修回日期: 2002-05-01
接受日期: 2002-05-11
在线出版日期: 2003-02-15
目的: 研究丙型肝炎病毒非结构区3(HCV NS3)蛋白对正常人源肝细胞的转化及MAPK磷酸化调节的作用.
方法: 利用脂质体介导转染技术和G418筛选得到稳定表达NS3蛋白的正常人源性肝细胞QSG7701, PCR和免疫组化S-P法检测细胞中NS3的表达; 细胞记数和软琼脂实验鉴定其生物学性质; 抗磷酸化MAPK抗体和抗MAPK抗体Western blot检测转染细胞MAPK活性及表达.
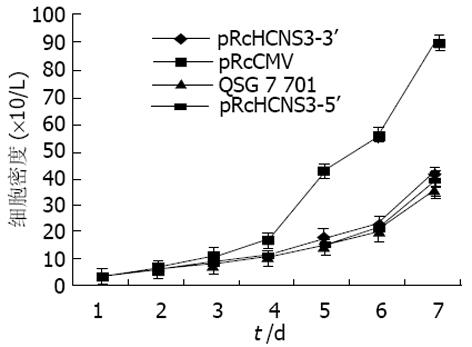
结果: HCV NS3转染的QSG7701肝细胞, 其NS3蛋白过度表达于细胞质; 真核表达质粒pRcHCNS3-5'转染细胞倍增时间为12 h, 较pRcHCNS3-3'、pRcCMV转染细胞和未转染的QSG7701明显缩短(分别24 h, 26 h和28 h). pRcHCNS3-5'、pRcHCNS3-3'和pRcCMV转染细胞及正常肝细胞在软琼脂中的克隆形成率分别为33%、1.33%、1.46%、1.11%, pRcHCNS3-5'形成的克隆明显高于其他三种细胞(P<0.01). pRcHCNS3-5'转染细胞MAPK磷酸化程度明显高于其他三种细胞(分别为8 858±877, 5 612±656, 2 212±245和989±188). (P<0.01), 而MAPK的表达量没有差异(P>0.05).
结论: 人源性正常肝细胞QSG7701是研究HCV NS3蛋白致病机制的较好的细胞系; HCV NS3 蛋白N端多肽具有促进细胞增生和改变细胞表型的作用; HCV NS3 蛋白N端多肽能上调MAPK活性, 不影响MAPK的表达量.
引文著录: 孙意, 程瑞雪, 冯德云, 欧阳小明, 郑晖. HCVNS3蛋白对正常人源肝细胞生长及MAPK磷酸化的影响. 世界华人消化杂志 2003; 11(2): 173-177
Revised: May 1, 2002
Accepted: May 11, 2002
Published online: February 15, 2003
AIM: To study effects of HCV NS3 protein on proliferation and transformation of normal human liver cell line.
METHODS: QSG7701 cells were transfected with pRcHCNS3-5' pRcHCNS3-3'and pRcCMV using liposome transfecting technique and selected with G418; Expression of HCV NS3 protein was determined by immunohistochemistry; Biological characters of transfected cells were evaluated by population doubling time and soft agar assays; activation of MAPK was analyzed by western blot.
RESULTS: QSG7701 cells transfected with pRcHCNS3-5'showed strong intracellular expression of HCVNS3 protein, and the positive signal was localized in cytoplasm. The level of expressed HCVNS3 protein in pRcHCNS3-3'transfected cells was lower than that in pRcHCNS3-5'transfected cells. The population doubling time in pRcHCNS3-5'ransfected cells (12 h) was significantly shorter than that in pRcHCNS3-3'ransfected cells (24 h), pRcCMV transfected cells (26h) and normal cells (28 h) (P < 0.01). The cells transfected with pRcHCNS3-5'showed much more anchorage independent colonies than those with pRcHCNS3-3'and pRcCMV (P < 0.01). The cloning efficiencies of transfected cells with pRcHCNS3-5' pRcHCNS3-3' pRcCMV and controls were 33%, 1.33%, 1.46%, 1.11%, respectively. The level of phosphorylated MAPK in cells with pRcHCNS3-5'was much higher than those with pRcHCNS3-3'nd cell transfected with pRcCMV and normal cells (8 858 ± 877, 5 612 ± 656, 2 212 ± 245, 989 ± 188, P < 0.01).
