Published online Jul 21, 2021. doi: 10.3748/wjg.v27.i27.4383
Peer-review started: January 28, 2021
First decision: March 29, 2021
Revised: April 12, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: July 21, 2021
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy that is best treated in a multidisciplinary fashion using surgery, chemotherapy, and radiation. Adjuvant chemotherapy has shown to have a significant survival benefit in patients with resected PDAC. However, up to 50% of patients fail to receive adjuvant chemotherapy due to postoperative complications, poor patient performance status or early disease progression. In order to ensure the delivery of chemotherapy, an alternative strategy is to administer systemic treatment prior to surgery. Precision oncology refers to the application of diverse strategies to target therapies specific to characteristics of a patient’s cancer. While traditionally emphasized in selecting targeted therapies based on molecular, genetic, and radiographic biomarkers for patients with metastatic disease, the neoadjuvant setting is a prime opportunity to utilize personalized approaches. In this article, we describe the current evidence for the use of neoadjuvant therapy (NT) and highlight unique opportunities for personalized care in patients with PDAC undergoing NT.
Core Tip: Neoadjuvant therapy (NT) is an increasingly utilized approach that maximizes the receipt of multimodality therapy, improves margin-negative resection rates, and potentially increases survival durations. In the era of personalized medicine, the neoadjuvant period can also be used to emphasize precision oncology. Already, current methods of anatomically staging, molecularly profiling, and monitoring response to therapy can be used to personalize neoadjuvant treatment for localized pancreatic ductal adenocarcinoma (PDAC). In this article, we describe the current evidence for the use of NT and highlight unique opportunities for personalized care in patients with PDAC undergoing NT.
- Citation: Hamad A, Brown ZJ, Ejaz AM, Dillhoff M, Cloyd JM. Neoadjuvant therapy for pancreatic ductal adenocarcinoma: Opportunities for personalized cancer care. World J Gastroenterol 2021; 27(27): 4383-4394
- URL: https://www.wjgnet.com/1007-9327/full/v27/i27/4383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i27.4383
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related deaths worldwide with a 5-year overall survival (OS) rate of only 10%[1]. Despite surgical resection being the only hope for cure, only a small proportion of patients present with resectable disease and the majority of patients will develop locoregional or metastatic recurrence after surgery[2,3]. Adjuvant chemotherapy has shown to have a significant survival benefit in patients with resected PDAC[3]. However, up to 50% of patients fail to receive adjuvant chemotherapy due to postoperative complications, poor patient performance status or early disease progression[4-6].
In order to ensure the delivery of chemotherapy, an alternative strategy is to administer systemic treatment prior to surgery. Neoadjuvant therapy (NT) has been shown to confer several clinical benefits such as improved margin-negative resection rates, decreased lymph node positivity, early treatment of presumed micro-metastatic disease, an optimal window of time to provide prehabilitation before surgery, and the ability to measure in vivo response to therapy histologically after resection[7-9]. Moreover, increasing evidence from randomized controlled trials (RCT) suggests NT may improve OS in patients with non-metastatic PDAC compared with upfront resection[10,11]. Despite the advantages of NT, its use in the United States has remained relatively low[12,13]. While NT is now the recommended treatment strategy for borderline resectable (BR) or locally advanced cancer, current NCCN guidelines support either upfront surgical resection or NT for patients with resectable disease[14].
There is growing interest in emphasizing personalized approaches to multidisciplinary cancer care that reflects not only unique differences in cancer biology but also individual circumstances and treatment goals. Precision oncology refers to the application of diverse strategies to target therapies specific to characteristics of a patient’s cancer. While traditionally emphasized in selecting targeted therapies based on molecular, genetic, and radiographic biomarkers for patients with metastatic disease, the neoadjuvant setting is a prime opportunity to utilize personalized approaches. In this article, we describe the current evidence for the use of NT and highlight unique opportunities for personalized care in patients with PDAC undergoing NT.
Large cohort and population-based studies have shown that as many as 50% of patients who undergo surgical resection for PDAC are unable to receive adjuvant therapy due to postoperative complications, poor performance status, or early disease progression[4-6,15,16]. Even among healthy patients enrolled in clinical trials, a substantial proportion of patients are unable to initiate adjuvant therapy due to the morbidity of pancreatic surgery[4]. An even greater proportion of patients fail to complete all intended cycles of adjuvant therapy[5]. Thus, the ability to ensure receipt of systemic therapy and facilitate multimodality therapy is one of the strongest reasons to recommend NT. Other advantages that support the use of NT include the early treatment of presumed micrometastatic disease, the ability to potentially downstage BR disease improving the chances of R0 resection, and improved patient selection by avoiding surgery in those patients with rapid disease progression during preoperative treatment. Additionally, well-oxygenated, non-devascularized tissue is more susceptible to the effects of chemoradiation, which theoretically increases the efficacy of chemoradiation if given prior to surgery[17,18].
These advantages must be carefully weighed against the potential disadvantages of pursuing NT. First, unlike in a surgery-first approach, tissue diagnosis and biliary decompression are uniformly required. These procedures may delay the initiation of treatment and are associated with small, but non-zero, risks. Second, NT is inherently multi-disciplinary and require careful coordination among providers. Third, and most importantly, delivering aggressive chemotherapy and/or radiation prior to surgery can lead to severe toxicity that, in extreme cases, can preclude subsequent surgical resection. Indeed, a systematic review by the Dutch Pancreatic Cancer Group calculated a Grade III or higher toxicity rate of 64% among patients undergoing NT[19]. The recent SWOG S1505 trial of NT for resectable PDAC found that nearly 13% that started NT were unable to undergo surgery because of performance status decline[20]. Finally, while distant progression while on NT is far more common, a small risk of local progression that leads to unresectability exists. These challenges highlight the importance not only of personalizing treatment decisions regarding NT, but also of emphasizing research that improves the delivery of NT for patients with localized PDAC.
