Copyright
©The Author(s) 2021.
World J Gastroenterol. Jun 28, 2021; 27(24): 3609-3629
Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3609
Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3609
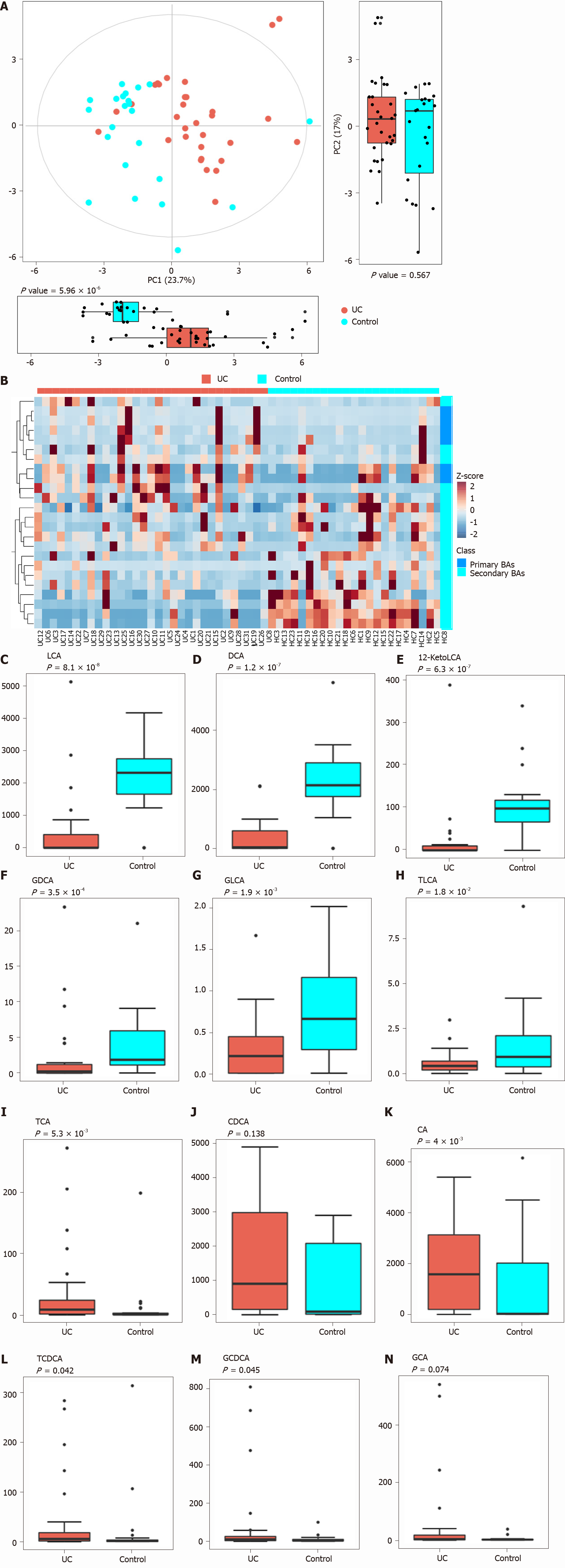
Figure 3 Analysis of difference in fecal bile acids between the ulcerative colitis and control groups.
A: Principal component analysis was performed to evaluate the similarity of the fecal BAs of the two groups. Twenty-four BAs clearly distinguished the ulcerative colitis (UC) group from the control group; B: Heatmap showing the individual BA concentrations in the samples (log-transformed). Shades of red and blue represent high and low BA concentrations, respectively (see color scale); C-H: Fecal secondary BAs in UC patients, such as lithocholic acid, deoxycholic acid, glyco-deoxycholic acid, glyco-lithocholic acid, and tauro-lithocholate, were significantly lower than those in healthy controls; I and K-M: The primary BAs such as tauro-cholic acid, cholic acid, tauro-chenodeoxycholic acid, and glyco-chenodeoxycholic acid were significantly higher than those in healthy controls; J and N: The concentrations of chenodeoxycholic acid and glyco-cholic acid showed a tendency to increase in UC patients but the increases were not significant. UC: Ulcerative colitis; PCA: Principal component analysis; BAs: Bile acids; LCA: Lithocholic acid; DCA: Deoxycholic acid; GDCA: Glyco-deoxycholic acid; GLCA: Glyco-lithocholic acid; TLCA: Tauro-lithocholate; TCA: Tauro-cholic acid; CA: Cholic acid; TCDCA: Tauro-chenodeoxycholic acid; GCDCA: Glyco-chenodeoxycholic acid; CDCA: Chenodeoxycholic acid; GCA: Glyco-cholic acid.
- Citation: Yang ZH, Liu F, Zhu XR, Suo FY, Jia ZJ, Yao SK. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J Gastroenterol 2021; 27(24): 3609-3629
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3609