成纤维细胞等体内回输或接种的方法治疗肿瘤[31-34]. 我们选择逆转录病毒介导的体外基因转移方法进行基因治疗的研究, 要求基因转移的靶细胞既要容易获得, 又要容易回输到人体, 因此, 我们采用PBMCs作为基因转移的靶细胞. 由于目前所常用的基因转移方法[35-41](包括逆转录病毒载体-包装细胞转移系统)效率均不高, 获得大量的靶细胞是重要的先决条件, 因此, 要求该细胞易于体外培养, 而且具有一定的分裂和增生能力. 此外, 逆转录病毒载体对处于活跃分裂状态的细胞感染率和基因转移率较高, 而对未分化的分裂不十分活跃的细胞感染率和基因转移率较低, 因此, 基因治疗中应尽量选择处于活跃分裂状态的细胞作为靶细胞. PBMCs具备上述的各项条件, 目前是一种较理想的基因治疗靶细胞[42-44]. 虽然有研究表明, TIL抗肿瘤的活性是PBMCs的50-100倍, 而且具有良好的肿瘤病灶趋向性和浸润性[45-49], 但由于其不易分离和培养, 在某些肿瘤如肝癌中TIL数量极少, 无法满足基因治疗中反复操作的要求, 因而其应用受到很大的限制.
我们分离出正常人PBMCs, 并用PHA和IL-2进行体外刺激培养, 用携带分泌型抗肝癌单链免疫毒素基因的重组逆转录病毒感染, 使其表达并分泌针对人肝癌细胞的sFv-TNF-α融合蛋白, 再经尾静脉输入荷肝癌裸鼠体内, 观察转导的PBMCs/PST对荷瘤裸鼠体内肿瘤生长的抑制作用. 本实验结果表明, 经重组逆转录病毒转导的PBMCs/PST在裸鼠体内对人肝癌细胞系SMMC-7721移植瘤具有一定的生长抑制作用, 这种抑瘤作用可能是由于转导的PBMCs/PST作为外源目的基因的载体, 在体内能够不断表达并分泌针对人肝癌细胞的免疫毒素-TNF-α融合蛋白, 这种靶向性融合蛋白分子在肝癌移植瘤局部富集, TNF-α通过与肝癌细胞表面的受体结合而导致跨膜信号的传导, 表现出TNF-α的生物学活性作用, 从而直接或间接发挥对肝癌细胞的抑制作用[50-53]. 此外, PBMCs/PST分泌的靶向性sFv-TNF-α融合蛋白的抑瘤作用不仅针对原发灶肿瘤细胞, 而且对转移至身体任何部位甚至进入血管及淋巴管的肿瘤细胞同样具有生长抑制作用, 是集免疫、基因治疗、靶向治疗和过继免疫治疗为一体的一种新的免疫基因治疗方法, 为肝癌的靶向基因治疗提供一条新的途径.
| 1. | Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:7-11. [PubMed] |
| 2. | Yang LJ, Wang WL. Preparation of monoclonal antibody against apoptosis-associated antigens of hepatoma cells by subtractive immunization. World J Gastroenterol. 2002;8:808-814. [PubMed] [DOI] |
| 3. | Wang FS, Liu MX, Zhang B, Shi M, Lei ZY, Sun WB, Du QY, Chen JM. Antitumor activities of human autologous cytokine-induced killer (CIK) cells against hepatocellular carcinoma cells in vitro and In vivo. World J Gastroenterol. 2002;8:464-468. [PubMed] [DOI] |
| 4. | Zhang G, Long M, Wu ZZ, Yu WQ. Mechanical properties of hepatocellular carcinoma cells. World J Gastroenterol. 2002;8:243-246. [PubMed] [DOI] |
| 5. | Sun ZJ, Pan CE, Liu HS, Wang GJ. Anti-hepatoma activity of resveratrol in vitro. World J Gastroenterol. 2002;8:79-81. [PubMed] [DOI] |
| 6. | El-Serag HB. Hepatocellular carcinoma and hepatitis C in the united states. Hepatology. 2002;36:S74-83. [PubMed] [DOI] |
| 7. | Liu WW. Etiological studies of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:93-95. |
| 8. | Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells in vitro induce efficient and special anti-tumor immune response. Shijie Huaren Xiaohua Zazhi. 1999;7:161-163. |
| 9. | Chen HB, Zhang JK, Huang ZL, Sun JL, Zhou YQ. Effects of cytokines on dendriti c cells against human hepatoma cell line. Shijie Huaren Xiaohua Zazhi. 1999;7:191-193. |
| 10. | Liu LH, Xiao WH, Yang JL. Effects of 5 Aza 2 deoxycytidine on growth of hu man hepatocellular carcinoma cell lines. Shijie Huaren Xiaohua Zazhi. 2000;8:420-423. |
| 11. | Fu JM, Yu XF, Shao YF. Telomerase and primary liver cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:461-463. |
| 12. | Du QY, Wang FS, Xu DP, Liu H, Lei ZY, Liu MX, Wang YD, Chen JM, Wu ZZ. Cytotoxic effects of CIK against hepatocellular carcinoma cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:863-866. |
| 13. | Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, Rodes J, Bruix J. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123-128. [DOI] |
| 14. | Llovet JM, Sala M, Bruix J. Nonsurgical treatment of hepatocellular carcinoma. Liver Transpl. 2000;6:S11-15. [PubMed] [DOI] |
| 15. | Bruix J, Llovet JM. Locoregional treatments for hepatocellular carcinoma. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:611-622. [PubMed] [DOI] |
| 16. | Xia SS. Current status of liver transplantation in China. Shijie Huaren Xiaohua Zazhi. 1999;7:645-646. |
| 17. | Chen HY, Liu WH, Qin SK. Induction of arsenic trioxide on apoptosis of hepatocarcinoma cell lines. Shijie Huaren Xiaohua Zazhi. 2000;8:532-535. |