Support for the use of NT for localized PDAC has largely come from small prospective trials, single-institutional series, and cancer registries[21]. These studies largely demonstrated its feasibility and proposed NT as an acceptable approach for PDAC. Suggestions of improved outcomes compared to upfront surgery have been limited by study design. However, the completion of several RCT in recent years comparing NT to upfront surgery have generated increased support for NT. For example, two RCTs found improved margin-negative resection rates and OS among patients with BR PDAC[22] who received neoadjuvant CRT compared to immediate surgery. Furthermore, the PACT-15 and Prep-02/JSAP-05 RCTs found improved OS among patients with resectable PDAC treated with neoadjuvant chemotherapy compared to those who underwent immediate surgery[16,23].
In a recent meta-analysis of only prospective RCTs, Cloyd et al[13] showed that the OS of patients with resectable or BR PDAC who received NT was nearly 30% better than that of patients who underwent surgery upfront using an intention-to-treat design. Furthermore, the meta-analysis found that NT improved R0 resection rate and decreased lymph node positivity rate. Since then, preliminary results from the ESPAC-5 trial, a four-arm RCT comparing patients undergoing surgery upfront, neoadjuvant gemcitabine/capecitabine (GEMCAP), neoadjuvant FOLFIRINOX, and neoadjuvant capecitabine-based radiation, showed improved one-year OS among patients receiving NT[24]. As a limitation of previous RCTs is the use of non-traditional neoadjuvant regimens (e.g., folfirinox or gemcitabine-abraxane), the long-term results of this trial and other contemporary studies comparing NT to immediate surgery are anxiously awaited.
Several factors make the neoadjuvant period an optimal scenario to emphasize precision oncology (Table 1). First, PDAC is anatomically and genetically heterogeneous as is the clinical presentation of patients with localized disease. Second, patient and tumor response to NT differs significantly. Evaluating and responding to this dynamic staging offers an opportunity to personalize subsequent treatment.
| Concept | Description | Examples | Outcome |
| Anatomic staging | Characterization of local extent and vascular involvement of tumor | Locally advanced/unresectable; Borderline resectable; Potentially resectable | Anatomic staging can influence the recommended duration and components (e.g., preoperative radiation) of neoadjuvant therapy |
| Molecular staging | Identification of tumor/germline genetic and molecular markers | BRCA mutations; Mismatch repair-deficiency; Molecular markers | Specific tumor/germline mutations may identify opportunity for targeted therapies (e.g., immunotherapy, PARP inhibitors) Standard chemotherapy may be influenced by molecular markers (e.g., resistance/sensitivity to traditional flouropyridamine or gemcitabine-based therapy) |
| Dynamic staging | Measuring biochemical, radiographic, and histologic response of the tumor to neoadjuvant therapy | Carbohydrate antigen 19-9; Response evaluation criteria in solid tumors response; Pathologic response | Measuring response to neoadjuvant therapy can influence treatment strategies (e.g., changing neoadjuvant regimen, use of radiation, recommendations for adjuvant therapy) |
Using high-quality cross-sectional imaging, localized PDAC is classified as resectable, BR or locally advanced (LA; also termed unresectable) according to its relationship with major vascular structures. While several organizations have published staging criteria with only slight differences, the SSO/SSAT/AHPBA consensus definitions are commonly employed. As these criteria reflect the likelihood of achieving a margin-negative resection with upfront surgery, the development of a uniform anatomic staging system has been pivotal for improved clinical protocol standardization specifically concerning the use of NT[25] (Table 2).
| Resectable | Borderline | Locally advanced | |
| SMV-PV | Uninvolved with tumor with clear fat planes around vessels | Abutment, encasement, or occlusion of short segment of vein | Occlusion, thrombosis, or encasement extending several centimeters |
| SMA | Uninvolved | Tumor abutment < 180° | Tumor abutment > 180° (encasement) or thrombosis of artery |
| Celiac axis | Uninvolved | Uninvolved celiac axis; short segment encasement or abutment of common hepatic artery may be amenable to resection and reconstruction | Abutment or encasement of celiac axis indicates unresectability |
LA PDAC truly represents unresectable disease, typically because of arterial encasement or non-reconstructable venous involvement. Still, recent studies have found that a small but significant proportion of patients can be converted to resectable disease after aggressive NT[26]. For example, a large retrospective study by Hackert et al[27] showed that 61% of patients with LA PADC receiving FOLFIRINOX as NT underwent successful surgical resection with a 40.8% R0 resection rate. Another study by Gemenetzis et al[28] showed that FOLFIRINOX-based therapy and stereotactic body radiation therapy correlated with increased probability of resection (P = 0.006); patients who eventually underwent surgical resection had higher median OS compared with those who did not (35.3 mo vs 16.3 mo; P < 0.0001). Given the vascular involvement and low likelihood of achieving negative microscopic margins, preoperative radiation therapy is commonly employed after induction systemic chemotherapy prior to attempts at surgical resection. This approach is logical since consolidative radiation is frequently administered for patients with LA disease who are not surgical candidates to enhance locoregional control[29].