CONCLUSION: QSG7701 is the good human liver cell line for investigating the pathogenesis of HCV NS3 protein. 5'region of the HCV genome segment encoding NS3 is involved in cell growth and cell phenotype. N-terminal peptide of HCV NS3 protein may up-regulate the activation of MAPK.
- Citation: Sun Y, Cheng RX, Feng DY, Ouyang XM, Zheng H. Effect of HCV NS3 on proliferation and phosphorylation of MAPK in human hepatocytes. Shijie Huaren Xiaohua Zazhi 2003; 11(2): 173-177
- URL: https://www.wjgnet.com/1009-3079/full/v11/i2/173.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i2.173
丙型肝炎病毒非结构区3(HCV NS3)基因位于HCV基因组非结构区3 420-5 312核苷酸区段, 可编码含有631个氨基酸的p72蛋白. HCV NS3具有多种潜在的生物学功能, 例如蛋白酶、解旋酶活性、介导细胞免疫反应、反式激活端粒酶、调节p53活性及参与PKA (protein kinase A)和STAT(signal transducers and activators of transcription)信号转导等[1-9]. 但目前其确切的作用机制仍不十分清楚. 我们利用真核表达质粒pRcHCNS3转染人肝细胞株QSG7701, 观察HCV NS3蛋白对人肝细胞转化作用及MAPK(mitogen-activated protein kinase)信号通路的影响, 从信号转导的角度探讨HCV致癌性.
正常人源肝细胞株QSG7701购自上海细胞生物所; 真核表达质粒pRcHCNS3-5'(表达HCV NS3蛋白N多肽)和pRcHCNS3-3'(表达HCV NS3蛋白C多肽)由日本Takegami教授惠赠[6]; 空白质粒pRcCMV购于Sigma公司; 脂质体LipofectamineTM kit和G418 系Gibco公司产品; XbaⅠ及其buffer、PCR试剂盒和Marker购于华美生物工程公司; 抗HCV NS3蛋白单克隆抗体和SP免疫组化试剂盒分别购自武汉博士德公司和福州迈新生物技术公司; 抗磷酸化MAPK抗体系New England Biolabs产品, 抗MAPK抗体及ECL化学发光剂购自Santa Cruz公司; HCV NS3-5'基因序列的PCR引物 (正义: 5'-CGGGC ACGTT GTAGG CATC-3'; 反义: 5'-AACGG ACGGC TTTAG GACGA-3')由上海生工公司合成.
