修回日期: 2002-12-30
接受日期: 2003-01-03
在线出版日期: 2003-10-15
探讨溃疡性结肠炎肠黏膜内Th1型细胞分泌的细胞因子TNF-α、IL-2, Th2型细胞分泌的细胞因子IL-4、IL-10的作用.
住院重症溃疡性结肠炎患者30例, 男24例, 女6例, 年龄18-59(平均45±13)岁. 全部患者均经电子结肠镜检查及组织学检查确诊, 并连续粪培养2次排除细菌感染, 同时排除阿米巴肠病, 血吸虫病, 肠道肿瘤和内分泌疾病. 病变部位包括全结肠型15例, 乙状结肠9例, 直肠型6例; 临床类型包括复发型20例, 持续型7例和初发型3例. 急性期患者均服用强的松30 mg, 柳氮磺胺嘧啶500 mg 2次/d. 治疗8 wk后复查纤维结肠镜. 另选健康对照20名, 男14名, 女6名, 年龄22-61(平均43±12)岁, 排除胃肠道和内分泌疾病. 应用RT-PCR检测溃疡性结肠炎患者黏膜内细胞因子的表达.
溃疡性结肠炎患者急性期应用柳氮磺胺吡啶和糖皮质激素可降低TNF-α(急性期1.22±0.02, 慢性期0.78±0.08, P<0.01)、IL-2(急性期0.82±0.06, 慢性期0.47±0.04, P<0.01)的表达; 提高IL-10 (急性期0.68±0.03, 慢性期0.91±0.02, P<0.01)的表达.
溃疡性结肠炎患者以分泌Th1细胞因子为主, 存在Th1和Th2细胞因子的平衡漂移. 提示他们在溃疡性结肠炎的发生发展中起了重要作用.
引文著录: 崔海宏, 陈村龙, 杨玉捷, 张祚建, 张耀东, 崔耀升. 溃疡性结肠炎患者肠黏膜Th1/Th2类细胞因子m-RNA的表达. 世界华人消化杂志 2003; 11(10): 1524-1527
Revised: December 30, 2002
Accepted: January 3, 2003
Published online: October 15, 2003
To investigate the effects of the expression of Th1/Th2 cytokines in intestinal mucosa with ulcerative colitis.
Thirty patients (24 males, 6 females, age 18-79 years) with severe ulcerative colitis (UC) were examined by colonoscope, the diagnosis was confirmed by histological method. Bacterial infection was excluded through consecutive stool cultures twice. And ameba, schistosomiasis, gastroen-terological cancer and endocrine diseases were also excluded. Ulcerative colitis was found in omni-colon (n = 15) , sigmoid (n = 9), rectum (n = 6), respectively. Its clinical categories included relapse (n = 20), persistent (n = 7) and initial (n = 3).They were treated by Sulphasalazine (SASP) and glucocorticoid after histological diagnosis. Eight weeks later, they were re-examined by colonoscope. The expression of cytokines in the intestinal mucosa of UC patients were detected by a semi-quantitative assay, reverse transcription-polymerase chain reaction(RT-PCR) before and after treatment respectively.
Comparison with control groups, the expression of TNF-α, IL-2 was increased but IL-4 was decreased in the intestinal mucosa in acute stage. Sulphasalazine (SASP) and glucocorticoid inhibited inflammation by reducing the expression of TNF-α from 1.22±0.02 to 0.78±0.08 (P<0.01) and of IL-2 from 0.82±0.06 to 0.47±0.04 (P<0.01), and elevate the expression of IL-10 from 0.68±0.03 to 0.91±0.02(P<0.01).
There is an imbalance of Th1 and Th2 phenotype cytokine in patients with ulcerative colitis.
- Citation: Cui HH, Chen CL, Yang YJ, Zhang ZJ, Zhang YD, Cui YS. Expression of Th1/Th2 cytokines in intestinal mucosa of ulcerative colitis. Shijie Huaren Xiaohua Zazhi 2003; 11(10): 1524-1527
- URL: https://www.wjgnet.com/1009-3079/full/v11/i10/1524.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i10.1524
溃疡性结肠炎(UC)的发病机制仍未明确. 目前认为与遗传因素、细菌的感染、机体免疫反应、环境因素及精神因素有关[1-6], 其中肠道免疫系统调节异常可能在其发生发展中起重要作用. 研究表明在UC患者体内促炎症因子水平升高, 抑炎症细胞因子水平降低. 当前他的治疗仍以糖皮质激素类、柳氮磺胺类和免疫抑制剂等药物为主, 检测细胞因子表达的变化, 旨在从分子水平上探讨UC发病机制.