| 18. | Ishikawa M, Yogita S, Miyake H, Fukuda Y, Harada M, Wada D, Tashiro S. Differential diagnosis of small hepatocellular carcinoma and borderline lesions and therapeutic strategy. Hepatogastroenterology. 2002;49:1591-1596. [PubMed] |
| 19. | Peipp M, Kupers H, Saul D, Schlierf B, Greil J, Zunino SJ, Gramatzki M, Fey GH. A recombinant CD7-specific single-chain immunotoxin is a potent inducer of apoptosis in acute leukemic T cells. Cancer Res. 2002;62:2848-2855. [PubMed] |
| 20. | Ueno A, Arakawa F, Abe H, Matsumoto H, Kudo T, Asano R, Tsumoto K, Kumagai I, Kuroki M, Kuroki M. T-cell immunotherapy for human MK-1-expressing tumors using a fusion protein of the superantigen SEA and anti-MK-1 scFv antibody. Anticancer Res. 2002;22:769-776. [PubMed] |
| 21. | Pennell CA, Erickson HA. Designing immunotoxins for cancer therapy. Immunol Res. 2002;25:177-191. [DOI] |
| 22. | Tur MK, Sasse S, Stocker M, Djabelkhir K, Huhn M, Matthey B, Gottstein C, Pfitzner T, Engert A, Barth S. An anti-GD2 single chain Fv selected by phage display and fused to Pseudomonas exotoxin A develops specific cytotoxic activity against neuroblastoma derived cell lines. Int J Mol Med. 2001;8:579-584. [DOI] |
| 23. | Matthey B, Engert A, Barth S. Recombinant immunotoxins for the treatment of Hodgkin's disease (Review). Int J Mol Med. 2000;6:509-514. [DOI] |
| 24. | Rosenblum MG, Horn SA, Cheung LH. A novel recombinant fusion toxin targeting HER-2/NEU-over-expressing cells and containing human tumor necrosis factor. Int J Cancer. 2000;15; 88267-88273. [DOI] |
| 25. | Vallera DA, Kuroki DW, Panoskaltsis-Mortari A, Buchsbaum DJ, Rogers BE, Blazar BR. Molecular modification of a recombinant anti-CD3epsilon-directed immunotoxin by inducing terminal cysteine bridging enhances anti-GVHD efficacy and reduces organ toxicity in a lethal murine model. Blood. 2000;96:1157-1165. [PubMed] |
| 26. | Tsutsumi Y, Onda M, Nagata S, Lee B, Kreitman RJ, Pastan I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc Natl Acad Sci USA. 2000;97:8548-8553. [PubMed] [DOI] |
| 27. | Bera TK, Kennedy PE, Berger EA, Barbas CF, Pastan I. Specific killing of HIV-infected lymphocytes by a recombinant immunotoxin directed against the HIV-1 envelope glycoprotein. Mol Med. 1998;4:384-391. [DOI] |
| 28. | Cheng H, Liu YF, Zhang HZ, Shen WA. Construction of the recombinant retroviral vector encoding anti-HCC single-chain bifunctional antibody and establishment of a stable virus producing PA317 cell line. Shijie Huaren Xiaohua Zazhi. 2000;8:708-709. |
| 29. | Chen SY, Yang AG, Chen JD, Kute T, King CR, Collier J, Cong Y, Yao C, Huang XF. Potent antitumour activity of a new class of tumour-specific killer cells. Nature. 1997;385:78-80. [PubMed] [DOI] |
| 30. | Yang AG, Chen SY. A new class of antigen-specific killer cells. Nat Biotechnol. 1997;15:46-51. [PubMed] [DOI] |
| 31. | Dai YM. Targeting chemotherapy: a new focus in gene therapy vesearch. Shijie Hu aren Xiaohua Zazhi. 1999;7:469-472. |
| 32. | Barzon L, Bonaguro R, Castagliuolo I, Chilosi M, Gnatta E, Parolin C, Boscaro M, Palu G. Transcriptionally targeted retroviral vector for combined suicide and immunomodulating gene therapy of thyroid cancer. J Clin Endocrinol Metab. 2002;87:5304-5311. [PubMed] [DOI] |
| 33. | Indraccolo S, Habeler W, Tisato V, Stievano L, Piovan E, Tosello V, Esposito G, Wagner R, Uberla K, Chieco-Bianchi L. Gene transfer in ovarian cancer cells: A comparison between retroviral and lentiviral vectors. Cancer Res. 2002;62:6099-6107. [PubMed] |
| 34. | Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267-275. [DOI] |
| 35. | Mochizuki H, Miura M, Shimada T, Mizuno Y. Adeno-associated virus-mediated antiapoptotic gene delivery: In vivo gene therapy for neurological disorders. Methods. 2002;28:248-252. [DOI] |