A similar approach should be considered for patients with BR PDAC yet with higher likelihood of undergoing surgical resection. Current practice is to typically begin with induction systemic chemotherapy. For example, the Alliance for Clinical Trials in Oncology group A021101, demonstrated a 93% R0 resection rate for patients with BR PDAC after receiving FOLFIRINOX followed by capecitabine-based chemoradiation as NT[30]. Similarly, a single-arm prospective trial of neoadjuvant FOLFIR
The use of NT remains the most controversial in patients with resectable PDAC and wide variation in practices exist[21]. Nevertheless, the use of NT continues to increase given the previously described rationale and increasing evidence that highlights improved outcomes. However, given the lesser need for downstaging and higher likelihood of achieving an R0 resection, current neoadjuvant approaches tend to utilize systemic chemotherapy alone. For example, the SWOG S1505 trial was a recent RCT of either neoadjuvant mFOLFIRINOX or gemcitabine/nab-paclitaxel prior to surgical resection for resectable PDAC as NT[33]. In addition, the recently opened Alliance A021806 will randomize patients with resectable PDAC to either perioperative mFOLFIRINOX or surgery upfront followed by adjuvant mFOLFIRINOX[34].
The last decade has seen dramatic advances in our understanding of the genetic underpinnings of PDAC pathogenesis. With advanced tumor profiling and next-generation sequencing, recent studies have characterized the frequency of genetic and molecular alterations in PDAC tumors[35]. This information, if obtained routinely via tissue biopsy or surgical specimen, can contribute to a more personalized approach to treatment via molecular profiling[36]. In a study by Krepline et al[37], 73 out of 78 resected PDAC specimens were found to have a pathogenic variant on NGS of which 18% were potentially actionable. For example, patients with BRCA1/BRCA2 mutations are known to benefit from platinum-based chemotherapy and PARP inhibitors[38,39]. Pancreatic cancer that is mismatch repair-deficient (dMMR), a small but clinically relevant proportion, are known to respond to immunotherapy[40,41]. Finally, the efficacy of standard chemotherapy regimens can be modified depending on thymidylate synthase (TYMS), excision repair cross-complementing (ERCC1) protein, ribonucleotide reductase M1 (RMM1), secreted protein acid and rich in cysteine (SPARC), topoisomerase I (TOP1), and human equilibrative nucleoside transported 1 (hENT1) levels. Low TYMS, ERCC1 protein, and RRM1 Levels predict efficacy of 5-FU based therapies and capecitabine, cisplatin, and gemcitabine respectively[42-44]. Elevated SPARC level predicted sensitivity to nab-paclitaxel while low levels of hENT1 have been associated with gemcitabine resistance[43,45].
These molecular biomarkers, if obtained from preoperative endoscopic ultrasound biopsies, can thus be used to tailor NT. In a recent prospective trial, Tsai et al[46] delivered neoadjuvant systemic therapy to 130 patients based on the molecular profile results. In this trial, 6 molecular targets were utilized to predict chemosensitivity: TYMS, ERCC1, RMM1, SPARC, TOP1, and hENT1. Of the 92 patients with predictive molecular profiling, 74 (80%) received fluoropyrimidine-based systemic therapy and 18 (20%) received gemcitabine-based systemic therapy[46]. Of the 130 overall patients enrolled, 107 (82%) completed all intended NT followed by surgical resection including 56 (92%) with resectable PDAC and 51 (74%) with BR PDAC. The importance of this trial was to demonstrate the increased rate of resectability, which suggests that molecular profiling potentially improved the efficacy of NT[46]. Furthermore, the Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) trial documented the feasibility of acquiring and screening pancreatic tumor tissue for HER2 amplification, KRAS mutation, and mutations in BRCA1, BRCA2, PALB2, and ATM[47]. A pilot study using molecular profiling in a wide variety of metastatic cancers demonstrated a longer PFS in 27% of patients receiving molecular profiling-based systemic therapy[36]. The scarcity of studies in this field suggest that this method of personalized care is under-utilized and warrants further investigation.
In contrast to standard adjuvant therapy, administering nonoperative therapies prior to surgery provides a unique opportunity to measure the tumor response to treatment in vivo. Traditional measures of tumor response to NT include biochemical, radiogra
Restaging with cross-sectional imaging is routinely performed during and following NT, mostly to rule out disease progression but also to assess the response of the primary tumor to treatment. While the Response Evaluation Criteria in Solid Tumors (RECIST) grading system has historically felt to under-represent treatment response[53], Perri et al[54] recently found in a large retrospective study that RECIST partial response and a reduction in tumor volume after NT were independently associated with pathologic response in patients with localized PDAC. In addition to serologic and radiographic measures, the response to NT can be measured histologically in surgical specimens as the proportion of active cancer cells. Unfortunately, previous studies have found that only a major pathologic response (defined as < 5% active cancer cells) is associated with improved prognosis[8] and that pathologic complete responses are rare[55].
