修回日期: 2002-11-08
接受日期: 2002-11-13
在线出版日期: 2003-04-15
探讨体外培养的肠巨噬细胞分泌TNFα的规律及地塞米松、 TNFa单克隆抗体及复方大承气汤对LPS诱导的肠巨噬细胞TNFα产生的影响.
体外分离培养大鼠肠巨噬细胞. 设对照组、脂多糖组、地塞米松处理组、TNFα单抗处理组和复方大承气汤处理组. 每组分别在3 h, 6 h, 12 h和24 h取上清液, 用放免法检测TNFα浓度. 并分别收集肠巨噬细胞, 提取RNA, 采用RT-PCR法相对定量TNFα mRNA .
肠巨噬细胞经脂多糖(LPS)诱导后, 各时相TNFα分泌及TNFmRNA表达均明显增加; 地塞米松、TNFα单克隆抗体及复方大承气汤处理后, 各时相TNFα分泌都较脂多糖(LPS) 诱导组显著下降, 各处理组TNFα mRNA表达在3 h时与脂多糖(LPS) 诱导组比较无明显差异, 6 h、12 h、24 h, 地塞米松、复方大承气汤处理组显著降低, 而TNFα单克隆抗体处理组无明显差异.
LPS是肠巨噬细胞分泌TNFα的有效激活剂, 地塞米松、复方大承气汤能从蛋白质及核酸水平抑制TNFα的产生, 而TNFα单克隆抗体只能从蛋白质水平抑制TNFα的产生.
引文著录: 陈海龙, 王辉, 李文利, 范琦. 大鼠肠巨噬细胞TNF-α表达及复方大承气汤的影响. 世界华人消化杂志 2003; 11(4): 442-445
Revised: November 8, 2002
Accepted: November 13, 2002
Published online: April 15, 2003
To explore the rule of producing TNF-a in gut macrophages through respects of gene expression and protein synthesis of TNF α and to observe the effect of dexamethasone (DEX), monocolonal antibody to tumor necrosis factor alpha (TNF α moAb) and Fufang Dachengqi Decoction compound on TNF α production.
The cultured rat gut macrophages were divided into five groups: control group (group C), lipopolysaccharide (LPS) group (group L), DEX treated group (group L+D), TNF α-MoAb treated group (group L+M), LPS plus Fufang Da Chengqi Decoction treated group (group L+F). Each group was divided into four phases: cultured for 3, 6, 12, and 24 h. The supernatants were collected and frozen at -70℃ until TNF α was determined, and the cells were used to isolate the RNA. TNF α levels were determined by radioimmunoassay method. The expression of TNF α mRNA was evaluated by RT-PCR.
The level of TNFα and expression of TNFα mRNA significantly increased at each phase in group L.The level of TNFα significantly decreased at each phase in group L+D, group L+M and group L+F compared with group L. The expression of TNFα mRNA in each treatment group had no obvious difference, compared with group L in 3 h, but group L+D and group L+F significantly decreased in 6, 12, and 24h. Group L+M still showed no obvious difference.
Gut macrophage induced by LPS can produce more TNFα through its gene expression and protein synthesis. DEX, Fufang Dachengqi Decoction can suppress the TNFα mRNA transcription and protein synthesis of TNF α; TNFα -moAb only lowered the level of TNFα protein. The three drugs have protective effects in inflammatory mediators reaction, gut barrier damage and multiple organ dysfunction syndrome (MODS).