| 36. | Kim SH, Lechman ER, Kim S, Nash J, Oligino TJ, Robbins PD. Ex vivo gene delivery of IL-1Ra and soluble TNF receptor confers a distal synergistic therapeutic effect in antigen-induced arthritis. Mol Ther. 2002;6:591-600. [DOI] |
| 37. | Wang F, Xia X, Hu H, Li L, Tian Y, Chen X, Huang Q. Liposome-mediated gene transfer into retina. Chunghua Yenko Tsachih. 2002;38:520-522. |
| 38. | Pan X, Pan W, Ke CW, Zhang B, Cao GW, Qi ZT. Tetracycline controlled DT/VEGF system gene therapy mediated by adenovirus vector. Shijie Huaren Xiaohua Zazhi. 2000;8:1121-1126. |
| 39. | Guo JC, Li JC, Fan DM, Qiao TD, Zhang XY. Regulation of HSP70 expression in huma n gastric cancer cell line SGC7901 by gene transfection. Shijie Huaren Xiaohua Zazhi. 1999;7:773-776. [DOI] |
| 40. | Ewert K, Ahmad A, Evans HM, Schmidt HW, Safinya CR. Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J Med Chem. 2002;45:5023-5029. |
| 41. | Hurez V, Hautton RD, Oliver J, Matthews RJ, Weaver CK. Gene delivery into primary T cells: overview and characterization of a transgenic model for efficient adenoviral transduction. Immunol Res. 2002;26:131-141. [DOI] |
| 42. | MacGregor RR, Ginsberg R, Ugen KE, Baine Y, Kang CU, Tu XM, Higgins T, Weiner DB, Boyer JD. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. AIDS. 2002;16:2137-2143. [DOI] |
| 43. | Keating SM, Bollinger RC, Quinn TC, Jackson JB, Carruth LM. Cross-clade T lymphocyte-mediated immunity to HIV Type 1: implications for vaccine design and immunodetection assays. AIDS Res Hum Retroviruses. 2002;18:1067-1079. [PubMed] [DOI] |
| 44. | Papageorgiou K, Isenberg D, Latchman D. Optimisation of herpes simplex virus-based vectors for delivery to human peripheral blood mononuclear cells. J Immunol Methods. 2002;270:235. [DOI] |
| 45. | Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarriere N. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539-546. [PubMed] [DOI] |
| 46. | Labarriere N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dreno B, Jotereau F. Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunothe. 2002;51:532-538. [PubMed] [DOI] |
| 47. | Parker C, Milosevic M, Panzarella T, Banerjee D, Jewett M, Catton C, Tew-George B, Gospodarowicz M, Warde P. The prognostic significance of the tumour infiltrating lymphocyte count in stage I testicular seminoma managed by surveillance. Eur J Cancer. 2002;38:2014. [DOI] |
| 48. | Paul S, Calmels B, Acres RB. Improvement of adoptive cellular immunotherapy of human cancer using ex-vivo gene transfer. Curr Gene Ther. 2002;2:91-100. [DOI] |
| 49. | Donskov F, Bennedsgaard KM, Von Der Maase H, Marcussen N, Fisker R, Jensen JJ, Naredi P, Hokland M. Intratumoural and peripheral blood lymphocyte subsets in patients with metastatic renal cell carcinoma undergoing interleukin-2 based immunotherapy: association to objective response and survival. Br J Cancer. 2002;87:194-201. [PubMed] [DOI] |
| 50. | Zhang JK, Sun JL, Chen HB, Zeng Y, Qu YJ. Influence of granulocyte macrophage colony stimulating factor and tumor necrosis factor on anti-hepatoma activities of human dendritic cells. World J Gastroenterol. 2000;6:718-720. [DOI] |
| 51. | Cheng H, Liu YF, Zhang HZ, Shen WA, Zhang SZ. Construction and expression of anti-HCC immunotoxin of sFv-TNF-α and GFP fusion proteins. Shijie Huaren Xiaohua Zazhi. 2001;9:640-644. |
| 52. | Zhang GQ, Yu H, Zhou XQ, Liao D, Xie Q, Wang B. TNF-αinduced apoptosis and necrosis of mice hepatocytes. Shijie Huaren Xiaohua Zazhi. 2000;8:303-306. |
| 53. | Liang WJ, Huang ZY, Ding YQ, Zhang WD. Lovo cell line apoptosis induced by cyclo heximide combined with TNFα. Shijie Huaren Xiaohua Zazhi. 1999;7:326-328. |