重症急性期住院治疗UC患者30例, 均经纤维结肠镜和病理学检查确诊. 男24例, 女6例, 年龄18-79 (45±13)岁, 平均45岁, 15例为全结肠型, 9例为乙状结肠型, 6例为直肠炎型. 诊断符合标准[7], 根据病情分为急性期和缓解期, 急性期患者均服用强的松30 mg , 柳氮磺胺嘧啶500 mg 2次/d. 治疗8 wk后复查纤维结肠镜. 对照组为健康自愿者, 男14例, 女6例. 年龄22-61(平均43±12)岁. 每例患者各取50 mg活检组织, 并立即贮存在液氮中备用.
RT-PCR半定量法检测TNF-α、IL-2、IL-4、IL-10 的表达. TNF-α、IL-2, IL-4, IL-10引物的设计参照文献[8]和GeneBank序列, 确定的序列交上海生工公司合成(表1). 提取总RNA采用异硫氰酸胍二步法. RNA沉淀溶1 g/L DEPC水中, 采用紫外分光光度计测RNA浓度. A260/A280比值需在1.8-2.0, 并稀释至1 g/L. 在20 mL逆转录体系中加入模板RNA 1 μL, 总RNA达0.1-1.0 μg, Oligo (dT) 18 (0.2 g/L) 4 μL, 用DEPC处理的双蒸水补足体积至15.5 μL. 