Published online Jun 7, 2022. doi: 10.3748/wjg.v28.i21.2291
Peer-review started: December 13, 2021
First decision: January 23, 2022
Revised: February 1, 2022
Accepted: April 25, 2022
Article in press: April 25, 2022
Published online: June 7, 2022
Clinical manifestations and progression of primary sclerosing cholangitis (PSC) are heterogeneous, and its pathogenesis is poorly understood. The importance of gut-liver interactions in the pathogenesis has been clinically confirmed and highlighted in different theories. Recent advances regarding biomarkers of biliary-gut crosstalk may help to identify clinically relevant PSC subgroups assisting everyday clinical work-up (e.g., diagnosis, disease stratification, or surveillance) and the exploration of potential therapeutic targets. Alkaline phosphatase produced by the biliary epithelium is consistently associated with prognosis. However, its level shows natural fluctuation limiting its use in individual patients. Inflammatory, cell activation, and tissue remodeling markers have been reported to predict clinical outcome. Elevated immunoglobulin (Ig) G4 level is associated with a shorter transplantation-free survival. IgG type atypical perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCAs) are non-specific markers of various autoimmune liver diseases and may reflect an abnormal B-cell response to gut microbial antigens. IgG type atypical P-ANCA identifies PSC patients with particular clinical and genetic (for human leukocyte antigens) characteristics. The presence of IgA type anti-F-actin antibody (AAA) may predict a progressive disease course, and it is associated with enhanced mucosal immune response to various microbial antigens and enterocyte damage. IgA type anti-glycoprotein 2 (GP2) antibodies identify patients with a severe disease phenotype and poor survival due to enhanced fibrogenesis or development of cholangiocarcinoma. Elevated soluble vascular adhesion protein-1 (sVAP-1) level is associated with adverse disease outcomes in PSC. High sVAP-1 levels correlate with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression in the liver that contributes to gut activated T-cell homing to the hepatobiliary tract. In the present paper, we review the evidence on these possible serological markers that could potentially help address the unmet clinical needs in PSC.
Core Tip: Recent advances in biomarker research may help clinicians identify relevant subgroups of primary sclerosing cholangitis (PSC) and assist everyday clinical work-up. However, a diagnostic biomarker is still an unmet need. On the other hand, several biomarkers have been reported to predict outcome in PSC; however, most of them have not been validated by subsequent studies. The IgA type anti-glycoprotein 2 antibody is the first one to be supported by a satisfactory number of clinical studies and could be incorporated into clinical practice. These discoveries also reveal different aspects of PSC providing with potential therapeutic targets.
- Citation: Tornai D, Ven PL, Lakatos PL, Papp M. Serological biomarkers for management of primary sclerosing cholangitis. World J Gastroenterol 2022; 28(21): 2291-2301
- URL: https://www.wjgnet.com/1007-9327/full/v28/i21/2291.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i21.2291
Primary sclerosing cholangitis (PSC) is a chronic and progressive inflammatory disease of the bile ducts, leading to the formation of intermitting bile duct strictures and dilatations, with periductal fibrosis that in most cases progresses to cirrhosis and decompensated disease stage. Moreover, PSC is associated with the development of cholangiocarcinoma (CCA) and colorectal cancer[1]. The etiology of PSC is poorly understood. However, the gut-liver interaction seems to be a driving factor in its development. It has been hypothesized that the interaction of the gut microbiome and certain genetically determined factors [such as certain human leukocyte antigens (HLA)] leads to the development of disrupted immunological pathways, as a result of an autoimmune reaction to yet unknown antigen(s). Most ab
The diagnosis of PSC is difficult, and no biomarker specific to the disease has been identified so far. In the presence of chronic cholestasis, the diagnosis is based on magnetic resonance cholangiography (MRCP) or, much less frequently today, endoscopic retrograde cholangiopancreatography (ERCP) with confirmation of biliary lesions and strictures. Careful exclusion of known causes of secondary sclerosing cholangitis (SSC) is also necessary. Immunoglobulin (Ig) G elevation and the presence of atypical perinuclear ANCA (P-ANCA) in serum are common but are not disease-specific serological alterations[3]. Routine liver biopsy is no longer routinely performed in the diagnosis of PSC and is only necessary in suspected cases of small duct PSC, when the cholangiogram is normal, or to confirm overlap syndrome with autoimmune hepatitis.
The disease course of PSC is variable. There are a few promising biomarkers to predict disease activity and progression, yet almost none has been validated in subsequent studies. A recently discovered serological biomarker [IgA type antibodies against pancreatic glycoprotein 2 (anti-GP2 IgA)][4,5] is the first that has predictive value in PSC confirmed in multiple studies[6,7]. However, it has not been integrated into clinical practice. Consequently, the question of accurate risk assessment, disease stratification, and follow-up strategy for patients with PSC is still unresolved[8].
Studying biomarkers characterizing the dialogue between the biliary tract and the intestine in PSC may not only identify clinically relevant subgroups for disease stratification, but can also unravel new pathogenesis-relevant correlations. This could in turn lead to the discovery of new therapeutic targets.
While biomarkers from other body fluids (e.g., bile) can reveal important aspects of the pathogenesis, blood is more readily available for clinicians and can be obtained in a highly controlled fashion (in contrast to, e.g., stool). This makes serological biomarkers the most appropriate for everyday clinical use, therefore these are the markers that we focus on in this review.