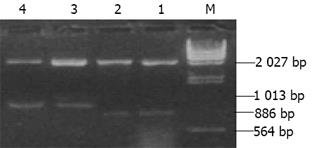
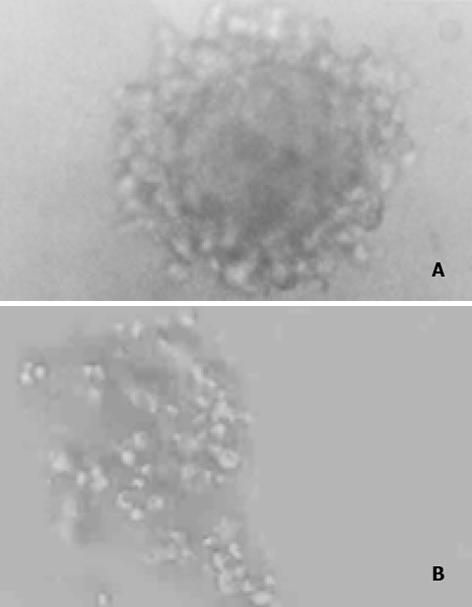
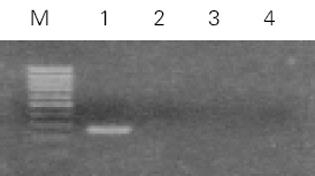
实验分为4组: 未转染QSG7701细胞组; 空白质粒pRcCMV转染细胞组; 质粒pRcHCNS3-3'转染细胞组; 质粒pRcHCNS3-5'转染细胞组. 质粒pRcCMV、pRcHCNS3-3'和pRcHCNS3-5'制备、纯化及鉴定用CaCl2法制备感受态大肠杆菌MC1061, 分别将pRcHCNS3-5'、pRcHCNS3-3'及pRcCMV导入细菌内扩增, 碱裂解法小量制备质粒, XbaI酶切, 于15g/L琼脂糖上以60 V电泳45 min, 质粒pRcHCNS3-3'和pRcHCNS3-5'分别出现1 031 bp和886 bp的电泳带, 与HCV NS3-3'基因和HCV NS3-5'基因片段大小-致(图1). 质粒经酶切鉴定后, 用碱裂解法大量抽提纯化质粒, 用于转染QSG7701细胞. HCV NS3基因导入QSG7701细胞及其克隆筛选和鉴定用Lipofectamine reagent试剂盒按产品说明书将质粒pRcCMV、pRcHCNS3-3'和pRcHCNS3-5'转染至正常人肝细胞株QSG7701, 传代于含400 mg/L G418的选择培基中筛选2 wk, 形成阳性克隆, 其中挑选质粒pRcCMV阳性克隆5个, pRcHCNS3-3'和pRcHCNS3-5'阳性克隆分别为8个和9个(图2). 抽提DNA, 并在PE480/PE DNA扩增仪上进行PCR扩增: 总反应液体积为50 ul: 亚沸水37.5 mL, 10×buffer 5 mL, 2 mmol/L dNTPs 5 mL, 25 mmol/ L引物各0.5 mL, 模板DNA 1 mL l(100 ng), Taq DNA聚合酶0.5 mL(5MU/ L); 95℃预变性90 s, 循环参数为: 94℃ 30 s, 57℃ 30 s, 72℃40 s, 35个循环后再72℃延伸5 min. PCR产物在8 g/L Agarose凝胶上以80U电压电泳30 min, 观察到pHCNS3-5'转染细胞DNA扩增出HCV特异性的257 bp片段, 电泳条带清晰, 无杂带及拖尾[6]; pRcHCNS3-3'转染细胞DNA、pRcCMV转染细胞DNA及未转染QSG7701细胞DNA均未扩出相应产物(图3). 质粒pRcHCNS3-3'和pRcHCNS3-5'转染细胞中HCV NS3蛋白表达利用S-P免疫组化方法检测质粒pRcHCNS3-5', pRcHCNS3-3'及pRcCMV转染细胞中HCV NS3蛋白的表达. 用PBS缓冲液分别代替-抗或二抗作空白对照; 用空白质粒转染细胞作阴性对照; 未转染细胞作空白对照.
1.1.1 转染细胞生物学行为的观察: (1)生长曲线的测定: 未转染细胞和转染细胞(6×104)接种于24孔培养板, 每隔24 h消化计数, 每次每组计数3孔, 取均值, 共检测7 d, 绘制生长曲线并计算出细胞的倍增时间. 实验重复3次. (2)停泊非依赖性生长实验: 未转染细胞和转染细胞以2×103 /L的密度接种于3.5 g/L的顶层琼脂中, 底层琼脂浓度为7 g/L. 连续培养4 wk, 记数>50细胞的克隆数, 求出克隆形成率(克隆形成率 = 克隆数/接种细胞数×100%). 实验重复3次.