于70 °C加热5 min. 迅速置冰上, 短暂离心. 上述微量离心管内加入: 10×反应缓冲液2 μL, 4×d NTP mix (10 mmol/L each) 1 μL, RNA酶抑制剂(40 ku/L) 0.5 μL, M-MuLV逆转录酶(200 ku/L)1 μL, 38 °C恒温60 min, 进行逆转录反应. 70 °C加热10 min, 灭活逆转录酶活性. 取逆转录产物10 μL作PCR扩增. PCR参数为94 °C 45 s, 56 °C 45 s, 72 °C 1 min, 30循环, 最后72 °C延长6 min, 指标均以G3PDH作内参照. PCR产物5 μL作20 g/L琼脂糖凝胶电泳, 溴化乙淀染色, 用图像分析仪进行分析.
| 引物 | 序列 | 扩增片段(bp) | 循环次数 |
| G3PDH | S ACCACAGTCCATGCCATCAC | 452 | 29 |
| A TCCACCACCCTGTTGCTGTA | |||
| TNF-α | S ATGAGCACTGAAAGCATGATCCGG | 695 | 30 |
| A GCAATGATCCCAAAGTAGACCTGCCC | |||
| IL-2 | S CATTGCACTAAGTCTTGCACTTGTCA | 305 | 30 |
| A CGTTGATATTGCTGATTAAGTCCCTG | |||
| IL-4 | S CGGCAACTTTGACCACGGACACAAGTGCGATA | 344 | 30 |
| A ACGTACTCTGGTTGGCTTCCTTCACAGGACAG | |||
| Il-10 | S AAGCTGAGAACCAAGACCCAGACATCAAGGCG | 328 | 30 |
| A AGCTATCCCAGAGCCCCAGATCCGATTTTGG |
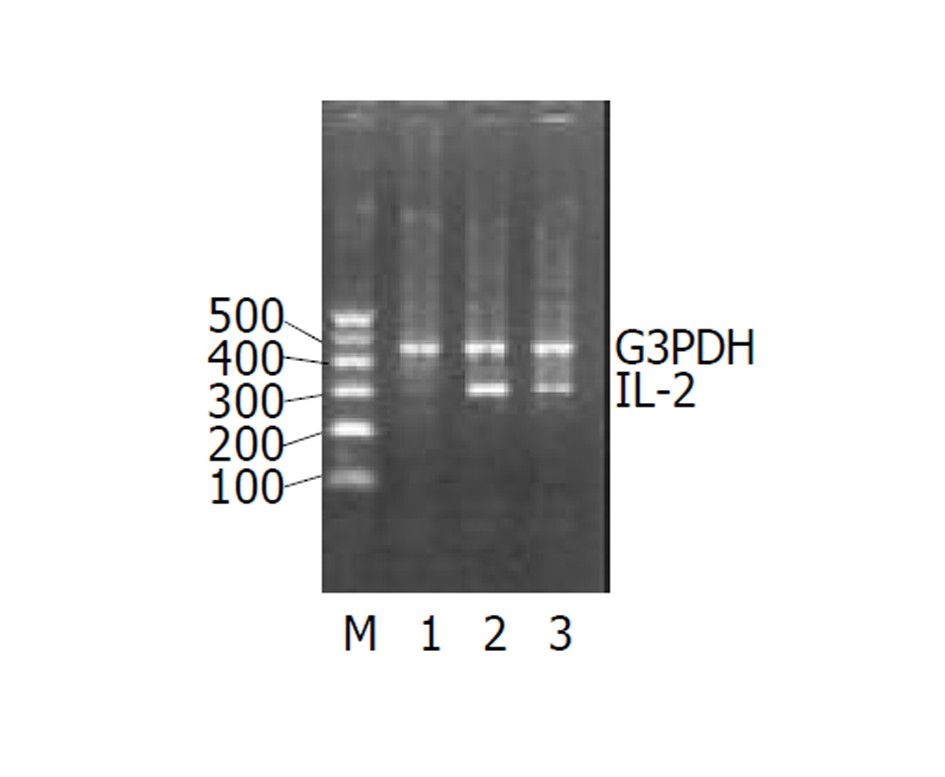
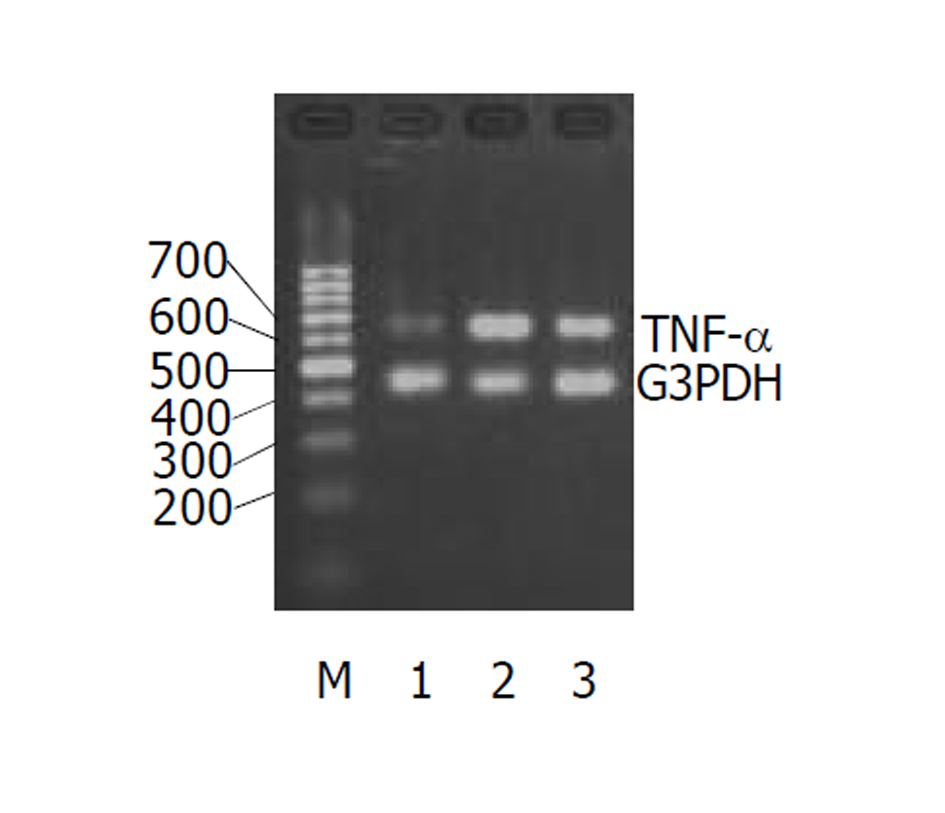
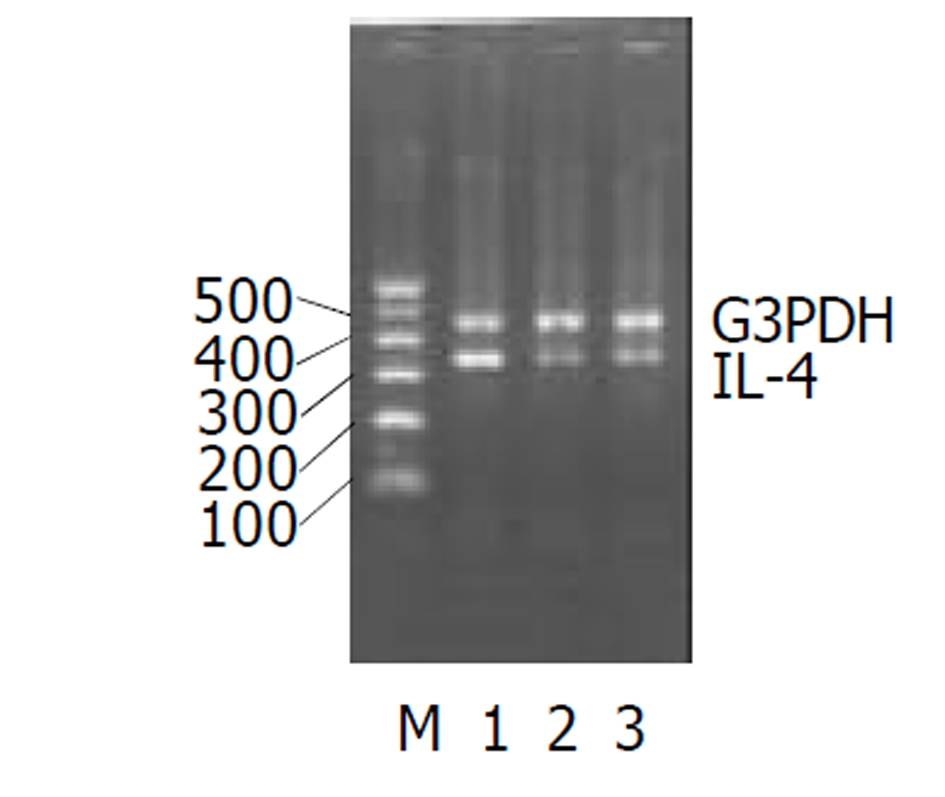
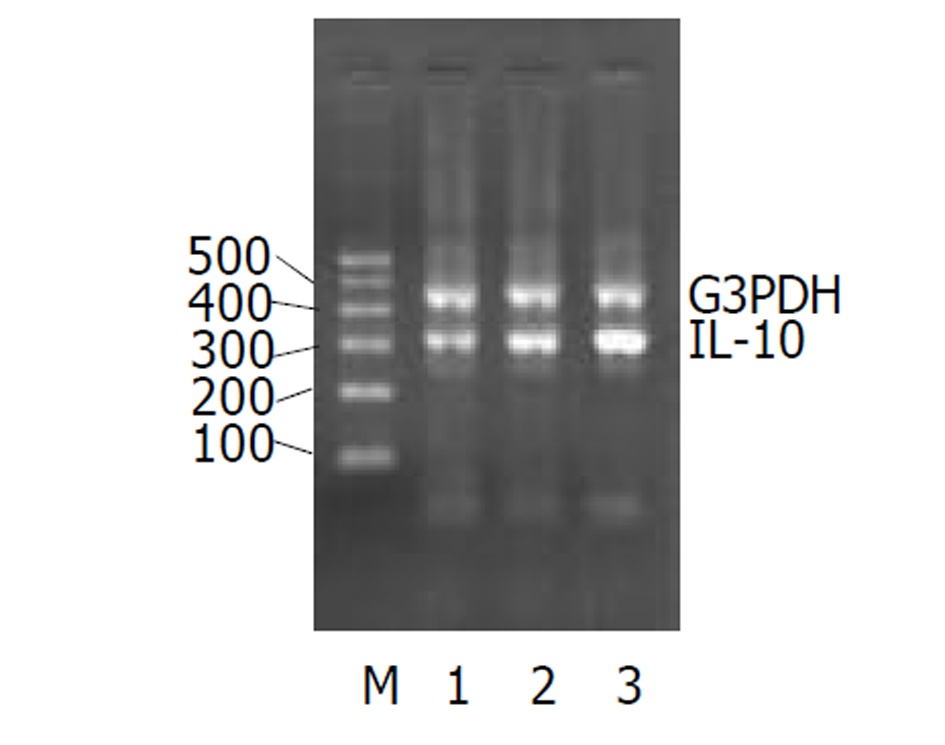
UC急性期IL-2、TNF-α的(图1-2)表达较正常对照组均显著增加, IL-4图3表达减少. 经过柳氮磺胺吡啶和糖皮质激素治疗后, 二者的表达明显下降, 而IL-10显著增加图4(表2).