Various clinical studies have consistently reported a correlation between alkaline phosphatase (ALP) levels and disease progression, but its individual application is difficult. In PSC, the fluctuation pattern of ALP is different from that observed in primary biliary cholangitis, and the degree of elevation is influenced by the development of various biliary complications (cholangitis, gallstones, or the appearance of a dominant stricture). Thus, it is not surprising that the ALP thresholds or values of change associated with disease outcomes in certain studies were not confirmed by other studies[9]. However, certain studies highlighted the importance of ALP fluctuation in CCA development free survival. The risk of developing CCA was significantly higher in patients with constantly high ALP levels without reduction[10-13]. Thus, further studies are needed to determine the significance of ALP level variation also in transplant free survival (due to progression of fibrosis) and treatment response.
In the absence of individual predictive markers, researchers tried to develop predictive models incorporating multiple markers. These scoring systems, however, also contain clinical parameters besides serological markers. The first and still the most widely used clinical prognostic model is the Mayo risk score (MRS), which, as modified in 2000, no longer includes invasive or subjective parameters[14]. Abnormal value of the factors included in the MRS [serum bilirubin, albumin, and aspartate aminotransferase (AST) levels, and esophageal variceal bleeding], with the exception of age, represents advanced liver disease. Thus, the discriminatory potential of this scoring system is inadequate in the early stages of PSC. In addition, the predictable time period for disease progression is only around 4 years. The model was also not able to predict the adverse disease outcome associated to high-dose ursodeoxycholic acid treatment (28-30 mg/kg/d) either[15].
The Amsterdam-Oxford model (AOM) is another prognostic scoring system for PSC[16], which includes seven objective parameters (PSC subtype, age at diagnosis, platelet count, serum albumin, ALP, AST, and bilirubin levels). This scoring system was developed to overcome the limitations of MRS in the prediction of transplant-free survival. It can be used to estimate a longer-term prognosis of PSC (15 years) and can be recalculated at a later timepoint. However, the discriminative power of the model was only moderate (C-statistic: 0.68). In contrast, it has the advantage of being much more widely applicable (the reliability of the model was consistent in the two populations and at different timepoints) as it was developed using population-based data rather than data from transplant centers.
The UK-PSC scores were also developed to predict transplant-free survival[17]. Two models were created for 2-year and for 10-year prediction, derived from a cohort of 1001 PSC patients and validated in two independent cohorts adding up to 451 patients. The incorporated variables were serum bilirubin, ALP, albumin, platelets, presence of extrahepatic biliary disease, and variceal hemorrhage. “Both short- and long-term UK-PSC risk scores had better performance than MRS and AST-to-platelet ratio index when predicting outcomes [C-statistics for 2-year survival (short-term) were 0.81 vs 0.75 and 0.81 vs 0.63, respectively; for 10-year survival (long-term) were 0.80 vs 0.79 and 0.80 vs 0.59, respectively].”
PSC risk estimate tool (PREsTo) was developed to predict the advent of hepatic decompensation (ascites, variceal hemorrhage, or encephalopathy). The model was derived from data of a multicenter North American cohort and validated in an international multicenter cohort including 509 and 278 subjects, respectively. Individuals with advanced PSC or CCA at baseline were excluded. PREsTo incorporates nine variables: Bilirubin, albumin, serum ALP times the upper limit of normal, platelets, AST, hemoglobin, sodium, patient age, and number of years since the diagnosis of PSC. In predicting hepatic decompensation, PREsTo performed better than model of end stage of liver disease (MELD) score and MRS (C-statistics: 0.90 vs 0.72 and 0.90 vs 0.85, respectively)[18]. The equation is not provided, and only an online calculator is available.
The enhanced liver fibrosis (ELF) test (Siemens ADVIA Centaur) is based on purely serological measurements summarizing three direct components of fibrogenesis (hyaluronic acid, tissue inhibitor of metalloproteinase 1, and type III procollagen amino-terminal propeptide). It has been reported to be a strong predictor of prognosis (mortality and liver transplantation) in PSC independently of MRS. Validation of this result was successful in multiple studies (C-statistic: 0.79-0.81)[19,20]. In a recent study, the ELF score slightly increased in PSC patients over a 5-year follow-up period and showed low intrapersonal variability supporting the consistency of this score[21].
About 10% of patients have elevated serum IgG4 levels without IgG4-associated disease. Recently, IgG4 determination is recommended at least once in all patients with PSC[22]. Elevated serum IgG4 levels (> 140 mg/dL) in PSC patients were associated with higher values of liver function tests, higher MRS, and shorter time to liver transplantation, which clearly indicates a more severe disease course in this subgroup of patients[23]. It is unknown whether the addition of a systemic glucocorticoid to treatment in the high IgG4 subtype of PSC may be beneficial in curbing disease progression[24].
Various other serum autoantibodies have been described in PSC patients; however, they are considered unspecific, since they may be present also in other diseases. The most frequently observed antibodies in PSC are ANCAs, but antinuclear antibodies and anti-smooth muscle antibodies were also reported to be frequently associated with PSC[25].
The occurrence of IgG isotype atypical P-ANCAs in PSC are common (up to 80% of cases). The putative role of intestinal bacteria in ANCA formation (homology between human beta-tubulin isotype 5 and bacterial FtsZ protein, molecular mimicry) has been described[26]. Early, small scale, case-control clinical studies[27] found no correlation between ANCA formation and clinical or genetic features of PSC. Hov et al[28], however, in a large comprehensive case-control study, demonstrated that risk genes with a strong association to PSC formation (HLA-B*08 and DRB1*03) were also associated with ANCA formation. Interestingly, association between the presence of PSC related HLA alleles (B*08, C*07, and DRB1*03) and increased risk of acute rejection syndrome in liver transplant recipients has also been reported (while DRB1*04 was found to be a protecting factor)[29].