- Citation: Chen HL, Wang H, Li WL, Fan Q. TNF-α expression and effects of Dachengqi Decoctionin compound in gut macrophages. Shijie Huaren Xiaohua Zazhi 2003; 11(4): 442-445
- URL: https://www.wjgnet.com/1009-3079/full/v11/i4/442.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i4.442
在严重感染(急性胰腺炎、急性胆道感染、外科腹内感染等)和创伤(烧伤等)应激状态下, 肠道屏障常受到损害, 产生肠源性感染和内毒素血症, 引起机体严重的全身反应和病理损害, 导致MODS而死亡[1-9]. 单核巨噬细胞系统(包括腹腔巨噬细胞、肝脏Kupffer细胞、肺巨噬细胞等)分泌的肿瘤坏死因子(TNFα)是机体炎症反应的主要致病因子. 炎症反应的正确调控成为目前研究的热点. 肠巨噬细胞是其中一部分, 也是肠道屏障的重要功能细胞. 但是单独分离出来的肠巨噬细胞分泌TNFa的规律的研究尚未见报道. 我们通过体外分离和培养大鼠肠巨噬细胞, 研究其分泌TNFα的规律, 特别是在脂多糖刺激下TNFα mRNA表达及地塞米松、TNFα单克隆抗体特别是通里攻下中药复方大承气汤对LPS诱导的肠巨噬细胞TNFα分泌及TNFa mRNA表达产生的影响, 以进一步明确肠道巨噬细胞在肠道屏障功能中的地位, 在机体全身和肠道局部炎症反应中的作用, 为临床上正确调控炎症反应, 保护肠道屏障提供理论依据.
♂SD健康大鼠, 质量 220-250 g, 由大连医科大学实验动物中心提供; RPMI 1 640培养基购自Gibco公司; 等渗细胞分离液(Percoll solution, 由1份10×Dulbecco's磷酸盐缓冲生理盐水和9份细胞分离液构成.不同浓度的Percoll溶液用1× Dulbecco's磷酸盐缓冲生理盐水稀释; 胶原酶IV及脂多糖(购自Sigma公司); RNA提取试剂盒、RT-PCR试剂盒(大连宝生物公司); 大鼠TNFa放免检测试剂盒(解放军总医院科技开发中心放免所); 复方大承气汤: 由大黄、川朴、枳实、芒硝、连翘、栀子和牡丹皮等组成, 其中大黄后下, 芒硝冲用. 由大连医科大学中西医结合急腹症研究所制备, 以原液再经抽提、过滤制成精制药液, 含生药1 kg/L.
肠巨噬细胞分离和培养参照文献[10]: 大鼠以密闭CO2笼处死, 迅速取全肠. 用冷PBS(pH = 7.4)液冲洗肠腔, 纵向剖开, 置入含1 g/L EDTA 的Hanks平衡盐溶液中, 37 ℃水浴振荡60 min. 弃上清, 用5 g/L胶原酶IV消化2 h. 所得细胞悬液以尼龙网过滤. 再以Hanks液洗涤, 重悬于500 g/L等渗细胞分离液, 离心, 2 000 r.min-1, 4 ℃, 15 min. 收集沉淀, 得肠巨噬细胞, 以无钙、镁Hanks液洗涤3遍. 用胎盘蓝染色, 细胞活力约为90%. 计数巨噬细胞, 以RPMI 1 640培养基调细胞浓度至2×108/L .加入24孔培养板, 每孔1 mL, 置于37℃, 50 mL/L CO2培养箱中培养.第1组: 对照组, 细胞上清液中加入培养基0.05 mL. 第2组: 脂多糖诱导组, 加脂多糖(LPS, 终浓度10 mg/L)0.05 mL.第3组: 地塞米松处理组, 加脂多糖(LPS, 终浓度10 mg/L)0.025 mL, 同时加入地塞米松(终浓度10-6 mmol/L)0.025 mL. 第4组: TNFα单抗处理组, 加脂多糖(LPS, 终浓度10 mg/L)0.025 mL, 同时加入TNFα单抗( 终浓度10-1 mmol/L)0.025 mL. 第5组: 复方大承气汤处理组, 加脂多糖(LPS, 终浓度10 mg/L)0.025 mL, 同时加入复方大承气汤( 终浓度1: 100)0.025 mL.每组分4个时相: 3 h, 6 h, 24 h, 每时相6个孔, 分别取上清液. -70 ℃保存, 用于检测TNFα. 细胞用于提取RNA, RT-PCR法检测TNFα mRNA. TNFα检测用放免法.