UC患者由肠道细菌等因素激活核因子[9,10], 可使肠道的黏膜细胞、固有层淋巴细胞和浸润的炎症细胞产生大量细胞因子, 直接或间接造成组织损害[11,12]. 炎症发生时, 先激活的前炎症因子如: IL-1β, TNF-α, IL-2, IFN-α等. 越来越多的证据显示, UC的发生是由多因素共同作用的结果. 免疫因素是重要的因素之一. 细胞因子在调节肠道免疫中扮演重要角色[13-16], 有诱导炎症(如: TNF-α, IL-1β, IL-6, IL-8, IL-12等)和抗炎(如: IL-10, IL-4, IL-5等)作用. 对于UC患者Th1和Th2细胞因子的改变, 研究者们意见不一. Sawa et al [17]通过实时RT-PCR检测患者肠道中UC患者肠道各种细胞因子mRNA的扩增, 发现Th1和Th2细胞因子IL-1β, IL-4, IL-5, IL-8, IL-10, IL-12p40, IFN-γ and TNF-α等mRNA表达都明显比正常对照组高, 这可能说明体液免疫和细胞免疫同时作用于UC的发病过程. Lakatos et al [18]认为UC患者体内以Th2细胞因子增多为主. Hata et al [19]用表达HLA-27的转基因大鼠在SP条件下模仿人的UC模型. 8 wk后用RT-PCR的方法检测细胞因子发现: Th1细胞因子IFN-α, IL-2等明显高于Th2细胞因子如IL-10, IL-4等. Sun et al [20]通过检测三硝基苯硫甲酸诱导的大鼠结肠炎中的细胞因子的变化发现Th1细胞分泌的细胞因子在炎症的发生发展中推持炎症细胞的浸润和活化、引起组织损伤. 这些细胞因子的表达有时间依赖性. TNF-α是一种前炎症因子可诱导多种细胞产生炎症因子. 特别有炎症发生的初期, 参与UC炎症反应和免疫应答. IL-2是由IL-1激活T细胞, 促进IL-2受体的表达分泌的. IL-2可剌激T细胞和B细胞的增值, 引起体液免疫反应. IL-2还可帮助激活炎症部位的巨噬细胞的活化. 他们同属于Th1型细胞因子. Garrelds et al [23]用IL-2缺陷的小鼠制成UC模型发现, 24 wk后, IL-2缺陷的小鼠比没缺陷的小鼠的肠黏膜中IL-1β明显增高可能是因为细胞毒性T细胞参与了UC的发生和发展过程. 然而IL-6和IL-10的浓度没有明显变化. 石蜡包埋的小鼠脾脏中, CD8+细胞明显下降. 我们的实验结果表明在炎症的急性期TNF-α, IL-2明显上升. 经柳氮磺胺吡啶和糖皮质激素治疗后, 有所下降, 可能是阻断了TNF-α, IL-2mRNA的表达[24-26], 可做为疾病治疗的观测指标.
大量研究资料证实IL-4能抑制单核巨噬细胞分泌氧自由基的能力, 而且存在剂量效应关系. IL-4还能抑制前列腺素E2和IL-8的产生. 而且, IL-4能诱导IL-1β产生, 提高IL-1α/IL-1β的比例. 许多实验发现UC患者的IL-4分泌细胞数减少, IL-4mRNA表达及蛋白分泌明显减少[27-30]. 我们的实验结果显示, UC组IL-4水平显著低于正常对照组, 提示IL-4与UC的发病有关, 而且可作为监测疾病程度的一个指标. 此外经药物治疗后, 缓解组与未缓解组间IL-4水平无差别. IL-10在黏膜免疫中是一种重要的细胞因子调节剂. IL-10基因敲除的大鼠易患结肠炎症. 早期用抗生素治疗可以缓解炎症的程度[31]. 本实验发现急性期炎症IL-10表达有所增加, 缓解期后IL-10仍持续增高. 可能是由于炎症刺激Th2型细胞分泌细胞因子引起增多. 现在人们开始尝试用IL-10来冶疗UC来逆转肠道的症状[32,33], IL-10缺陷可能做为UC的发病机制之一引起严重的症状[34].
目前, UC的发病机制尚未搞清, 他是由多因素导致的结果. 但免疫反应在UC的发生发展中起了重要作用. Th1和Th2型细胞因子存在分泌偏移. 研究这些细胞因子可了解他们在UC发病中的作用, 并可做为临床治疗的监测指标.
| 2. | Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237-242. [PubMed] [DOI] |
| 3. | Schwab M, Schaeffeler E, Marx C, Fromm MF, Kaskas B, Metzler J, Stange E, Herfarth H, Schoelmerich J, Gregor M. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology. 2003;124:26-33. [PubMed] [DOI] |
| 4. | Su C, Lichtenstein GR. Recent developments in inflammatory bowel disease. Med Clin North Am. 2002;86:1497-1523. [DOI] |
| 5. | Williams JP, Meyers JA. Immune-mediated inflammatory disorders (I.M.I.D.s): the economic and clinical costs. Am J Manag Care. 2002;8:S664-681. [PubMed] |
| 6. | Karban A, Eliakim R, Brant SR. Genetics of inflammatory bowel disease. Isr Med Assoc J. 2002;4:798-802. [PubMed] |
| 7. | Jiang XL, Quan QZ, Wang ZK. Diagnosis, typing and effect criteria of ulcerative colitis. Shijie Huaren Xiaohua Zazhi. 2000;8:332-334. |
| 8. | Sun KH, Yu CL, Tang SJ, Sun GH. Monoclonal anti-double-stranded DNA autoantibody stimulates the expression and release of IL-1beta, IL-6, IL-8, IL-10 and TNF-alpha from normal human mononuclear cells involving in the lupus pathogenesis. Immunology. 2000;99:352-360. [PubMed] [DOI] |
| 9. | Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034-1041. [PubMed] [DOI] |
| 10. | MacDonald TT, Pettersson S. Bacterial regulation of intestinal immune responses. Inflamm Bowel Dis. 2000;6:116-122. [PubMed] [DOI] |
| 11. | Stevceva L. Cytokines and their antagonists as therapeutic agents. Curr Med Chem. 2002;9:2201-2207. [DOI] |
| 12. | Ardizzone S, Porro GB. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med. 2002;252:475-496. [PubMed] [DOI] |
| 13. | Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820-2828. [PubMed] [DOI] |
| 14. | Rutgeerts P, Geboes K. Understanding inflammatory bowel disease-the clinician's perspective. Eur J Surg Suppl. 2001;586:66-72. [DOI] |
| 15. | Ikeda Y, Akbar SM, Matsui H, Onji M. Antigen-presenting dendritic cells in ulcerative colitis. J Gastroenterol. 2002;37:53-55. [PubMed] [DOI] |
| 16. | Bamias G, Marini M, Moskaluk CA, Odashima M, Ross WG, Rivera-Nieves J, Cominelli F. Down-regulation of intestinal lymphocyte activation and Th1 cytokine production by antibiotic therapy in a murine model of Crohn's disease. J Immunol. 2002;169:5308-5314. [DOI] |
| 17. | Sawa Y, Oshitani N, Adachi K, Higuchi K, Matsumoto T, Arakawa T. Comprehensive analysis of intestinal cytokine messenger RNA profile by real-time quantitative polymerase chain reaction in patients with inflammatory bowel disease. Int J Mol Med. 2003;11:175-179. [DOI] |
| 18. | Lakatos L. Immunology of inflammatory bowel diseases. Acta Physiol Hung. 2000;87:355-372. [PubMed] |
| 19. | Hata K, Andoh A, Sato H, Araki Y, Tanaka M, Tsujikawa T, Fujiyama Y, Bamba T. Sequential changes in luminal microflora and mucosal cytokine expression during developing of colitis in HLA-B27/beta2-microglobulin transgenic rats. Scand J Gastroenterol. 2001;36:1185-1192. [PubMed] [DOI] |
| 20. | Sun FF, Lai PS, Yue G, Yin K, Nagele RG, Tong DM, Krzesicki RF, Chin JE, Wong PY. Pattern of cytokine and adhesion molecule mRNA in hapten- induced relapsing colon inflammation in the rat. Inflammation. 2001;25:33-45. [DOI] |
| 21. | Sandborn WJ. Strategies for targeting tumour necrosis factor in IBD. Best Pract Res Clin Gastroenterol. 2003;17:105-117. [DOI] |
| 22. | Su C, Salzberg BA, Lewis JD, Deren JJ, Kornbluth A, Katzka DA, Stein RB, Adler DR, Lichtenstein GR. Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitis. Am J Gastroenterol. 2002;97:2577-2584. [PubMed] [DOI] |
| 23. | Garrelds IM, Heiligers JP, Van Meeteren ME, Duncker DJ, Saxena PR, Meijssen MA, Zijlstra FJ. Interleukin-2-Deficient mice: effect on cytokines and inflammatory cells in chronic colonic disease. Dig Dis Sci. 2002;47:503-510. [DOI] |
| 24. | Hasko G, Szabo C, Nemeth ZH, Deitch EA. Sulphasalazine inhibits macrophage activation: inhibitory effects on inducible nitric oxide synthase expression, interleukin-12 production and major histocompatibility complex II expression. Immunology. 2001;103:473-478. [DOI] |
| 25. | Oshima T, Pavlick K, Grisham MB, Jordan P, Manas K, Joh T, Itoh M, Alexander JS. Glucocorticoids and IL-10, but not 6-MP, 5-ASA or sulfasalazine block endothelial expression of MAdCAM-1: implications for inflammatory bowel disease therapy. Aliment Pharmacol Ther. 2001;15:1211-1218. [DOI] |
| 26. | Vohra P. Inflammatory bowel disease. Indian J Pediatr. 2000;67:747-756. [DOI] |
| 27. | Klein W, Tromm A, Griga T, Fricke H, Folwaczny C, Hocke M, Eitner K, Marx M, Duerig N, Epplen JT. Interleukin-4 and interleukin-4 receptor gene polymorphisms in inflammatory bowel diseases. Genes Immun. 2001;2:287-289. [PubMed] [DOI] |
| 28. | Bisping G, Lugering N, Lutke-Brintrup S, Pauels HG, Schurmann G, Domschke W, Kucharzik T. Patients with inflammatory bowel disease (IBD) reveal increased induction capacity of intracellular interferon-gamma (IFN-gamma) in peripheral CD8+ lymphocytes co-cultured with intestinal epithelial cells. Clin Exp Immunol. 2001;123:15-22. [DOI] |
| 29. | Rogy MA, Beinhauer BG, Reinisch W, Huang L, Pokieser P. Transfer of interleukin-4 and interleukin-10 in patients with severe inflammatory bowel disease of the rectum. Hum Gene Ther. 2000;11:1731-1741. [PubMed] [DOI] |
| 30. | Schmit A, Van Gossum A, Carol M, Houben JJ, Mascart F. Diversion of intestinal flow decreases the numbers of interleukin 4 secreting and interferon gamma secreting T lymphocytes in small bowel mucosa. Gut. 2000;46:40-45. [DOI] |
| 31. | Madsen KL. Inflammatory bowel disease: lessons from the IL-10 gene-deficient mouse. Clin Invest Med. 2001;24:250-257. [PubMed] |
| 32. | Ishizuka K, Sugimura K, Homma T, Matsuzawa J, Mochizuki T, Kobayashi M, Suzuki K, Otsuka K, Tashiro K, Yamaguchi O. Influence of interleukin-10 on the interleukin-1 receptor antagonist/interleukin-1 beta ratio in the colonic mucosa of ulcerative colitis. Digestion. 2001;63:22-27. [PubMed] [DOI] |
| 33. | Bristol IJ, Farmer MA, Cong Y, Zheng XX, Strom TB, Elson CO, Sundberg JP, Leiter EH. Heritable susceptibility for colitis in mice induced by IL-10 deficiency. Inflamm Bowel Dis. 2000;6:290-302. [DOI] |
| 34. | Kanauchi O, Mitsuyama K, Araki Y, Andoh A. Anti-TNF therapy for crohn's disease. Curr Pharm Des. 2003;9:289-294. [DOI] |