The antibody against serine proteinase-3 (PR3) is a type of cytoplasmic ANCA (C-ANCAs). PR3-ANCA is typically observed in small vessel vasculitis[30] but recently has been reported in ulcerative colitis (UC)[31], which prompted researchers to investigate this antibody in PSC as well. Interestingly, in the setting of PSC, PR3-ANCA seems to be associated with worse liver biochemistry rather than with a co-diagnosis of UC[32]. In a recent study by Wunsch et al[7], besides worse liver function tests and MELD score, PR3-ANCA has been shown to be associated with CCA development [hazard ratio (HR) = 9.8, 95% confidence interval (CI) = 1.3-74.5; P = 0.03] and risk of shorter transplant free survival (HR = 1.8; 95%CI = 1.3-2.8; P = 0.01). However, the predictive capacity of PR3-ANCA was not confirmed in the validation cohort where the frequency of PR3-ANCA was found to be surprisingly low (5% in contrast to 54% in the discovery cohort) and CCA development was also less prevalent[7].
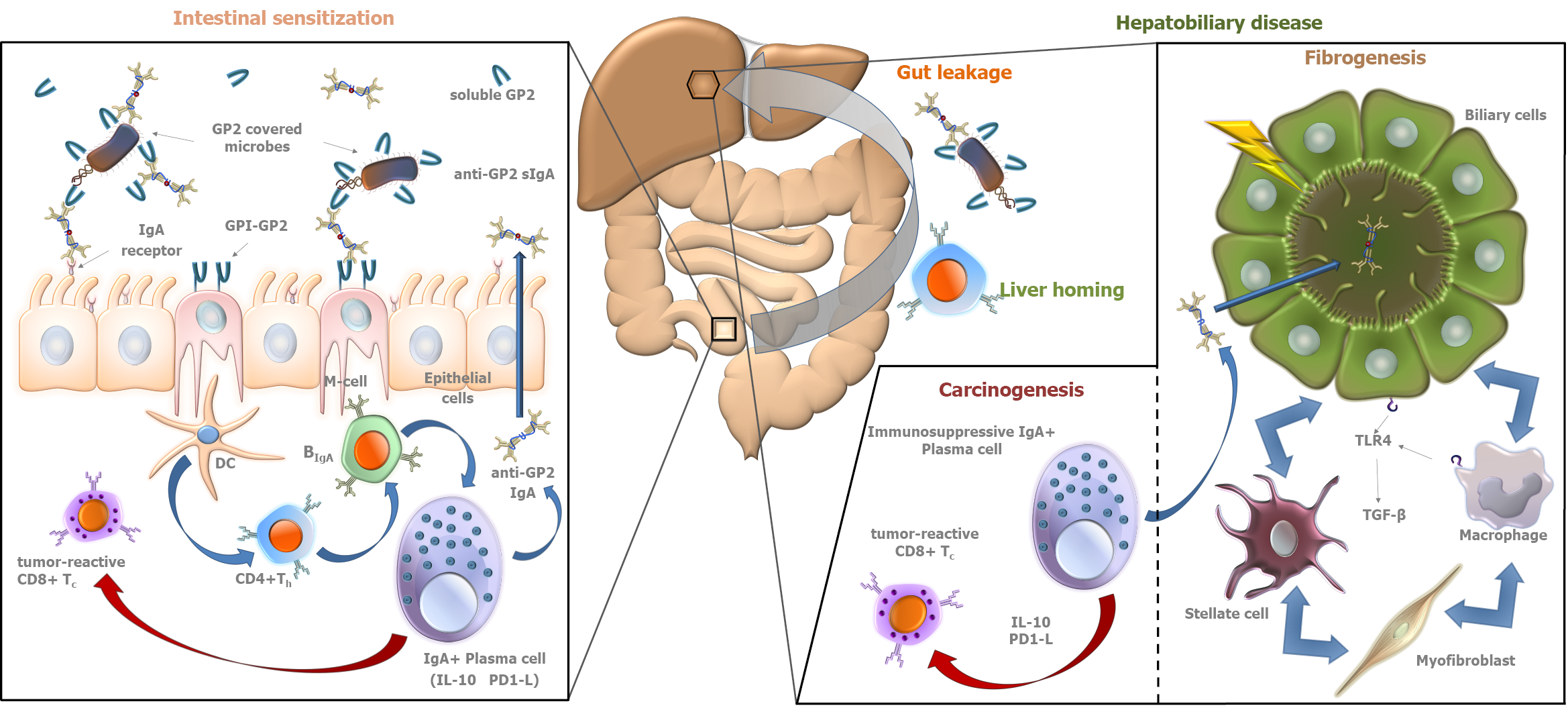
IgA is the most important immunoglobulin isotype involved in mucosal immunity[33]. Since the gut-liver interaction plays a central role in the pathogenesis of PSC, the formation of IgA type autoreactive antibodies is a characteristic feature of the disease. Under physiological conditions, monomeric IgA in serum inhibits immunological processes (so-called immunosuppressive effect). However, in pathological conditions, immune complexes formed by aggregation of monomeric IgA molecules can activate immunological processes by binding to myeloid cells[34]. IgA, produced by plasma cells lining the gut and biliary epithelium, is a highly abundant immunoglobulin in bile and plays a central role in the defense against intestinal pathogens. Biliary epithelial cells (that are the cellular targets of injury in PSC) and intestinal epithelial cells transport the IgA molecules into the biliary and intestinal lumens, respectively (→secretory IgA)[35-37]. However, in the past, most studies on serological antibodies in autoimmune liver diseases, like in the case of ANCA, have focused mainly on IgG isotype antibodies, and the IgA isotype is only recently gaining attention[38].
The work of Berglin et al[39] is an example, which highlighted the described importance of IgA isotype antibody related pathogenetic pathways in PSC. They demonstrated that in the presence of IgA isotype autoantibodies against biliary epithelial cells, disease progression was faster than in their absence. No similar correlation was observed for IgG isotype antibodies.