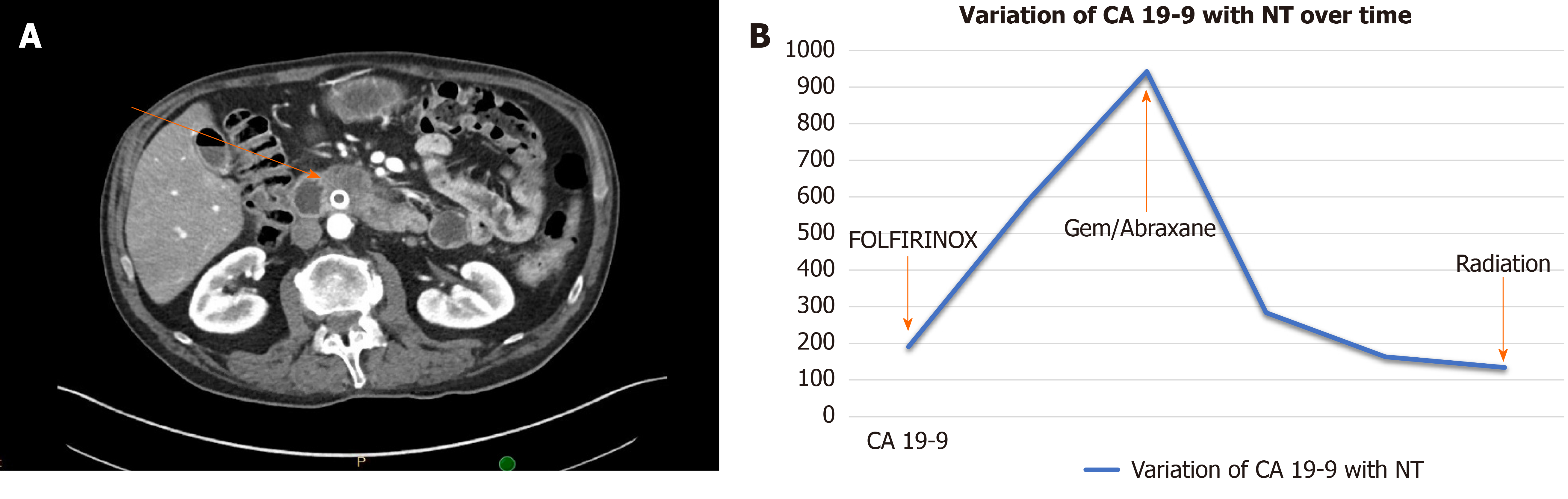
A dynamic assessment of tumor response to treatment may help personalize treatment in several ways. First, non-responders to induction systemic chemotherapy can be switched to alternative regimens. In a study by Vreeland et al[56], of 25 BR or LA PDAC patients who did not respond to FOLFIRINOX after 4 mo of treatment as NT, 21 (84%) showed a serologic or radiographic response after switching to gemcitabine/nab-paclitaxel and 11 of them underwent surgical resection (Figure 1). In contrast, responders who are tolerating therapy may be selected to continue this regimen particularly if CA 19-9 has not normalized yet or additional downstaging is required. Second, reassessment of anatomic location may assist decision making regarding the role of preoperative radiation. Third, information on response to NT may guide the use of adjuvant therapy. For example, Liu et al[50] showed that in a cohort of patients in whom CA 19-9 normalized with a decrease > 50% after NT, adjuvant therapy was not associated with additional survival benefit whereas in patients with no normalization of CA 19-9 or decrease of > 50%, receipt of adjuvant therapy was in fact associated with a survival benefit[50]. Additional research is needed to determine whether alternative adjuvant regimens (e.g., gemcitabine-based chemotherapy) should be utilized in patients who do not respond to first-line neoadjuvant chemotherapy (e.g., FOLFIRINOX).
With an improved understanding of tumor biology, cancer care is becoming increasingly personalized. Integrated genomic analysis has revealed several molecular tumor subtypes of PDAC as well as subsets of the tumor microenviroment (TME)[57,58]. The clinical applications of such classification systems are still in development but perhaps this data can be used to inform about prognosis or aid in treatment decisions[59]. A shift toward personalized NT will depend greatly on the development and validation of novel biomarkers. As an example, SPARC is a protein that is overly expressed in the TME of PDAC tumors. SPARC expression was associated with an inferior survival in patients receiving gemcitabine-based chemotherapy while no association was detected for patients receiving fluoropyrimidine-based chemotherapy suggesting SPARC expression might act as a negative predictive biomarker in patients treated with gemcitabine-based chemotherapy[60]. However, SPARC expression was not associated with survival or response to gemcitabine-based chemotherapy in patients with metastatic PDAC[61]. Similarly, hENT1 is a receptor that is upregulated on the surface of PDAC cells. Gemcitabine exerts its cytotoxic effects after its cellular uptake by hENT1. Therefore, hENT1 expression could potentially predict therapeutic activity of gemcitabine while its under-expression would be a mechanism for resistance[62,63]. hENT1 was studied as a predictor of response to gemcitabine in the adjuvant setting[64,65], but again could not be validated in the metastatic setting[66]. Additionally, microRNA, serum metabolism profiling, or methylation patterns may prove to be useful biomarkers for diagnosis and potential response to therapy in PDAC[67-69].
As systemic therapies for PDAC improves, more novel and sophisticated methods of monitoring tumor response are needed. Response to therapy is routinely based on imaging obtained during the course of treatment. Changes in the tumor-parenchyma interface may serve as an early predictor of response to therapy based on computed tomography imaging[70]. Other novel methods such as detection of circulating tumor cells and circulating tumor DNA (ctDNA) are on the horizon for both diagnosis and markers of response to therapy[71,72]. In patients with PDAC, ctDNA levels have been found to correlate with tumor burden and serial monitoring of ctDNA may provide a method to monitor early response to chemotherapy[73]. Patients who received NT had significantly lower circulating tumor cells compared to patients who were eligible for upfront resection who did not receive NT. Interestingly, alterations in circulating tumor cells were not only observed in response to treatment but also seen before disease recurrence[74]. Future studies will be needed to apply and validate them in the neoadjuvant setting.
As the use of NT increases for all stages of PDAC, a greater emphasis on optimizing patient-centered outcomes is necessary and likely to be best achieved through a personalized approach[75]. Despite the advantages of NT, recent evidence has highlighted that some patients will be unable to complete NT and undergo surgery, most commonly because of disease progression or a decrease in their performance status. Recent interest in prehabilitation and advanced nutritional strategies prior to pancreatectomy could be applied to this patient population to improve readiness for surgery[76,77]. Furthermore, although health-related quality of life appears to be preserved during NT, patient symptoms are common during treatment[78]. Prior literature has highlighted the value of monitoring and responding to changes in patient-reported outcomes which could be used to improve and personalize NT[79].