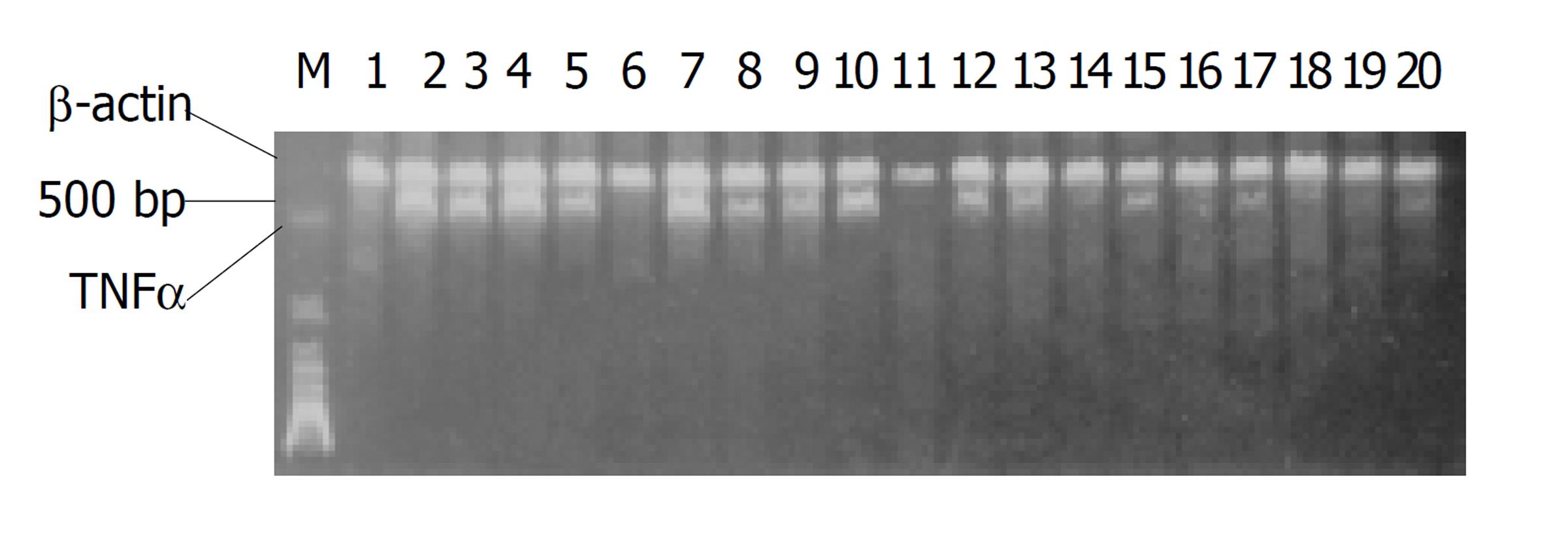
TNFα mRNA表达采用RT-PCR法. 细胞总RNA提取, 按试剂盒操作. 细胞总RNA稀释至1 g/L. 取1 mg用于合成cDNA单链. CDNA单链的合成, 反应条件: 30 ℃ 10 min, 42 ℃ 30 min, 95 ℃ 5 min, 循环一次. 产物取1 mL用于PCR反应. PCR反应TNFα 引物: P(T1) 5'-GGATCATCTTCTCAAAACTCG-3'P(T2)5'-TCACAGAGCAATGACTCCAAA-3'其扩增片段为348个碱基. β-肌动蛋白引物[11](β-actin作为内参照, 用于TNFα mRNA相对定量): P(A1)5'-TAAAGACCTCTATGCCAACAC-3'P(A2)5'-TAAAGCCATGCCAAATGTCTC-3'其扩增片段为348个碱基. 扩增步骤: 变性温度 94 ℃, 45 s, 退火温度55 ℃ , 45 s, 延伸温度72 ℃, 1 min, 循环35次后, 72 ℃ , 延伸10 min. 产物取10 mL, 16 g/L凝胶电泳. 紫外灯下照相, 胶片经密度扫描, 得TNFα带与β-肌动蛋白(β-actin)带的密度比.
统计学处理 所有数据均以均数±标准差(mean±SD)表示, 结果以SPSS10.0统计软件进行方差分析, 以P<0.05为检验显著性标准.