In 2015, our group reported the association between PSC and the presence of anti- GP2 IgA antibodies in inflammatory bowel disease (IBD) patients[40]. Subsequently, anti-GP2 IgA positivity was demonstrated to be able to predict a progressive disease course in PSC by our and another group[4,5]. These results were confirmed later by further studies[6,7]. In total, 1111 PSC patients have been evaluated for the presence of anti-GP2 IgA and the prevalence was found between 30.8% and 52.2%[8]. Anti-GP2 IgA was consistently reported to be associated with a more severe PSC phenotype with a 2- to 5-fold higher risk to develop end stage liver disease with a need for liver transplantation (Figure 1). Consequently, anti-GP2 IgA may soon be integrated into clinical practice for risk assessment in PSC. Moreover, anti-GP2 IgA can also identify a subset of PSC patients with existing biliary tract cancer or with an increased risk of developing it during the disease course. This association between anti-GP2 IgA and CCA was reported by Jendrek et al[4] and confirmed by Wunsch et al[7] both evaluating two cohorts each, providing strong evidence that incorporation of anti-GP2 IgA into CCA surveillance protocols could be beneficial (Figure 1).
IgA autoantibody against F-actin has been also reported to be associated with enhanced mucosal immune response to various microbial antigens and enterocyte damage in PSC, and to identify PSC patients with progressive disease (HR = 4.54; 95%CI = 1.14-18.18; P = 0.032)[41]. Cytoskeletal F-actin initiates an extracellular damage-associated molecular pattern signaling pathway through DNGR-1/CLEC9A and Syk-SFK that results in antigen cross-presentation to CD8+ T cells[42-45]. CD8+ T cells, after activation in the gut, can be recruited to the liver and induce immune-mediated cholangitis in mice[46].
As a result of increased lipopolysaccharide (LPS) exposure, biliary epithelial cells produce interleukin-8 (IL-8), which has a proliferation-promoting effect and enhances fibrogenesis related gene expression. In the study by Vesterhus et al[47], using modern antibody array technology, elevated serum IL-8 showed the strongest association with poor transplant-free survival among pro-inflammatory markers. However, the predictive value of MRS and ELF test for clinical outcomes was found to be higher than that of IL-8.
Bossen et al[48] found that macrophage activation markers, soluble CD163 (sCD163) and mannose receptor (sMR), were increased according to disease severity in two independent PSC cohorts of 138 and 159 patients. In both cohorts, sCD163 and sMR levels rose in parallel with increasing liver enzymes, MRS, and ELF test. Patients with high baseline levels of sCD163 (> 3.86 mg/L) had decreased transplant-free survival during the 8-year follow-up in both cohorts (35.2% vs 83.0% in the combined cohort). sMR showed similar association only in one of the cohorts with more severe disease features. In Cox regression, sCD163 also performed better in the more severe cohort (HR = 3.15 and 2.89) but it lost significance in the multivariate model against ELF test and AOM score.
In a study investigating serum markers of BT, serum levels of zonulin, intestinal fatty acid binding protein, soluble CD14 (sCD14), LPS, and LPS-binding protein (LBP) were measured in 166 PSC patients and 100 healthy controls[49]. LBP and sCD14 levels were higher, whilst zonulin levels were lower in PSC patients compared to controls. This latter difference disappeared with the exclusion of PSC patients with elevated prothrombin time (indicating advanced liver disease). In patients with CCA, sCD14 levels were higher compared to patients without it. High sCD14 and LBP values (> 1638 ng/mL and > 13942 ng/mL, respectively) were associated with reduced transplantation-free survival independently of MRS (HR = 2.26; 95%CI = 1.15-4.43; P = 0.018 and HR = 2.00; 95%CI = 1.17-3.43; P = 0.011, respectively). Further studies are needed to validate these findings.
In a cohort of 138 large-duct PSC patients (74% with IBD) using 52 UC patients as controls, Nielsen et al[50] investigated the predictive potential of four extracellular remodeling markers related to collagen formation (PRO-C3 and PRO-C5) and collagen degradation (C3M and C4M). All markers were elevated in PSC compared to UC patients, with PRO-C3 showing the most robust difference. High serum levels of three markers (with the exception of C3M) were associated with a shorter transplant-free survival during a 4-year follow-up period. In the univariate Cox regression model (using tertiles of the markers), PRO-C3 had the highest HR (HR = 3.02; 95%CI = 1.96-4.67, P < 0.001) but it failed to predict survival in the multivariate model including ELF test, while PRO-C5 remained an independent predictor (multivariate HR = 1.92; 95%CI = 1.28-2.88; P = 0.002). The combination of PRO-C3 and PRO-C5 resulted in a significantly improved odds ratio (OR = 47.3; P < 0.001) compared to the individual markers and ELF test. However, in this latter test, the authors compared the outcomes associated with the first (lowest) tertile of the markers to those associated with the third (highest) tertile. Further studies are needed to confirm the importance of these findings as well.
MicroRNA-122 (miR-122) is the most abundant microRNA in the liver, orchestrating molecular pathways in hepatocytes and regulating liver functions including lipid and cholesterol homeostasis. MiR-122 deficiency leads to inflammation, cholestasis, and ultimately fibrosis of the liver[51]. Friedrich et al[52] investigated the predictive potential of miR-122 in 114 PSC patients with a prospective follow-up of 10 years. They divided the population to patients with low and high miR-122 levels based on the median value of the marker (CT-value of 28.5). Low miR-122 levels were associated with a higher risk of death or need for liver transplantation (HR = 1.27; 95%CI = 1.04-1.39; P = 0.009) even in the multivariate Cox regression model including MRS, presence of dominant stricture, and IBD (HR = 1.19; 95%CI = 1.00-1.43; P = 0.045).