PDAC is an aggressive malignancy that is best treated in a multidisciplinary fashion using surgery, chemotherapy, and radiation. NT is an increasingly utilized approach that maximizes the receipt of multimodality therapy, improves margin-negative resection rates, and potentially increases survival durations. In the era of personalized medicine, the neoadjuvant period can also be used to emphasize precision oncology. Already, current methods of anatomically staging, molecularly profiling, and monitoring response to therapy can be used to personalize neoadjuvant treatment for localized PDAC. Ongoing efforts in developing novel biomarkers, innovative methods of measuring response, and patient-reported outcome measurements will expand opportunities for precision oncology during NT. These efforts, along with the development of novel treatment options for this aggressive disease, offer hope for improved multidisciplinary, patient-centered, cancer care.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Stifter K S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13300] [Cited by in F6Publishing: 14452] [Article Influence: 2890.4] [Reference Citation Analysis (2)] |
| 2. | Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 3. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1945] [Cited by in F6Publishing: 1818] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 4. | Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, Talamonti MS. Multimodality therapy for pancreatic cancer in the U.S. : utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Altman AM, Wirth K, Marmor S, Lou E, Chang K, Hui JYC, Tuttle TM, Jensen EH, Denbo JW. Completion of Adjuvant Chemotherapy After Upfront Surgical Resection for Pancreatic Cancer Is Uncommon Yet Associated With Improved Survival. Ann Surg Oncol. 2019;26:4108-4116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Aloia TA, Lee JE, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, Abbruzzese JL, Crane CH, Evans DB, Pisters PW. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Cloyd JM, Katz MH, Prakash L, Varadhachary GR, Wolff RA, Shroff RT, Javle M, Fogelman D, Overman M, Crane CH, Koay EJ, Das P, Krishnan S, Minsky BD, Lee JH, Bhutani MS, Weston B, Ross W, Bhosale P, Tamm EP, Wang H, Maitra A, Kim MP, Aloia TA, Vauthey JN, Fleming JB, Abbruzzese JL, Pisters PW, Evans DB, Lee JE. Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: a 25-Year Single-Institution Experience. J Gastrointest Surg. 2017;21:164-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Cloyd JM, Wang H, Egger ME, Tzeng CD, Prakash LR, Maitra A, Varadhachary GR, Shroff R, Javle M, Fogelman D, Wolff RA, Overman MJ, Koay EJ, Das P, Herman JM, Kim MP, Vauthey JN, Aloia TA, Fleming JB, Lee JE, Katz MHG. Association of Clinical Factors With a Major Pathologic Response Following Preoperative Therapy for Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2017;152:1048-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Cloyd JM, Nogueras-González GM, Prakash LR, Petzel MQB, Parker NH, Ngo-Huang AT, Fogelman D, Denbo JW, Garg N, Kim MP, Lee JE, Tzeng CD, Fleming JB, Katz MHG. Anthropometric Changes in Patients with Pancreatic Cancer Undergoing Preoperative Therapy and Pancreatoduodenectomy. J Gastrointest Surg. 2018;22:703-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 11. | Sugimoto M, Takahashi N, Farnell MB, Smyrk TC, Truty MJ, Nagorney DM, Smoot RL, Chari ST, Carter RE, Kendrick ML. Survival benefit of neoadjuvant therapy in patients with non-metastatic pancreatic ductal adenocarcinoma: A propensity matching and intention-to-treat analysis. J Surg Oncol. 2019;120:976-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Youngwirth LM, Nussbaum DP, Thomas S, Adam MA, Blazer DG 3rd, Roman SA, Sosa JA. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18 243 patients. J Surg Oncol. 2017;116:127-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Cloyd JM, Heh V, Pawlik TM, Ejaz A, Dillhoff M, Tsung A, Williams T, Abushahin L, Bridges JFP, Santry H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Benson AB 3rd, Binder E, Cardin DB, Cha C, Chiorean EG, Chung V, Czito B, Dillhoff M, Dotan E, Ferrone CR, Hardacre J, Hawkins WG, Herman J, Ko AH, Komanduri S, Koong A, LoConte N, Lowy AM, Moravek C, Nakakura EK, O'Reilly EM, Obando J, Reddy S, Scaife C, Thayer S, Weekes CD, Wolff RA, Wolpin BM, Burns J, Darlow S. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 658] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 15. | Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, Pitt HA, Lillemoe KD, Cameron JL. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621-33; discussion 633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 437] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, Matsuyama Y, Unno M; Study Group of Preoperative Therapy for Pancreatic Cancer (Prep) and Japanese Study Group of Adjuvant Therapy for Pancreatic cancer (JSAP). Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 vs upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 17. | Quiros RM, Brown KM, Hoffman JP. Neoadjuvant therapy in pancreatic cancer. Cancer Invest. 2007;25:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Lim KH, Chung E, Khan A, Cao D, Linehan D, Ben-Josef E, Wang-Gillam A. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 2012;17:192-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, van Eijck CHJ, Groot Koerkamp B, Rasch CRN, van Tienhoven G; Dutch Pancreatic Cancer Group. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105:946-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 331] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 20. | Sohal DPS, Duong M, Ahmad SA, Gandhi NS, Beg MS, Wang-Gillam A, Wade JL 3rd, Chiorean EG, Guthrie KA, Lowy AM, Philip PA, Hochster HS. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021;7:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 21. | Brown ZJ, Cloyd JM. Trends in the utilization of neoadjuvant therapy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2021;123:1432-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, Oh DY, Chie EK, Lee JM, Heo JS, Park JO, Lim DH, Kim SH, Park SJ, Lee WJ, Koh YH, Park JS, Yoon DS, Lee IJ, Choi SH. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg. 2018;268:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 23. | Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, Pinelli D, Mosconi S, Doglioni C, Chiaravalli M, Pircher C, Arcidiacono PG, Torri V, Maggiora P, Ceraulo D, Falconi M, Gianni L. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3:413-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 24. | Ghaneh P, Palmer DH, Cicconi S, Halloran C, Psarelli EE, Rawcliffe CL, Sripadam R, Mukherjee S, Wadsley J, Al-Mukhtar A, Jiao LR, Wasan HS, Carter R, Graham JS, Ammad F, Evans J, Tjaden C, Hackert T, Buchler MW, Neoptolemos JP. ESPAC-5F: Four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pan. J Clin Oncol. 2020;15:4505. [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 25. | Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Heinrich S, Besselink M, Moehler M, van Laethem JL, Ducreux M, Grimminger P, Mittler J, Lang H, Lutz MP, Lesurtel M; Scientific and Research Committee of the E-AHPBA and the EORTC pancreas working group. Opinions and use of neoadjuvant therapy for resectable, borderline resectable, and locally advanced pancreatic cancer: international survey and case-vignette study. BMC Cancer. 2019;19:675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, Strobel O, Jäger D, Ulrich A, Büchler MW. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg. 2016;264:457-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 28. | Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, Fishman EK, Hruban RH, Yu J, Burkhart RA, Cameron JL, Weiss MJ, Wolfgang CL, He J. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg. 2019;270:340-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 241] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 29. | Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, Borbath I, Bouché O, Shannon J, André T, Mineur L, Chibaudel B, Bonnetain F, Louvet C; LAP07 Trial Group. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 622] [Cited by in F6Publishing: 659] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 30. | Katz MHG, Shi Q, Ahmad SA, Herman JM, Marsh R de W, Collisson EA, Schwartz LH, Martin RCG, Conway WC, Truty M, Kindler HL, Lowy AM, Philip PA, Bekaii-Saab TS, Cardin DB, LoConte NK, Venook AP. Preoperative modified FOLFIRINOX (mFOLFIRINOX) followed by chemoradiation (CRT) for borderline resectable (BLR) pancreatic cancer (PDAC): Initial results from Alliance Trial A021101. J Clin Oncol. 2015;15:4008. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Faris JE, Zhu AX, Goyal L, Lillemoe KD, DeLaney TF, Fernández-Del Castillo C, Ferrone CR, Hong TS. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:963-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 379] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 32. | Katz MHG, Shi Q, Meyers JP, Herman JM, Choung M, Wolpin BM, Ahmad S, Marsh R de W, Schwartz LH, Behr S, Frankel WL, Collisson EA, Leenstra JL, Williams TM, Vaccaro GM, Venook AP, Meyerhardt JA, O’Reilly EM. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol. 2021;39:377. [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 33. | Ahmad SA, Duong M, Sohal DPS, Gandhi NS, Beg MS, Wang-Gillam A, Wade JL 3rd, Chiorean EG, Guthrie KA, Lowy AM, Philip PA, Hochster HS. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg. 2020;272:481-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 34. | Ferrone CR. Alliance for Clinical Trials in Oncology. A Phase III Trial of Perioperative Versus Adjuvant Chemotherapy for Resectable Pancreatic Cancer. [accessed 2021 January 25]. In: ClinicalTrials.gov [Internet]. Mobile (AL): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04340141 ClinicalTrials.gov Identifier: NCT04340141. [Cited in This Article: ] |
| 35. | Wood LD, Hruban RH. Pathology and molecular genetics of pancreatic neoplasms. Cancer J. 2012;18:492-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Von Hoff DD, Stephenson JJ Jr, Rosen P, Loesch DM, Borad MJ, Anthony S, Jameson G, Brown S, Cantafio N, Richards DA, Fitch TR, Wasserman E, Fernandez C, Green S, Sutherland W, Bittner M, Alarcon A, Mallery D, Penny R. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877-4883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 458] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 37. | Krepline AN, Bliss L, Geurts J, Akinola I, Christians KK, George B, Ritch PS, Hall WA, Erickson BA, Evans DB, Tsai S. Role of Molecular Profiling of Pancreatic Cancer After Neoadjuvant Therapy: Does it Change Practice? J Gastrointest Surg. 2020;24:235-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Blair AB, Groot VP, Gemenetzis G, Wei J, Cameron JL, Weiss MJ, Goggins M, Wolfgang CL, Yu J, He J. BRCA1/BRCA2 Germline Mutation Carriers and Sporadic Pancreatic Ductal Adenocarcinoma. J Am Coll Surg 2018; 226: 630-637. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 39. | Wattenberg MM, Asch D, Yu S, O'Dwyer PJ, Domchek SM, Nathanson KL, Rosen MA, Beatty GL, Siegelman ES, Reiss KA. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122:333-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 40. | Connor AA, Denroche RE, Jang GH, Timms L, Kalimuthu SN, Selander I, McPherson T, Wilson GW, Chan-Seng-Yue MA, Borozan I, Ferretti V, Grant RC, Lungu IM, Costello E, Greenhalf W, Palmer D, Ghaneh P, Neoptolemos JP, Buchler M, Petersen G, Thayer S, Hollingsworth MA, Sherker A, Durocher D, Dhani N, Hedley D, Serra S, Pollett A, Roehrl MHA, Bavi P, Bartlett JMS, Cleary S, Wilson JM, Alexandrov LB, Moore M, Wouters BG, McPherson JD, Notta F, Stein LD, Gallinger S. Association of Distinct Mutational Signatures With Correlates of Increased Immune Activity in Pancreatic Ductal Adenocarcinoma. JAMA Oncol. 2017;3:774-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 41. | Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, Choti MA, Yeo CJ, McCue P, White MA, Knudsen ES. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 763] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 42. | Miyoshi T, Kondo K, Toba H, Yoshida M, Fujino H, Kenzaki K, Sakiyama S, Takehisa M, Tangoku A. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur+uracil) in patients with non-small cell lung cancer. Anticancer Res. 2007;27:2641-2648. [PubMed] [Cited in This Article: ] |
| 43. | Chen G, Tian X, Liu Z, Zhou S, Schmidt B, Henne-Bruns D, Bachem M, Kornmann M. Inhibition of endogenous SPARC enhances pancreatic cancer cell growth: modulation by FGFR1-III isoform expression. Br J Cancer. 2010;102:188-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Akita H, Zheng Z, Takeda Y, Kim C, Kittaka N, Kobayashi S, Marubashi S, Takemasa I, Nagano H, Dono K, Nakamori S, Monden M, Mori M, Doki Y, Bepler G. Significance of RRM1 and ERCC1 expression in resectable pancreatic adenocarcinoma. Oncogene. 2009;28:2903-2909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, Cass C, Lai R, Mackey JR. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956-6961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 46. | Tsai S, Christians KK, George B, Ritch PS, Dua K, Khan A, Mackinnon AC, Tolat P, Ahmad SA, Hall WA, Erickson BA, Evans DB. A Phase II Clinical Trial of Molecular Profiled Neoadjuvant Therapy for Localized Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;268:610-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Chantrill LA, Nagrial AM, Watson C, Johns AL, Martyn-Smith M, Simpson S, Mead S, Jones MD, Samra JS, Gill AJ, Watson N, Chin VT, Humphris JL, Chou A, Brown B, Morey A, Pajic M, Grimmond SM, Chang DK, Thomas D, Sebastian L, Sjoquist K, Yip S, Pavlakis N, Asghari R, Harvey S, Grimison P, Simes J, Biankin AV; Australian Pancreatic Cancer Genome Initiative (APGI); Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial Management Committee of the Australasian Gastrointestinal Trials Group (AGITG). Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res. 2015;21:2029-2037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 48. | Al Abbas AI, Zenati M, Reiser CJ, Hamad A, Jung JP, Zureikat AH, Zeh HJ 3rd, Hogg ME. Serum CA19-9 Response to Neoadjuvant Therapy Predicts Tumor Size Reduction and Survival in Pancreatic Adenocarcinoma. Ann Surg Oncol. 2020;27:2007-2014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Bartlett DL, Zureikat AH, Bahary N, Zeh HJ 3rd. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21:4351-4358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 50. | Liu H, Zenati MS, Rieser CJ, Al-Abbas A, Lee KK, Singhi AD, Bahary N, Hogg ME, Zeh HJ 3rd, Zureikat AH. CA19-9 Change During Neoadjuvant Therapy May Guide the Need for Additional Adjuvant Therapy Following Resected Pancreatic Cancer. Ann Surg Oncol. 2020;27:3950-3960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Rose JB, Edwards AM, Rocha FG, Clark C, Alseidi AA, Biehl TR, Lin BS, Picozzi VJ, Helton WS. Sustained Carbohydrate Antigen 19-9 Response to Neoadjuvant Chemotherapy in Borderline Resectable Pancreatic Cancer Predicts Progression and Survival. Oncologist. 2020;25:859-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Tzeng CW, Balachandran A, Ahmad M, Lee JE, Krishnan S, Wang H, Crane CH, Wolff RA, Varadhachary GR, Pisters PW, Aloia TA, Vauthey JN, Fleming JB, Katz MH. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford). 2014;16:430-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 53. | Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 565] [Cited by in F6Publishing: 592] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 54. | Perri G, Prakash L, Wang H, Bhosale P, Varadhachary GR, Wolff R, Fogelman D, Overman M, Pant S, Javle M, Koay E, Herman J, Kim M, Ikoma N, Tzeng CW, Lee JE, Katz MHG. Radiographic and Serologic Predictors of Pathologic Major Response to Preoperative Therapy for Pancreatic Cancer. Ann Surg. 2021;273:806-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 55. | Cloyd JM, Ejaz A, Shen C, Dillhoff M, Williams TM, Noonan A, Pawlik TM, Tsung A. Pathologic complete response following neoadjuvant therapy for pancreatic ductal adenocarcinoma: defining the incidence, predictors, and outcomes. HPB (Oxford). 2020;22:1569-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Vreeland TJ, McAllister F, Javadi S, Prakash LR, Fogelman DR, Ho L, Varadhachary G, Aloia TA, Vauthey JN, Lee JE, Kim MP, Katz MHG, Tzeng CD. Benefit of Gemcitabine/Nab-Paclitaxel Rescue of Patients With Borderline Resectable or Locally Advanced Pancreatic Adenocarcinoma After Early Failure of FOLFIRINOX. Pancreas. 2019;48:837-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, Quertinmont E, Svrcek M, Elarouci N, Iovanna J, Franchimont D, Verset L, Galdon MG, Devière J, de Reyniès A, Laurent-Puig P, Van Laethem JL, Bachet JB, Maréchal R. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology. 2018;155:1999-2013.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 58. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative; Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2480] [Cited by in F6Publishing: 2210] [Article Influence: 276.3] [Reference Citation Analysis (0)] |
| 59. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 1207] [Article Influence: 301.8] [Reference Citation Analysis (0)] |