正常细胞(对照组)培养上清液中TNFα浓度较低, 经LPS刺激后, 各时相均明显增高(P<0.01), 3 h增加明显, 6 h达到高峰, 12 h开始下降, 24 h逐渐降低. 应用地塞米松、TNFα单克隆抗体及复方大承气汤处理后, 各时相上清液TNFα浓度均较相应的脂多糖诱导组明显降低(P<0.01, 表1).
正常细胞(对照组)无明显TNFα mRNA表达, 经LPS诱导后TNFα mRNA表达增加, 3 h增加明显, 6 h达到高峰, 12-24 h逐渐降低. DEX处理组、复方大承气汤处理组TNFα mRNA表达与LPS诱导组比较, 3 h皆无显著性差异(P>0.05), 6 h, 12 h, 24 h则明显降低(P<0.05-0.01, 图1, 表2). TNFα单克隆抗体处理组与LPS诱导组比较, 各时相皆无明显差异.
肠巨噬细胞是肠道发挥屏障功能的重要组成部分, 同时他还是机体单核-巨噬细胞系统的成员之一, 也是肠产生TNFα的主要细胞[12]. 肠巨噬细胞受到内毒素等因素攻击可以释放大量的TNFα, 除了通过内分泌形式进入血循环, 作用于肝、肺、肾等产生远隔器官损害外, 还可通过旁分泌形式对邻近肠上皮细胞发生作用, 从而影响肠道的屏障功能[13-25]. 因此, 研究肠巨噬细胞表达和分泌炎性递质的规律及中西药物影响具有重要意义. 我们发现, 肠巨噬细胞在正常情况下有低量的TNFα分泌, 无明显基因表达. 在LPS(10 mg/L)作用下, TNFα分泌及TNFα mRNA表达都明显增加, 3 h, 6 h增加明显, 6 h相对较高, 12 h, 24 h逐渐降低, 但仍高于正常水平. 表明肠道巨噬细胞在肠道屏障中具有双重作用. 必须在不同时期进行正确调控, 才能发挥其保护作用, 减少或消除其损伤作用. 目前较多应用地塞米松[26-28]、TNFα单克隆抗体[29,30]及中药来阻断TNFα损伤作用[28-34]. 本实验探讨这三种不同中西药物对LPS诱导的肠巨噬细胞表达和分泌TNF的影响. 地塞米松对LPS诱导的肠巨噬细胞TNFα分泌及基因表达具有明显的抑制作用. TNFα单克隆抗体对LPS诱导的肠巨噬细胞TNFα蛋白质分泌有明显的抑制作用, 而对基因表达无抑制作用.
复方大承气汤可抑制LPS诱导的肠巨噬细胞TNFα的分泌及基因表达. 复方大承气汤是由经典通里攻下名方大承气汤加减而成. 由大连医科大学中西医结合急腹症研究所组方配制, 广泛应用于临床的常用方剂. 主要用于治疗急性肠梗阻、急性阑尾炎、急性胰腺炎、急性腹腔感染等疾病. 有关实验表明, 此方有增强胃肠道推进运动作用, 增加肠血流量, 降低血管通透性, 以及抑菌抗感染, 减轻肠源性内毒素血症, 保护胃肠黏膜等作用[31-34]. 但此药对肠巨噬细胞及其分泌TNFα的影响, 还未见深入研究. 本实验通过复方大承气汤直接作用于体外培养的肠巨噬细胞, 结果表明, 其可以较强抑制炎性递质TNFα的分泌和mRNA的表达. 作用机制尚不完全清楚, 估计可能与下调核转录因子(NF-kappaB)及调节细胞内cGMP和cAMP的比例等有关.
总之, 这三种药物都可不同程度抑制TNFα介导的炎症反应, 但从作用环节和药理效应方面看, 地塞米松又是一个对机体免疫功能具有一定损害作用的免疫抑制药, 而且主要作用于基因转录阶段, 当炎症进展, 蛋白质已经大量表达时就显得无能为力. TNFα单克隆抗体只是对已经表达的蛋白质具有中和作用, 对于炎症反应的初始步骤基因水平的阻抑则不起作用, 而且生产工艺复杂, 价格昂贵, 广泛应用于临床尚需时日.