Expression of vascular adhesion protein 1 (VAP-1) and amine oxidase enzyme activity in the liver endothelium are increased in PSC. The former results in an elevation of its soluble form (sVAP-1) in patients’ sera. Elevated levels of sVAP-1 (> 529 ng/mL) in PSC are associated with an adverse disease outcome, independent of the presence of cirrhosis (HR = 3.85; 95%CI = 1.57 to 9.34; P = 0.003)[53].
Increased VAP-1 activity results in the expression of mucosal addressin cell adhesion molecule (MAdCAM-1) in liver tissue, which is otherwise expressed only in the intestinal endothelium. MAdCAM-1 binds to α4β7 integrin receptors on effector T lymphocytes, which is required for lymphocyte homing. Thus, abnormally expressed MAdCAM-1 in the liver results in the entry of gut activated effector T lymphocytes into liver tissue[53]. The increased expression and intrahepatic activity of VAP-1 may be attributed to the altered intestinal flora and the increased amine influx into the portal tract due to the inflamed, permeable bowel. The pathological amine substrate of VAP-1 is cysteamine[53], which causes colitis and colorectal carcinoma in mice. The Escherichia genus, which is able to produce cysteamine in vitro, is upregulated in the mucosa-associated microbiota population in PSC. However, the intestinal epithelium itself is capable of cysteamine production via the ectoenzyme vanin-1. The observation that cysteamine enhances colonic inflammation and carcinogenesis is consistent with the increased colon carcinoma formation observed in PSC. The aldehyde derivative formed during the metabolism of cysteamine causes abnormal collagen cross-binding, which could theoretically contribute to increased fibrogenesis. Increased VAP-1 activity in liver and colonic tissue is most likely an attempt to counteract the increased amine load resulting from colitis.
The newly discovered VAP-1 pathway is one explanation of how colitis and increased amine production lead to damage of liver tissue. VAP-1 may be a potential therapeutic target in the future due to its effect in promoting α4β7/MAdCAM-1 interaction. A VAP-1 antagonist may also be able to regulate the migration of effector T lymphocytes from the inflamed intestine to the liver and thereby inhibit fibrogenesis[53].
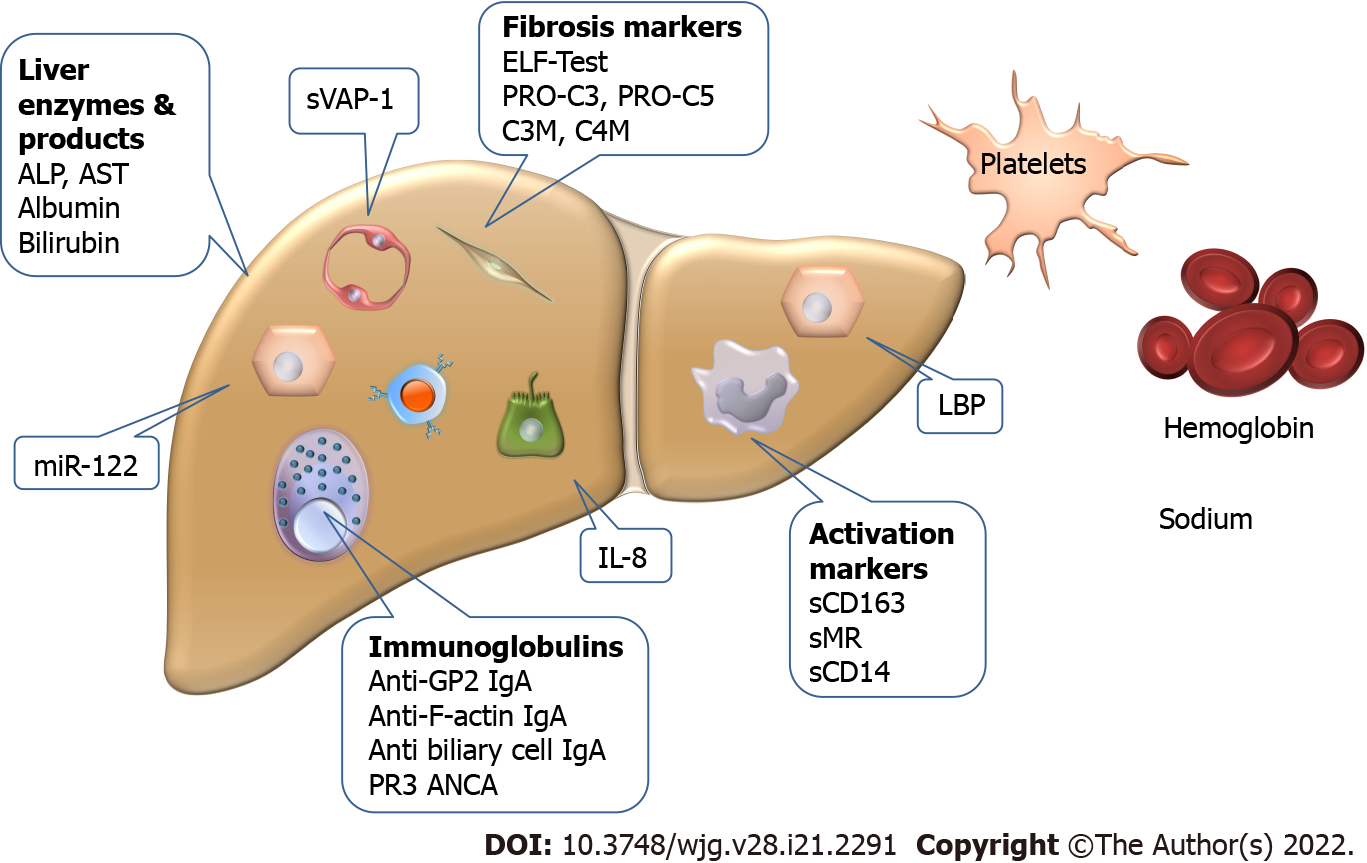
The biomarkers used individually or as parts of score systems are summarized in Figure 2.