| 60. | Ormanns S, Haas M, Baechmann S, Altendorf-Hofmann A, Remold A, Quietzsch D, Clemens MR, Bentz M, Geissler M, Lambertz H, Kruger S, Kirchner T, Heinemann V, Boeck S. Impact of SPARC expression on outcome in patients with advanced pancreatic cancer not receiving nab-paclitaxel: a pooled analysis from prospective clinical and translational trials. Br J Cancer. 2016;115:1520-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Hidalgo M, Plaza C, Musteanu M, Illei P, Brachmann CB, Heise C, Pierce D, Lopez-Casas PP, Menendez C, Tabernero J, Romano A, Wei X, Lopez-Rios F, Von Hoff DD. SPARC Expression Did Not Predict Efficacy of nab-Paclitaxel plus Gemcitabine or Gemcitabine Alone for Metastatic Pancreatic Cancer in an Exploratory Analysis of the Phase III MPACT Trial. Clin Cancer Res. 2015;21:4811-4818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 62. | Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, Sandvold ML. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Mori R, Ishikawa T, Ichikawa Y, Taniguchi K, Matsuyama R, Ueda M, Fujii Y, Endo I, Togo S, Danenberg PV, Shimada H. Human equilibrative nucleoside transporter 1 is associated with the chemosensitivity of gemcitabine in human pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncol Rep. 2007;17:1201-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, Crawford CR, Cass CE. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58:4349-4357. [PubMed] [Cited in This Article: ] |
| 65. | Bird NT, Elmasry M, Jones R, Psarelli E, Dodd J, Malik H, Greenhalf W, Kitteringham N, Ghaneh P, Neoptolemos JP, Palmer D. Immunohistochemical hENT1 expression as a prognostic biomarker in patients with resected pancreatic ductal adenocarcinoma undergoing adjuvant gemcitabine-based chemotherapy. Br J Surg. 2017;104:328-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Poplin E, Wasan H, Rolfe L, Raponi M, Ikdahl T, Bondarenko I, Davidenko I, Bondar V, Garin A, Boeck S, Ormanns S, Heinemann V, Bassi C, Evans TR, Andersson R, Hahn H, Picozzi V, Dicker A, Mann E, Voong C, Kaur P, Isaacson J, Allen A. Randomized, multicenter, phase II study of CO-101 vs gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: including a prospective evaluation of the role of hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol. 2013;31:4453-4461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 67. | Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, Davidson SM, Papagiannakopoulos T, Yang A, Dayton TL, Ogino S, Stampfer MJ, Giovannucci EL, Qian ZR, Rubinson DA, Ma J, Sesso HD, Gaziano JM, Cochrane BB, Liu S, Wactawski-Wende J, Manson JE, Pollak MN, Kimmelman AC, Souza A, Pierce K, Wang TJ, Gerszten RE, Fuchs CS, Vander Heiden MG, Wolpin BM. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 444] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 68. | Ding Z, Wu H, Zhang J, Huang G, Ji D. MicroRNAs as novel biomarkers for pancreatic cancer diagnosis: a meta-analysis based on 18 articles. Tumour Biol. 2014;35:8837-8848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, Calatayud D, Nielsen SE, Yilmaz M, Holländer NH, Andersen KK, Johansen JS. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311:392-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 321] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 70. | Amer AM, Zaid M, Chaudhury B, Elganainy D, Lee Y, Wilke CT, Cloyd J, Wang H, Maitra A, Wolff RA, Varadhachary G, Overman MJ, Lee JE, Fleming JB, Tzeng CW, Katz MH, Holliday EB, Krishnan S, Minsky BD, Herman JM, Taniguchi CM, Das P, Crane CH, Le O, Bhosale P, Tamm EP, Koay EJ. Imaging-based biomarkers: Changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer. 2018;124:1701-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol. 2017;11:40-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 72. | Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16:398-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 503] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 73. | Wei T, Zhang Q, Li X, Su W, Li G, Ma T, Gao S, Lou J, Que R, Zheng L, Bai X, Liang T. Monitoring Tumor Burden in Response to FOLFIRINOX Chemotherapy Via Profiling Circulating Cell-Free DNA in Pancreatic Cancer. Mol Cancer Ther. 2019;18:196-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 74. | Gemenetzis G, Groot VP, Yu J, Ding D, Teinor JA, Javed AA, Wood LD, Burkhart RA, Cameron JL, Makary MA, Weiss MJ, He J, Wolfgang CL. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate With Disease Status: Results of the Prospective CLUSTER Study. Ann Surg. 2018;268:408-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 107] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 75. | Cloyd JM, Tsung A, Hays J, Wills CE, Bridges JF. Neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma: The need for patient-centered research. World J Gastroenterol. 2020;26:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Ngo-Huang A, Parker NH, Bruera E, Lee RE, Simpson R, O'Connor DP, Petzel MQB, Fontillas RC, Schadler K, Xiao L, Wang X, Fogelman D, Sahai SK, Lee JE, Basen-Engquist K, Katz MHG. Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life. Integr Cancer Ther. 2019;18:1534735419894061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 77. | Jabłońska B, Mrowiec S. The Role of Immunonutrition in Patients Undergoing Pancreaticoduodenectomy. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Cloyd JM, Hyman S, Huwig T, Monsour C, Santry H, Wills C, Tsung A, Bridges JFP. Patient experience and quality of life during neoadjuvant therapy for pancreatic cancer: a systematic review and study protocol. Support Care Cancer. 2021;29:3009-3016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318:197-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1430] [Cited by in F6Publishing: 1306] [Article Influence: 186.6] [Reference Citation Analysis (0)] |