中药药源广泛、价廉、易得. 他的作用又是多方面多层次的, 不仅在基因转录、蛋白质表达水平, 还是在效应阶段都起作用. 另外他在整体水平上可以通过扶正祛邪、改善微循环、增强内稳态, 直接中和、拮抗抑制细菌内毒素、保护肠道屏障功能和其他脏器功能等发挥作用.如果与地塞米松、TNFα 单克隆抗体等配合应用, 将是一种很好的中西医结合形式.必将优势互补、相得益彰, 为临床上保护肠屏障, 防治MODS提供有效方法.
| 1. | Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg. 1992;216:117-134. [DOI] |
| 2. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. [PubMed] [DOI] |
| 3. | Goris RJ. MODS/SIRS: result of an overwhelming inflammatory response? World J Surg. 1996;20:418-421. [PubMed] [DOI] |
| 4. | Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:962-965. [DOI] |
| 5. | Zhao LF, Han DW. Clinical significance of endotoxemia in liver diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:391-393]. |
| 6. | Nolan JP, Camara DS. Intestinal endotoxins as co-factors in liver injury. Immunol Invest. 1989;18:325-337. [PubMed] [DOI] |
| 7. | Pugin J, Chevrolet JC. The intestine-liver-lung axis in septic syndrome. Schweiz Med Wochenschr. 1991;121:1538-1544. [PubMed] |
| 8. | Towfigh S, Heisler T, Rigberg DA, Hines OJ, Chu J, McFadden DW, Chandler C. Intestinal ischemia and the gut-liver axis: an in vitro model. J Surg Res. 2000;88:160-164. [PubMed] [DOI] |
| 9. | Koo DJ, Zhou M, Jackman D, Cioffi WG, Bland KI, Chaudry IH, Wang P. Is gut the major source of proinflammatory cytokine release during polymicrobial sepsis? Biochim Biophys Acta. 1999;1454:289-295. [DOI] |
| 10. | Ogle CK, Mao JX, Wu JZ, Ogle JD, Alexander JW. The 1994 lindberg award. The production of tumor necrosis factor, interleukin-1, interleukin-6, and prostaglandin E2 by isolated enterocytes and gut macrophages: effect of lipopolysaccharide and thermal injury. J Burn Rehabil. 1994;15:470-477. [DOI] |
| 11. | Nedel U, Zakut R, Shani M, Levy Z, Yaffe D, Neuman S. The nucleotide sequence of the rat cytoplasmicbeta-actin gene. Nucloic Acid Res. 1983;11:1759-1771. [DOI] |
| 12. | Yuan J, Xiao G, Zhou L. A study of the expression and localization of tumor necrosis factor mRNA in small intestine of rats after severe burn. Zhonghua Zhengxing Shaoshang Waike Zazhi. 1996;12:163-166. |
| 13. | Nathens AB, Rotstein OD, Dackiw AP, Marshall JC. Intestinal epithelial cells down-regulate macrophage tumor necrosis factor-alpha secretion: a mechanism for immune homeostasis in the gut-associated lymphoid tissue. Surgery. 1995;118:343-350. [DOI] |
| 14. | Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42:635-642. [PubMed] [DOI] |
| 15. | Tan X, Hsueh W, Gonzalez-Crussi F. Cellular localization of tumor necrosis factor(TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF gene expression by Paneth cells in intestinal eosinophils, and macrophages. Am J Pathol. 1993;142:1858-1865. [PubMed] |
| 16. | Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial cells are amajor site of macrophage inflammatory protein 3alpha(MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818-826. [DOI] |
| 17. | Cong B, Li SJ, Yao YX, Zhu GJ, Ling YL. Effect of cholecystokinin octapeptide on tumor necrosis factor alpha transcription and nuclear factor-kappaB activity induced by lipopolysaccharide in rat pulmonary interstitial macrophages. World J Gastrointerol. 2002;8:718-723. [DOI] |
| 18. | Fujiie S, Hieshima K, Izawa D, Nakayama T, Fujisawa R, Ohyanagi H, Yoshie O. Proinflammatory cytokines induce liver and activate regulated chemokine/macrophage inflammatory protein 3alpha/CCL20 in mucosal epithelial cells through NF- kappa B [ correction of NK-KappaB]. Int Immunol. 2002;13:1255-1263. [DOI] |
| 19. | O'Keeffe J, Lynch S, Whelan A, Jackson J, Kennedy NP, Feighery C. Flow cytometric measurement of intracellular migration inhibition factor and tumour necrosis factor alpha in the mucosa of patients with coeliac disease. Clin Exp Immunol. 2001;125:376-382. [DOI] |
| 20. | Koksoy C, Kuzu MA, Kuzui-Ergun H, Gurhan I. Role of tumor necrosis factor in lung injury caused by intestinal ischaemia-reperfusion. Br J Surg. 2001;88:464-468. [PubMed] [DOI] |
| 21. | Zareir M, Singh PK, Irvine EJ, Sherman PM, Mckay DM. Monocyte/macrophage activation by normal bacteria and bacterial products: implications for altered epithelial function in Crohn抯 disease. Am J Pathol. 2001;158:1101-1109. [DOI] |
| 22. | Woywodt A, Ludwig D, Neustock P, Kruse A, Schwarting K, Jantschek G, Kirchner H, Stange EF. Mucosal cytokine expression, cellular markers and adhesion molecules in inflammatory bowel disease. Eur J Gasteroenterol Hepatol. 1999;11:267-276. [DOI] |
| 23. | Sun Z, Olanders K, Lasson A, Dib M, Annborn M, Andersson K, Wang X, Andersson R. Effective treatment of gut barrier dysfunction using an antioxidant, a PAF inhibitor, and monoclonal antibodies against the adhesion molecule PECAM-1. J Surg Res. 2002;105:220-233. [DOI] |
| 24. | Forsythe RM, Xu DZ, Lu Q, Deitch EA. Lipopolysaccharide-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock. 2002;17:180-184. [DOI] |
| 25. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923-927. [PubMed] [DOI] |
| 26. | Beutler B, Krochin N, Milsark IW, Luede C, Cerami A. Control of cachectin ctumor necrosis factor synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977-982. [DOI] |
| 27. | Beutler B, Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987;316:379-384. [PubMed] [DOI] |
| 28. | Giroir BP. Mediators of septic shock: new approaches for interrupting the endogenous inflammatory cascade. Crit Care Med. 1993;21:780-789. [DOI] |
| 29. | Wanner GA, Muller PE, Ertel W, Bauer M, Menger MD, Messmer K. Differential effect of anti-TNF-alpha antibody on proinflammatory cytokine release by Kupffer cells following liver ischemia and reperfusion. Shock. 1999;11:391-395. [PubMed] |
| 30. | Yao YM. Protective effect of monoclonal antibody of tumor nercosis factor-alpha for vital organ in a model suffering from intestinal ischemia and reperfusion injury. Zhonghua Waike Zazhi. 1993;31:497-500. [PubMed] |
| 31. | Chen HL. Exploration on essence of yang-ming fu-shi syndrome from viewpoint of traditional Chinese medicine combined with Western medicine. Zhongguo Zhongxiyi Jiehe Zazhi. 1993;13:690-691. [PubMed] |
| 32. | Chen HL, Wu XZ, Gan FL. Protective effects of tongli gongxia herbs on gut barrier in rat with multiple organ dysfunction syndrome. Zhongguo Zhongxiyi Jiehe Zazhi. 2000;20:120-122. [PubMed] |
| 33. | Wu XZ. Traditional Chinease therapy of acute pancretitis. Shijie Huaren Xiaohua Zazhi. 2001;9:417-418. |
| 34. | Yue MX. Combined treatment of abdominal diseases with MODS by Chinese medicine. Shijie Huaren Xiaohua Zazhi. 2002;10:937-941. |