Carbohydrate antigen 19-9 (CA19-9) is the most commonly used serological marker for screening CCA, but its application is limited in PSC by the lack of comprehensive studies comparing PSC patients with and without CCA. Most studies were too small and ultimately provided inconsistent cut-off values[54]. A recent development in this field is the discovery of the role of fucosyltransferase (FUT) 2 and 3 in influencing the serum levels of CA19-9 in patients with PSC. These enzymes catalyze the final steps of CA19-9 biosynthesis. The study identified FUT genotypes with low, intermediate, and high CA19-9 synthesis capacity displaying distinct CA19-9 serum levels[55]. According to these data, CA19-9 is not an appropriate marker for CCA screening in patients with a low CA19-9 biosynthesis genotype since they do not synthetize CA19-9. This group can be identified by a Lewis-negative blood group which is also determined by these enzymes. In contrast, the high CA19-9 biosynthesis genotype might be a reason for false positive results[55]. In addition, bacterial cholangitis can be another explanation for increased CA19-9 level in PSC patients without CCA[56].
Finally, a study demonstrated a significant influence of FUT2 genotype also on CEA serum levels. The most prominent effect was observed in the subgroup incapable of CA19-9 biosynthesis[57]. Studies with other serological biomarkers, like angiopoetin-2[58] or cytokeratin fraction 21-1[59], have not reached satisfactory results in terms of identifying individuals with CCA among PSC patients.
Several promising discoveries have been made on biomarkers for PSC. The first validated prognostic biomarker (anti-GP2 IgA) is finally ready to be incorporated into clinical practice, and the investigations highlighted important pathophysiological mechanisms for future research that might open new avenues for medical therapy. However, we still lack specific serological markers that could support the diagnosis of PSC. Additionally, a serological marker with satisfactory discriminative power to aid the recognition of CCA in PSC patients is another unmet need.
Provenance and peer review: Invited Conference articles; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Losurdo G, Italy; Wen XL, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 2. | Trivedi PJ, Adams DH. Mucosal immunity in liver autoimmunity: a comprehensive review. J Autoimmun. 2013;46:97-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. Lancet. 2018;391:2547-2559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 4. | Jendrek ST, Gotthardt D, Nitzsche T, Widmann L, Korf T, Michaels MA, Weiss KH, Liaskou E, Vesterhus M, Karlsen TH, Mindorf S, Schemmer P, Bär F, Teegen B, Schröder T, Ehlers M, Hammers CM, Komorowski L, Lehnert H, Fellermann K, Derer S, Hov JR, Sina C. Anti-GP2 IgA autoantibodies are associated with poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Gut. 2017;66:137-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Tornai T, Tornai D, Sipeki N, Tornai I, Alsulaimani R, Fechner K, Roggenbuck D, Norman GL, Veres G, Par G, Par A, Szalay F, Lakatos PL, Antal-Szalmas P, Papp M. Loss of tolerance to gut immunity protein, glycoprotein 2 (GP2) is associated with progressive disease course in primary sclerosing cholangitis. Sci Rep. 2018;8:399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Sowa M, Kolenda R, Baumgart DC, Pratschke J, Papp M, Tornai T, Suchanski J, Bogdanos DP, Mytilinaiou MG, Hammermann J, Laass MW, Conrad K, Schramm C, Franke A, Roggenbuck D, Schierack P. Mucosal Autoimmunity to Cell-Bound GP2 Isoforms Is a Sensitive Marker in PSC and Associated With the Clinical Phenotype. Front Immunol. 2018;9:1959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Wunsch E, Norman GL, Milkiewicz M, Krawczyk M, Bentow C, Shums Z, Mahler M, Lopens S, Reinhold D, Franke A, Schramm C, Roggenbuck D, Milkiewicz P. Anti-glycoprotein 2 (anti-GP2) IgA and anti-neutrophil cytoplasmic antibodies to serine proteinase 3 (PR3-ANCA): antibodies to predict severe disease, poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2021;53:302-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Tornai D, Papp M. Editorial: serologic antibodies in primary sclerosing cholangitis - a tell-tale sign of compromised gut-liver immunity? Aliment Pharmacol Ther. 2021;53:350-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 449] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 10. | Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Hilscher M, Enders FB, Carey EJ, Lindor KD, Tabibian JH. Alkaline phosphatase normalization is a biomarker of improved survival in primary sclerosing cholangitis. Ann Hepatol. 2016;15:246-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 12. | Lindström L, Hultcrantz R, Boberg KM, Friis-Liby I, Bergquist A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11:841-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Stanich PP, Björnsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Kim WR, Therneau TM, Wiesner RH, Poterucha JJ, Benson JT, Malinchoc M, LaRusso NF, Lindor KD, Dickson ER. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Thorburn D. Prognostic scores and non-invasive markers in primary sclerosing cholangitis: good for patients or for papers? Lancet Gastroenterol Hepatol. 2017;2:774-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | de Vries EM, Wang J, Williamson KD, Leeflang MM, Boonstra K, Weersma RK, Beuers U, Chapman RW, Geskus RB, Ponsioen CY. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut. 2018;67:1864-1869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Goode EC, Clark AB, Mells GF, Srivastava B, Spiess K, Gelson WTH, Trivedi PJ, Lynch KD, Castren E, Vesterhus MN, Karlsen TH, Ji SG, Anderson CA, Thorburn D, Hudson M, Heneghan MA, Aldersley MA, Bathgate A, Sandford RN, Alexander GJ, Chapman RW, Walmsley M; UK-PSC Consortium, Hirschfield GM, Rushbrook SM. Factors Associated With Outcomes of Patients With Primary Sclerosing Cholangitis and Development and Validation of a Risk Scoring System. Hepatology. 2019;69:2120-2135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Eaton JE, Vesterhus M, McCauley BM, Atkinson EJ, Schlicht EM, Juran BD, Gossard AA, LaRusso NF, Gores GJ, Karlsen TH, Lazaridis KN. Primary Sclerosing Cholangitis Risk Estimate Tool (PREsTo) Predicts Outcomes of the Disease: A Derivation and Validation Study Using Machine Learning. Hepatology. 2020;71:214-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | de Vries EMG, Färkkilä M, Milkiewicz P, Hov JR, Eksteen B, Thorburn D, Chazouillères O, Pares A, Nygård S, Gilja OH, Wunsch E, Invernizzi P, Carbone M, Bernuzzi F, Boberg KM, Røsjø H, Rosenberg W, Beuers UH, Ponsioen CY, Karlsen TH, Vesterhus M. Enhanced liver fibrosis test predicts transplant-free survival in primary sclerosing cholangitis, a multi-centre study. Liver Int. 2017;37:1554-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Vesterhus M, Hov JR, Holm A, Schrumpf E, Nygård S, Godang K, Andersen IM, Naess S, Thorburn D, Saffioti F, Vatn M, Gilja OH, Lund-Johansen F, Syversveen T, Brabrand K, Parés A, Ponsioen CY, Pinzani M, Färkkilä M, Moum B, Ueland T, Røsjø H, Rosenberg W, Boberg KM, Karlsen TH. Enhanced liver fibrosis score predicts transplant-free survival in primary sclerosing cholangitis. Hepatology. 2015;62:188-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Fossdal G, Mjelle AB, Wiencke K, Bjørk I, Gilja OH, Folseraas T, Karlsen TH, Rosenberg W, Giil LM, Vesterhus M. Fluctuating biomarkers in primary sclerosing cholangitis: A longitudinal comparison of alkaline phosphatase, liver stiffness, and ELF. JHEP Rep. 2021;3:100328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 22. | Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646-59; quiz 660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 23. | Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, Chari S, Lindor KD. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070-2075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Björnsson E, Chari S, Silveira M, Gossard A, Takahashi N, Smyrk T, Lindor K. Primary sclerosing cholangitis associated with elevated immunoglobulin G4: clinical characteristics and response to therapy. Am J Ther. 2011;18:198-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Relevance. Front Immunol. 2018;9:609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Terjung B, Spengler U. Atypical p-ANCA in PSC and AIH: a hint toward a "leaky gut"? Clin Rev Allergy Immunol. 2009;36:40-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 109] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Hov JR, Boberg KM, Taraldsrud E, Vesterhus M, Boyadzhieva M, Solberg IC, Schrumpf E, Vatn MH, Lie BA, Molberg Ø, Karlsen TH. Antineutrophil antibodies define clinical and genetic subgroups in primary sclerosing cholangitis. Liver Int. 2017;37:458-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Fosby B, Næss S, Hov JR, Traherne J, Boberg KM, Trowsdale J, Foss A, Line PD, Franke A, Melum E, Scott H, Karlsen TH. HLA variants related to primary sclerosing cholangitis influence rejection after liver transplantation. World J Gastroenterol. 2014;20:3986-4000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Savige J, Gillis D, Benson E, Davies D, Esnault V, Falk RJ, Hagen EC, Jayne D, Jennette JC, Paspaliaris B, Pollock W, Pusey C, Savage CO, Silvestrini R, van der Woude F, Wieslander J, Wiik A. International Consensus Statement on Testing and Reporting of Antineutrophil Cytoplasmic Antibodies (ANCA). Am J Clin Pathol. 1999;111:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 361] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 31. | Mahler M, Bogdanos DP, Pavlidis P, Fritzler MJ, Csernok E, Damoiseaux J, Bentow C, Shums Z, Forbes A, Norman GL. PR3-ANCA: a promising biomarker for ulcerative colitis with extensive disease. Clin Chim Acta. 2013;424:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Stinton LM, Bentow C, Mahler M, Norman GL, Eksteen B, Mason AL, Kaplan GG, Lindkvist B, Hirschfield GM, Milkiewicz P, Cheung A, Janssen HL, Fritzler MJ. PR3-ANCA: a promising biomarker in primary sclerosing cholangitis (PSC). PLoS One. 2014;9:e112877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 34. | Monteiro RC. Role of IgA and IgA fc receptors in inflammation. J Clin Immunol. 2010;30:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Chen XM, O'Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol. 2008;86:497-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 731] [Cited by in F6Publishing: 754] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 37. | Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 38. | Schwarze C, Terjung B, Lilienweiss P, Beuers U, Herzog V, Sauerbruch T, Spengler U. IgA class antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and autoimmune hepatitis. Clin Exp Immunol. 2003;133:283-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Berglin L, Björkström NK, Bergquist A. Primary sclerosing cholangitis is associated with autoreactive IgA antibodies against biliary epithelial cells. Scand J Gastroenterol. 2013;48:719-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Papp M, Sipeki N, Tornai T, Altorjay I, Norman GL, Shums Z, Roggenbuck D, Fechner K, Stöcker W, Antal-Szalmas P, Veres G, Lakatos PL. Rediscovery of the Anti-Pancreatic Antibodies and Evaluation of their Prognostic Value in a Prospective Clinical Cohort of Crohn's Patients: The Importance of Specific Target Antigens [GP2 and CUZD1]. J Crohns Colitis. 2015;9:659-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Tornai T, Palyu E, Vitalis Z, Tornai I, Tornai D, Antal-Szalmas P, Norman GL, Shums Z, Veres G, Dezsofi A, Par G, Par A, Orosz P, Szalay F, Lakatos PL, Papp M. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J Gastroenterol. 2017;23:5412-5421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