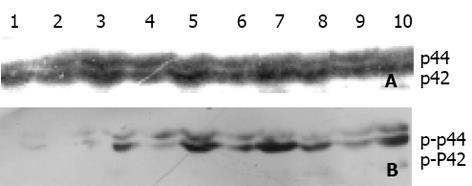
1.1.2 Western blot检测MAPK表达及其活性: 未转染和转染得到稳定克隆的QSG7701细胞, 胰酶消化后按5×105细胞/孔接种于6孔板中培养24 h, 加无血清培养基饥饿24 h使细胞生长同步化, 加含100 mL/L FCS培养基刺激细胞生长. 5 h后用冰预冷的PBS洗涤细胞2次, 每孔细胞直接裂解于样品缓冲液(62.5 mmol/L Tris-HCl, 20 g/L SDS, 100 mL/L glycerol, 50 mmol/L DTT, 1 g/L bromphenol blue)80 mL, 超声破碎5-7 s, 95-100℃煮5 min, 12 000 r/min离心5 min, 取上清分装, -70℃储存备用. 取20 mL于100 g/L SDS聚丙烯酰胺凝胶中电泳后, 转至硝酸纤维素膜上, 膜于50 g/L脱脂奶粉中封闭3 h, 然后和抗磷酸化MAPK抗体(1: 1 000稀释于脱脂奶粉中)于4℃反应过夜, 1g/L吐温20的TBS洗3次, 加入抗小鼠IgG+HRP室温孵育1h. ECL化学发光剂(Santa Cruz)检测阳性信号. 检测完磷酸化MAPK的膜在洗脱液(100 mmol/L β-ME, 20 g/L SDS, 62.5 mmol/L Tris-HCl, pH6.7)中50℃洗脱30 min, 然后用非磷酸化的MAPK单克隆抗体按上述程序检测非磷酸化的MAPK. 将所得X光片上的信号条带进行灰度扫描, 计算其积分光密度值. 实验重复3次.
统计学处理 采用F检验和t检验.
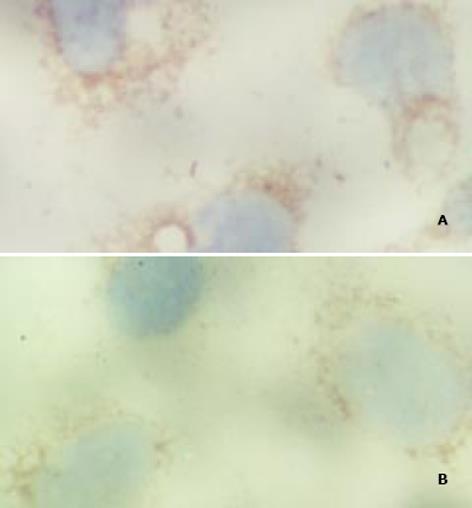
免疫组化检测显示pRcHCNS3-3'和pRcHCNS3-5'转染的细胞均呈HCV NS3蛋白阳性, 阳性信号位于细胞质. 空白对照组和阴性对照组均阴性; 阳性对照组阳性(图4)
对四种细胞分别测定生长曲线(图5), 并得到其倍增时间. 未转染细胞为28h, pRcCMV为26 h, pRcHCNS3-3'为24 h, pRcHCNS3-5'为12 h. 后者的生长速度明显增快. pRcHCNS3-5', pRcHCNS3-3', pRcCMV转染细胞及正常细胞的克隆形成率分别为33%、1.33%、1.46%、1.11%. pRcHCNS3-5'转染细胞形成的克隆明显高于其他三种细胞(P<0.01).
pRcHCNS3-5', pRcHCNS3-3', pRcCMV质粒转染细胞和未转染细胞的MAPK磷酸化经Western blot检测其值分别为8 858±877, 5 612±656, 2 212±245和989±188, 前者显著大于后3者(P<0.01), 而MAPK总蛋白的表达各组没有差异(P>0.05). pRcHCNS3-5'具有促进MAPK磷酸化的作用(图6).
QSG7701是一种永生化的正常人肝细胞. 我们利用真核表达质粒pRcHCNS3-5'和pRcHCNS3-3'转染该细胞系, 并在其强大的启动子SV40和CMV作用下成功地表达筛选标志和目的基因, 分别获得9个pRcHCNS3-5'阳性克隆和8个pRcHCNS3-3'阳性克隆, 经PCR和免疫组化证实, 目的基因在QSG7701细胞中得到稳定表达. 我们首次采用人肝细胞作为HCV NS3转染的受体细胞研究HCV的致癌性[10,11], 并获得满意结果, 这为研究HCV NS3的致癌机制建立了HCV NS3蛋白高度表达的细胞模型. HCV是一种正链RNA病毒, 无逆转录酶活性. 不能以"病毒基因组插入"方式致癌, 普遍推测HCV可能是通过其表达的蛋白质参与癌变[12-20]. HCV NS3蛋白是一种多功能蛋白, 定位于细胞质, 氨基端表现为蛋白酶, 羧基端为螺旋酶[21,22]. 我们发现表达HCV NS3蛋白N端多肽的QSG7701细胞的倍增明显时间缩短, 克隆形成率增高, 停泊非依赖性生长能力明显增强, 在-定程度上表现出肿瘤细胞的特性; 而表达HCV NS3蛋白C端多肽细胞, 阴性和空白对照组无明显差异. 表明HCV NS3蛋白N端多肽的蛋白酶功能有转化细胞表型的作用.
HCV NS3蛋白在分裂、加工病毒及其宿主细胞蛋白的过程中, 可能导致癌基因和信号转导因子的激活[2,4,5]. Ras-Raf-MAPK信号转导通路是与细胞生长和增生密切相关的主要通路, MAPK以磷酸化的方式激活, 其活性持续增高是细胞转化和癌变的关键[23-26]. 本资料显示HCV NS3蛋白N端多肽能明显上调MAPK磷酸化, 对总MAPK表达无影响, 且细胞呈现转化表型, 提示HCV NS3蛋白可能是通过激活MAPK信号转导通路促进细胞转化. HCV NS3蛋白上调MAPK磷酸化的具体机制尚不清楚, 但有关HCV NS3蛋白干扰蛋白磷酸化, 抑制cAMP依赖PKA信号转导途径的已有报道[4]. 在Ras-Raf-Mapk信号通路中, Ras和Raf间有特异性的结合位点, 对Raf的浆膜定位和活化十分重要. PKA通过对Raf-N末端丝氨酸43磷酸化, 在N末端形成帽状结构覆盖在Ras与Raf的作用位点上, 从而阻断Ras对Raf的激活[27-36]. HCV NS3蛋白1 487-1 500氨基酸存在一个富含精氨酸的序列, 该序列与PKA R亚基的底物识别位点高度同源, 作为识别信号介导PKA C亚基与HCV NS3蛋白结合, 导致了 PKA失活, 从而推测HCV NS3蛋白通过抑制PKA的负调控作用, 激活Ras-Raf-MAPK信号转导通路, 导致细胞增生和转化.
日本Kanazawa医科大学Takegami教授馈赠真核细胞表达质粒.
编辑: N/A
| 1. | Du MX, Johnson RB, Sun XL, Staschke KA, Colacino J, Wang QM. Comparative characterization of two DEAD-box RNA helicases in superfamily II: human translation-initiation factor 4A and hepatitis C virus non-structural protein 3 (NS3) helicase. Biochem J. 2002;363:147-155. [PubMed] [DOI] |
| 2. | Otsuka M, Kato N, Lan K, Yoshida H, Kato J, Goto T, Shiratori Y, Omata M. Hepatitis C virus core protein enhances p53 function through augmentation of DNA binding affinity and transcriptional ability. J Biol Chem. 2000;275:34122-34130. [PubMed] [DOI] |
| 3. | van der Most RG, Harrington LE, Giuggio V, Mahar PL, Ahmed R. Yellow fever virus 17D envelope and NS3 proteins are major targets of the antiviral T cell response in mice. Virology. 2002;296:117-124. [PubMed] [DOI] |
| 4. | Aoubala M, Holt J, Clegg RA, Rowlands DJ, Harris M. The inhibition of cAMP-dependent protein kinase by full-length hepatitis C virus NS3/4A complex is due to ATP hydrolysis. J Gen Virol. 2001;82:1637-1646. [PubMed] [DOI] |
| 5. | Heim MH, Moradpour D, Blum HE. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J Virol. 1999;73:8469-8475. [PubMed] |
| 6. | Zemel R, Gerechet S, Greif H, Bachmatove L, Birk Y, Golan-Goldhirsh A, Kunin M, Berdichevsky Y, Benhar I, Tur-Kaspa R. Cell transformation induced by hepatitis C virus NS3 serine protease. J Viral Hepat. 2001;8:96-102. [PubMed] [DOI] |
| 7. | Yang JM, Wang RQ, Bu BG, Zhou ZC, Fang DC, Luo YH. Effect of HCV infection on expression of several cancer-associated gene products in HCC. World J Gastroenterol. 1999;5:25-27. [PubMed] [DOI] |
| 8. | Mori K, Yamashita H, Nagao M, Horiguchi J, Yamawaki S. Effects of anticholinergic drug withdrawal on memory, regional cerebral blood flow and extrapyramidal side effects in schizophrenic patients. Pharmacopsychiatry. 2002;35:6-11. [PubMed] [DOI] |
| 9. | Du JH, Cha WZ. The research situation of relationship between HCV and HCC. Shijie Huaren Xiaohua Zazhi. 1999;7:176. [DOI] |
| 10. | Zhang SZ, Liang JJ, Qi ZT, Hu YP. Cloning of the non-structural gene 3 of hepatitis C virus and its inducible expression in cultured cells. World J Gastroenterol. 1999;5:125-127. [PubMed] [DOI] |
| 11. | Zhu FL, Lu HY, Li Z, Qi ZT. Cloning and expression of NS3 cDNA fragment of HCV genome of Hebei isolate in E.coli. World J Gastroenterol. 1998;4:165-168. [PubMed] [DOI] |
| 12. | Woitas RP, Petersen U, Moshage D, Brackmann HH, Matz B, Sauerbruch T, Spengler U. HCV-specific cytokine induction in monocytes of patients with different outcomes of hepatitis C. World J Gastroenterol. 2002;8:562-566. [PubMed] [DOI] |
| 13. | Levin MK, Patel SS. Helicase from hepatitis C virus, energetics of DNA binding. J Biol Chem. 2002;277:29377-29385. [PubMed] [DOI] |
| 14. | Egger D, Wölk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974-5984. [PubMed] [DOI] |
| 15. | Shi ST, Polyak SJ, Tu H, Taylor DR, Gretch DR, Lai MM. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology. 2002;292:198-210. [PubMed] [DOI] |
| 16. | Li J, Chen YF, Wang WL, Lin SG. Translocated expression of HCV core protein inhibits apoptosis in the tissue of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:579-582. [DOI] |
| 17. | Dai YM, Shou ZP, Ni CR, Wang NJ, Zhang SP. Localization of HCV RNA and capsid protein in human hepatocellular carcinoma. World J Gastroenterol. 2000;6:136-137. [PubMed] [DOI] |
| 18. | Caselmann WH, Serwe M, Lehmann T, Ludwig J, Sproat BS, Engels JW. Design, delivery and efficacy testing of therapeutic nucleic acidsused to inhibit hepatitis C virus gene expression in vitro and in vivo. World J Gastroenterol. 2000;6:626-629. [PubMed] [DOI] |
| 19. | Chen MY, Huang ZQ, Chen LZ, Gao YB, Peng RY, Wang DW. Detection of hepatitis C virus NS5 protein and genome in Chinese carcinoma of the extrahepatic bile duct and its significance. World J Gastroenterol. 2000;6:800-804. [PubMed] [DOI] |
| 20. | Zhu LX, Liu J, Li YC, Kong YY, Staib C, Sutter G, Wang Y, Li GD. Full-length core sequence dependent complex-type glycosylation of hepatitis C virus E2 glycoprotein. World J Gastroenterol. 2002;8:499-504. [PubMed] [DOI] |
| 21. | Tessmann K, Erhardt A, Häussinger D, Heintges T. Cloning and molecular characterization of human high affinity antibody fragments against Hepatitis C virus NS3 helicase. J Virol Methods. 2002;103:75-88. [PubMed] [DOI] |
| 22. | Ingallinella P, Fattori D, Altamura S, Steinkühler C, Koch U, Cicero D, Bazzo R, Cortese R, Bianchi E, Pessi A. Prime site binding inhibitors of a serine protease: NS3/4A of hepatitis C virus. Biochemistry. 2002;41:5483-5492. [PubMed] [DOI] |
| 23. | Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23:499-510. [PubMed] [DOI] |
| 24. | Kwon YW, Ueda S, Ueno M, Yodoi J, Masutani H. Mechanism of p53-dependent apoptosis induced by 3-methylcholanthrene: involvement of p53 phosphorylation and p38 MAPK. J Biol Chem. 2002;277:1837-1844. [PubMed] [DOI] |
| 25. | Rivedal E, Opsahl H. Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22:1543-1550. [PubMed] [DOI] |
| 26. | Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33-36. [PubMed] [DOI] |
| 27. | Borowski P, Kühl R, Laufs R, Schulze zur Wiesch J, Heiland M. Identification and characterization of a histone binding site of the non-structural protein 3 of hepatitis C virus. J Clin Virol. 1999;13:61-69. [PubMed] [DOI] |
| 28. | Borowski P, Heiland M, Feucht H, Laufs R. Characterisation of non-structural protein 3 of hepatitis C virus as modulator of protein phosphorylation mediated by PKA and PKC: evidences for action on the level of substrate and enzyme. Arch Virol. 1999;144:687-701. [PubMed] [DOI] |
| 29. | Borowski P, Schulze zur Wiesch J, Resch K, Feucht H, Laufs R, Schmitz H. Protein kinase C recognizes the protein kinase A-binding motif of nonstructural protein 3 of hepatitis C virus. J Biol Chem. 1999;274:30722-30728. [PubMed] [DOI] |
| 30. | Borowski P, Oehlmann K, Heiland M, Laufs R. Nonstructural protein 3 of hepatitis C virus blocks the distribution of the free catalytic subunit of cyclic AMP-dependent protein kinase. J Virol. 1997;71:2838-2843. [PubMed] |
| 31. | Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol. 2002;22:3237-3246. [PubMed] [DOI] |
| 32. | Soh JW, Lee EH, Prywes R, Weinstein IB. Novel roles of specific isoforms of protein kinase C in activation of the c-fos serum response element. Mol Cell Biol. 1999;19:1313-1324. [PubMed] [DOI] |
| 33. | Lo LW, Cheng JJ, Chiu JJ, Wung BS, Liu YC, Wang DL. Endothelial exposure to hypoxia induces Egr-1 expression involving PKCalpha-mediated Ras/Raf-1/ERK1/2 pathway. J Cell Physiol. 2001;188:304-312. [PubMed] [DOI] |
| 34. | Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963-969. [PubMed] |
| 35. | Hekman M, Hamm H, Villar AV, Bader B, Kuhlmann J, Nickel J, Rapp UR. Associations of B- and C-Raf with cholesterol, phosphatidylserine, and lipid second messengers: preferential binding of Raf to artificial lipid rafts. J Biol Chem. 2002;277:24090-24102. [PubMed] [DOI] |