| 42. | Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 547] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 43. | Brown GD. Immunology: Actin' dangerously. Nature. 2012;485:589-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Ahrens S, Zelenay S, Sancho D, Hanč P, Kjær S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, Batista F, Thompson B, Way M, Reis e Sousa C, Schulz O. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36:635-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 45. | Hanč P, Fujii T, Iborra S, Yamada Y, Huotari J, Schulz O, Ahrens S, Kjær S, Way M, Sancho D, Namba K, Reis e Sousa C. Structure of the Complex of F-Actin and DNGR-1, a C-Type Lectin Receptor Involved in Dendritic Cell Cross-Presentation of Dead Cell-Associated Antigens. Immunity. 2015;42:839-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Mathies F, Steffens N, Kleinschmidt D, Stuhlmann F, Huber FJ, Roy U, Meyer T, Luetgehetmann M, von Petersdorff M, Seiz O, Herkel J, Schramm C, Flavell RA, Gagliani N, Krebs C, Panzer U, Abdullah Z, Strowig T, Bedke T, Huber S. Colitis Promotes a Pathological Condition of the Liver in the Absence of Foxp3+ Regulatory T Cells. J Immunol. 2018;201:3558-3568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Vesterhus M, Holm A, Hov JR, Nygård S, Schrumpf E, Melum E, Thorbjørnsen LW, Paulsen V, Lundin K, Dale I, Gilja OH, Zweers SJLB, Vatn M, Schaap FG, Jansen PLM, Ueland T, Røsjø H, Moum B, Ponsioen CY, Boberg KM, Färkkilä M, Karlsen TH, Lund-Johansen F. Novel serum and bile protein markers predict primary sclerosing cholangitis disease severity and prognosis. J Hepatol. 2017;66:1214-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Bossen L, Vesterhus M, Hov JR, Färkkilä M, Rosenberg WM, Møller HJ, Boberg KM, Karlsen TH, Grønbæk H. Circulating Macrophage Activation Markers Predict Transplant-Free Survival in Patients With Primary Sclerosing Cholangitis. Clin Transl Gastroenterol. 2021;12:e00315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Dhillon AK, Kummen M, Trøseid M, Åkra S, Liaskou E, Moum B, Vesterhus M, Karlsen TH, Seljeflot I, Hov JR. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 2019;39:371-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 50. | Nielsen MJ, Thorburn D, Leeming DJ, Hov JR, Nygård S, Moum B, Saffioti F, Gilja OH, Boberg KM, Mazza G, Røsjø H, Pinzani M, Karlsen TH, Karsdal MA, Vesterhus M. Serological markers of extracellular matrix remodeling predict transplant-free survival in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2018;48:179-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Hu J, Xu Y, Hao J, Wang S, Li C, Meng S. MiR-122 in hepatic function and liver diseases. Protein Cell. 2012;3:364-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Friedrich K, Baumann C, Wannhoff A, Rupp C, Mehrabi A, Weiss KH, Gotthardt DN. Serum miRNA-122 is an independent biomarker of survival in patients with primary sclerosing cholangitis. J Gastrointestin Liver Dis. 2018;27:145-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Trivedi PJ, Tickle J, Vesterhus MN, Eddowes PJ, Bruns T, Vainio J, Parker R, Smith D, Liaskou E, Thorbjørnsen LW, Hirschfield GM, Auvinen K, Hubscher SG, Salmi M, Adams DH, Weston CJ. Vascular adhesion protein-1 is elevated in primary sclerosing cholangitis, is predictive of clinical outcome and facilitates recruitment of gut-tropic lymphocytes to liver in a substrate-dependent manner. Gut. 2018;67:1135-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Wannhoff A, Gotthardt DN. Recent developments in the research on biomarkers of cholangiocarcinoma in primary sclerosing cholangitis. Clin Res Hepatol Gastroenterol. 2019;43:236-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Rizvi S, Eaton JE, Gores GJ. Primary Sclerosing Cholangitis as a Premalignant Biliary Tract Disease: Surveillance and Management. Clin Gastroenterol Hepatol. 2015;13:2152-2165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9:434-9.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Wannhoff A, Folseraas T, Brune M, Rupp C, Friedrich K, Knierim J, Weiss KH, Sauer P, Flechtenmacher C, Schirmacher P, Stremmel W, Hov JR, Gotthardt DN. A common genetic variant of fucosyltransferase 2 correlates with serum carcinoembryonic antigen levels and affects cancer screening in patients with primary sclerosing cholangitis. United European Gastroenterol J. 2016;4:84-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Voigtländer T, David S, Thamm K, Schlué J, Metzger J, Manns MP, Lankisch TO. Angiopoietin-2 and biliary diseases: elevated serum, but not bile levels are associated with cholangiocarcinoma. PLoS One. 2014;9:e97046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Chapman MH, Sandanayake NS, Andreola F, Dhar DK, Webster GJ, Dooley JS, Pereira SP. Circulating CYFRA 21-1 is a Specific Diagnostic and Prognostic Biomarker in Biliary Tract Cancer. J Clin Exp Hepatol. 2011;1:6-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |