Published online May 7, 2021. doi: 10.3748/wjg.v27.i17.1959
Peer-review started: January 25, 2021
First decision: February 27, 2021
Revised: March 6, 2021
Accepted: April 12, 2021
Article in press: April 12, 2021
Published online: May 7, 2021
The association between chronic hepatitis C (CHC) infection and extrahepatic manifestations (EHMs), particularly cardiometabolic diseases, has been extensively examined. However, there has still been insufficient evaluation for these EHMs after virological cure. Several multidirectional mechanisms have been proposed explaining the ability of hepatitis C virus (HCV) developing EHMs, cardiometabolic ones, as well as the effect of antiviral therapy to resolve these EHMs. Data on these manifestations after achieving sustained virologic response (SVR) are still conflicting. However, current evidence suggests that reversal of hepatic steatosis and its coexistent hypocholesterolemia after successful viral eradication led to unfavorable lipid profile, which increases cardiovascular disease (CVD) risk. Additionally, most observations showed that metabolic alterations, such as insulin resistance and diabetes mellitus (DM), undergo some degree of reduction after viral clearance. These changes seem HCV-genotype dependent. Interferon-based antiviral therapy and direct acting antiviral drugs were shown to minimize incidence of DM. Large epidemiological studies that investigated the effect of SVR on CVD showed great discrepancies in terms of results, with predominant findings indicating that CVD events decreased in patients with SVR compared to non-responders or untreated ones. In this review, we present a summary of the current knowledge regarding extrahepatic sequelae of CHC following SVR, which may have an impact on healthcare providers’ clinical practice.
Core Tip: The implementation of direct acting antiviral drugs has dramatically changed the landscape of hepatitis C virus (HCV) treatment, with over 95% of patients achieving sustained virologic response (SVR). Although consistent evidence demonstrated better outcomes for both hepatic and extrahepatic complications after viral clearance, data on cardiometabolic manifestation showed inconsistent results. In this review, we are shading light on the latest findings about cardiometabolic extrahepatic manifestations post-SVR. These updates may guide clinicians engaged in HCV care to integrate in their management post-viral eradication risks and subsequent long-term care.
- Citation: Shengir M, Elgara M, Sebastiani G. Metabolic and cardiovascular complications after virological cure in hepatitis C: What awaits beyond. World J Gastroenterol 2021; 27(17): 1959-1972
- URL: https://www.wjgnet.com/1007-9327/full/v27/i17/1959.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i17.1959
Chronic hepatitis C (CHC) infection caused by hepatitis C virus (HCV) is associated with substantial morbidity and mortality globally, affecting approximately 2.5% individuals (equivalent to 177.5 million) worldwide[1]. It is currently one of the leading etiologies for hepatocellular carcinoma (HCC) and decompensated cirrhosis requiring liver transplantation in Western countries[2,3]. The primary goal of treatment is to achieve the cure of the infection or sustained virologic response (SVR), defined as undetectable HCV RNA in the serum 12 or 24 wk after the end of treatment[4,5]. Since the introduction of first-generation direct acting antiviral agents (DAAs), boceprevir and telaprevir, in 2011, there has been a rapidly expanding population of CHC patients achieving SVR[6]. Viral eradication has been associated with marked reduction in the risk of end-stage liver disease, need for liver transplantation, and decrease in both liver-related and overall mortality[7].
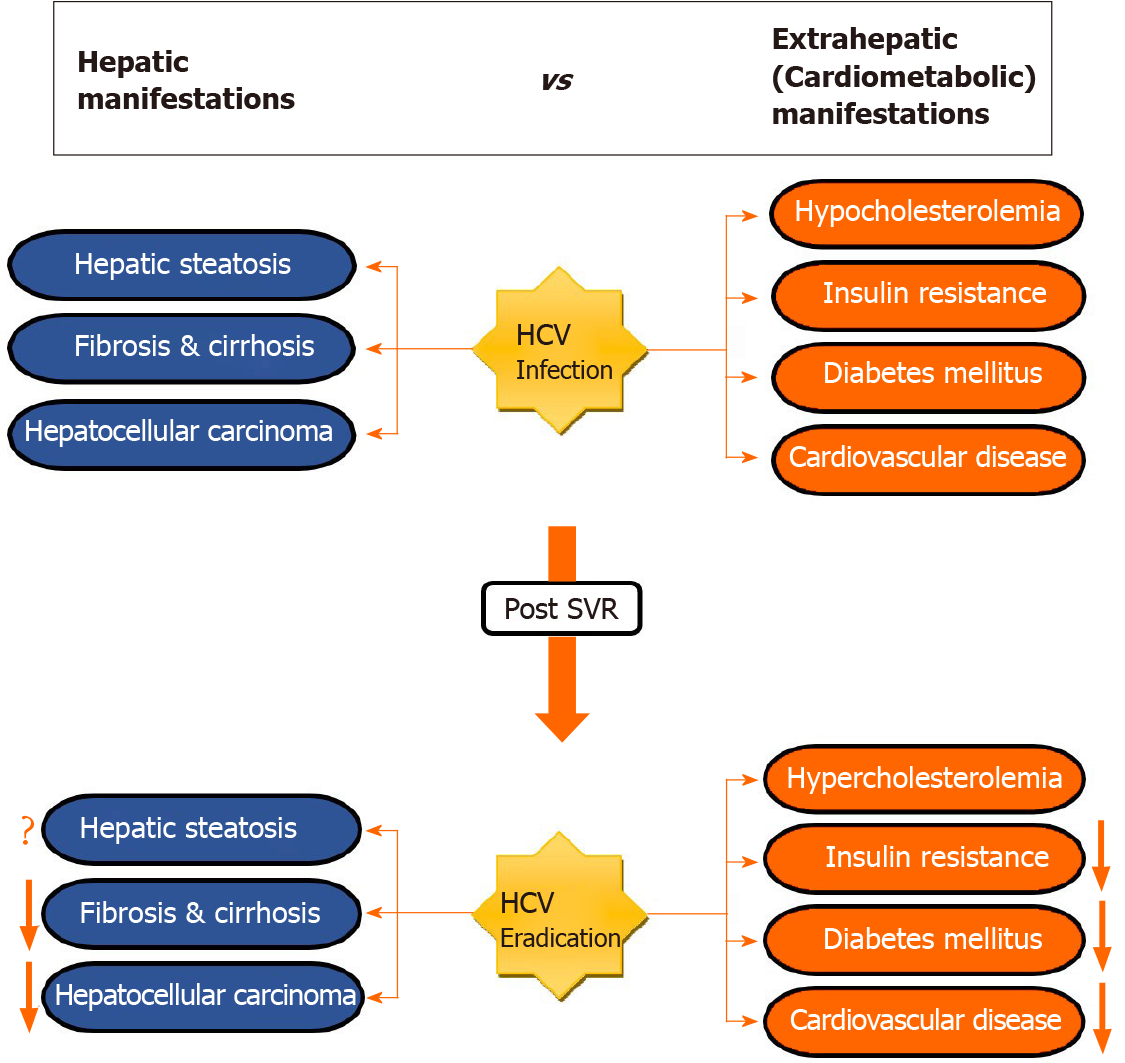
Although HCV is a hepatotropic virus, for two decades several studies described the association between HCV and a heterogeneous array of extrahepatic manifestations (EHMs)[8-10] (Figure 1). Yet, the mechanism by which the virus evokes the systemic diseases remain to be elucidated. Endocrine-metabolic alterations, which are most frequently found in CHC patients, are thought to be caused by direct and indirect effects of disturbing host lipid and glucose metabolism[11,12], as well as alteration in adipocytokines released from adipose tissue[13,14]. Likewise, CHC infection has also been identified as an independent predictor for cardiovascular events such as carotid artery atherosclerosis, stroke, myocardial ischemia and heart failure, all of which are linked with poor outcomes[12]. Nevertheless, the impact on cardiovascular disease (CVD) is not fully established[15]. In clinical settings, the prognosis of CHC is not only depending on liver-related outcomes but also on extrahepatic sequelae.
Recently, much attention is drawn toward EHMs that occur following viral cure. Nonetheless, whether the development of such manifestations is a long-term consequence of the viral infection itself or an effect of HCV medications remains unknown. In this context, various reports have shown ample evidence for high prevalence of CVD and metabolic alterations such as dyslipidemia, hepatic steatosis, insulin resistance (IR), obesity and diabetes mellitus (DM)[16-19] (Table 1). While the impact of CHC infection on liver-related outcomes pre- and post-treatment has been well studied, extrahepatic sequelae, especially in post-SVR setting, are less well known. This review article highlights the current knowledge regarding the effect of SVR on EHMs.
| Ref. | Antiviral regimen | The studied HCV-associated cardiometabolic manifestations | Post SVR outcomes |
| Fernández-Rodríguez et al[28], 2006 | NA1 | Lipid disturbances. Hepatic steatosis | Hypercholesterolemia in patients with genotype 3 |
| No change in genotype 3 non-responders and in patients with genotype 1 regardless of response | |||
| Decrease in steatosis | |||
| Giordanino et al[71], 2008 | IFN monotherapy or Peg-IFN + RBV (24-48 wk) | Glucose abnormalities (IFG or DM) | No significant reduction in the risk of glucose intolerance in long-term responders and non-responders |
| Arase et al[70], 2009 | IFN monotherapy or IFN + RBV2 | DM | Decreased incidence of DM in sustained responders. However, its development is associated with advanced liver disease |
| Corey et al[18], 2009 | NA1 | Lipid abnormalities | Increased LDL and total cholesterol from baseline compared to non-responders |
| Risk of CVD | Increased CVD risk profile | ||
| Conjeevaram et al[67], 2011 | Peg-IFN + RBV (24-48 wk) | IR | Decreased IR |
| Obesity | Decreased in BMI | ||
| Kuo et al[27], 2011 | Peg-IFN + RBV (24 wk) | Change in serum lipid | Total cholesterol and triglycerides levels significantly increased |
| No evident change in lipid profile occurred in non-SVR group | |||
| Aghemo et al[68], 2012 | Peg-IFN + RBV2 | IR in non-diabetic CHC patients | Baseline and posttreatment HOMA-IR values were similar in SVR patients |
| Significant increase in HOMA-IR was noted in non-SVR patients | |||
| Clark et al[25], 2012 | Albinterferon α-2b + RBV | Lipid abnormalities in genotypes 2,3 | Hypercholesterolemia |
| Thompson et al[66], 2012 | Albinterferon α-2b vs Peg-IFN + RBV (24-48 wk) | IR in genotypes 1,2,3 | Reduced IR in genotype 1 responders |
| No change in genotype 1 non-responders and genotype 2 and 3 regardless of the response | |||
| Chang et al[29], 2014 | eg-IFN + RBV (24/48 wk) | Lipids and IR in genotypes 2, 3 | Increased total cholesterol and triglycerides in sustained responders |
| Decreased HOMA-IR in patients with SVR and baseline IR | |||
| High HOMA-IR was found in patients without baseline IR (only in genotype 1) | |||
| Hsu et al[88], 2015 | Peg-IFN + RBV (16-48 wk) | Acute coronary syndrome and ischemic stroke | Improvement in both studied circulatory outcomes |
| Innes et al[89], 2015 | NA1 | CVD | Reduced hazard and absolute risk for CVD |
| Meissner et al[24], 2015 | SOF + RBV (24 wk) | Lipid disturbances in genotype 1 | Increased LDL level and particle size and decreased triglycerides concentration and VLDL particle size irrespective to treatment response |
| Increased intrahepatic lipid-related genes in sustained responders | |||
| Leone et al[72], 2016 | IFN-based regimen | DM and CVD | No significant risk reduction in DM and CVD in SVR group as opposed to non SVR |
| Yair-Sabag et al[39], 2016 | Peg-IFN + RBV (24-48 wk) | IFG and DM. Triglycerides. Hepatic steatosis | Lower IFG and DM, and higher triglycerides in sustained responders |
| Improvement in hepatic steatosis | |||
| Chang et al[16], 2017 | NA1 | Cardiovascular complications | An increased adipokine PAI-1 in SVR group, which accelerates cardiovascular risk, especially in vulnerable cases |
| Mahale et al[69], 2018 | IFN-based regimen2 | DM and CVD | Antiviral therapy associated with lower risk of DM and stroke whereas no significant effect on CVD |
| Nahon et al[90], 2017 | Peg-IFN + RBV (16-48 wk) or combination therapies3 | CVD | Lower risk of CVD in SVR subjects in comparison to non SVR |
| Stine et al[74], 2017 | DAAs2,3 | DM in genotypes 1, 2, 3 | Glycosylated hemoglobin was not affected in known diabetic patients |
| 1/3 of patients required escalation of anti-diabetic therapy during antiviral treatment | |||
| Carvalho et al[11], 2018 | SOF + LDV ± RBV (group 1) vs Peg-IFN + RBV (group 2) | Lipid levels. Serum glucose. IR | While total cholesterol increased in both groups, triglycerides levels decreased in group 1 and increased in group 2 |
| LDL elevated in group 1 and No change in group 2 | |||
| No significant variation in serum glucose | |||
| Significant increase in HOMA-IR only in group 2 | |||
| Kawagishi et al[17], 2018 | DAAs3 | Hepatic steatosis. Lipid abnormalities | Decrease in CAP and LDL in patients with high baseline values |
| Elevated sdLDL in patients who had dyslipidemia and hepatic steatosis at 24 wk | |||
| Li et al[73], 2018 | DAAs4 | DM | Lower risk of DM in SVR patients than in treatment failure group |
| Noureddin et al[46], 2018 | DAAs3 | Hepatic steatosis and fibrosis | High prevalence of fatty liver |
| Although fibrosis has been reduced in patients with and without steatosis compared to baseline, patients with steatosis continued to have clinically significant liver stiffness | |||
| Li et al[10], 2019 | IFN + RBV (48 wk) | Serum glucose level and IR | Reduced glucose level |
| Improved IR | |||
| Butt et al[87], 2019 | IFN + RBV2,3. DAAs2,3 | CVD | Lower incidence in treatment group, compared to controls |
| DAAs showed greater risk reduction than interferon-based regimen | |||
| SVR associated with decreased CVD risk | |||
| Abdo et al[75], 2020 | SOF + DCV (12-24 wk) | Glycemic status, IR, and lipid profile in CHC patients with DM | Improvement of glycemic state and HOMA-IR |
| Global worsening of lipid profile | |||
| Graf et al[45], 2020 | DAAs3 | IR, lipid perturbations, body weight changes, and hepatic steatosis | Lower HOMA-IR compared to baseline |
| Higher total cholesterol, LDL, and HDL | |||
| Higher CAP relative to baseline | |||
| BMI did not significantly change over time | |||
| Huang et al[31], 2020 | DAAs4 | Lipids and cardiovascular events | Increased total cholesterol and LDL |
| Higher cardio-cerebral diseases |
It has been known that HCV possesses a mutual relationship with host lipids and lipoproteins metabolisms, which the virus uses for multiple key steps in its life cycle[20,21]. HCV circulates as a lipid-rich particle, utilizing lipoprotein cell receptors to gain entry into the hepatocyte[22,23]. Within hepatocytes, it influences three mechanisms in lipid metabolism: It upregulates lipid biosynthesis, impairs mitochondrial β-oxidation and thus lipid degradation, and reduces apolipoprotein exportation, in particular very low-density lipoprotein cholesterol (LDL), resulting in significant intracellular lipid accumulation and circulating hypocholesterolemia and hypolipoproteinemia[13].
Several studies have linked successful HCV eradication with rebound rise in lipid levels. Meissner et al[24], who investigated the influence of DAAs, sofosbuvir and ribavirin, on serum lipid profiles and intrahepatic lipid-related genes expression in patients with genotype 1 CHC, reported that serum LDL level and molecular size increased early in therapy, whereas triglycerides concentration and very low-density lipoprotein cholesterol (VLDL) particle size decreased concomitantly, irrespective of treatment outcome. This observation likely reflects a direct effect on lipid metabolism associated with the inhibition of HCV replication[24]. This notion was further supported in several reports. Clark et al[25], used cholesterol metabolites as an indicator to evaluate the impact of HCV on lipid metabolism. In this study, genotype 3 but not genotype 2 showed a selected interference with late cholesterol synthesis pathway, resulting in hypocholesterolemia. However, this interference was resolved after SVR. Another Japanese study that included 100 subjects showed early rebound (within 28 d) in LDL level in CHC patients who underwent interferon-free DAA treatment. However, the elevation was regimen-specific, more prominent in the group who received daclatasvir and asunaprevir for 24 wk than in those received ledipasvir and sofosbuvir for 12 wk[26]. In addition, many reports correlated the rebound in lipid profile with treatment response status[27-29]. This was clearly demonstrated by Corey et al[18], which conducted a 2 steps study to evaluate the relationship between CHC infection and its treatment with lipid levels. After confirming that HCV infection is associated with significantly lower LDL concentrations in the first step, they found that remarkable hyperlipidemia was developed in patients who achieved viral clearance, compared to non-responders or those who relapsed. In the same context, some studies have further investigated the role HCV genotype on post-SVR hypercholesterolemia. In a study that included 215 patients, Fernández-Rodríguez et al[28] observed that increased serum cholesterol levels were associated with genotype 3 in patients who achieved SVR. In contrast, un-changed serum cholesterol figures were noted in genotype 3 non-responders and genotype 1 regardless of response[28]. Although the reversal of both hepatic steatosis and hypolipidemia has been reported only in genotype 3 in this study, there is accumulating evidence demonstrating that the reversal of hypolipidemia is not HCV-genotype specific[29,30].
Many reports proposed that atherosclerotic CVD risk increases after successful eradication of HCV due to the unfavorable lipid profile, which is a result of reversed hypolipidemia, represented in high serum LDL and small dense LDL. The latter has greater atherogenic potential and is a better marker for prediction of CVD than LDL[31,32]. The important question at this point is whether these patients require lipid-lowering treatment. According to the National Cholesterol Education Program Adult Treatment Plan Guideline III, patients should be put on lipid lowering agents for: (1) an LDL >100 mg/dL, if they have coronary heart disease or its equivalents[1]; (2) an LDL >130 mg/dL, if they have two or more major coronary heart disease risk factors[2]; and (3) an LDL >190 mg/dL, with none or one major risk factor[33]. Corey and his colleagues have found that 13% of their studied cohort had post-SVR LDL levels requiring lipid lowering therapy as these patients had values > 130 mg/dL plus presence of two or more major coronary heart disease risk factors. Nonetheless, before antiviral therapy, none of these patients had LDL readings requiring medications. Post-treatment lipid profile deterioration may reach clinically meaningful level requiring the consideration for cholesterol lowering therapy.
Hepatic steatosis is a frequent histological liver finding in patients with CHC[34]. Since HCV is known to hijack lipid metabolic pathways for virion maturation and secretion, several possible mechanisms of HCV-induced liver steatosis have been suggested. HCV induces lipogenesis by increasing intrahepatic fat milieu through sterol regulatory element binding protein 1c, which is a protein that overexpresses LDL receptors which in turn facilitates fatty acid uptake by hepatocytes, leading to higher intrahepatic fat content[35]. In contrast, HCV inhibits lipolysis by disturbing mito-chondrial β-oxidation[36], either directly by the virus itself or indirectly via downregulation of the enzyme carnitine palmitoyltransferase-1, which regulates fatty acids oxidation[37,38]. These two mechanisms further potentiated by HCV-induced IR[39]. Moreover, HCV core protein suppresses the activity of microsomal triacylglycerol transfer protein, which is used for the assembly and secretion of VLDL, resulting in increased intracytoplasmic lipid droplets and therefore steatosis[40]. Miyoshi et al[41], who studied the role of HCV core protein in development of steatosis in HCV genotype 2, revealed that core protein activates the enzyme δ-9 desaturase, fatty acid metabolizing enzyme, and therefore leads to accumulation of triglycerides. This lipid metabolism disorder was also associated with mitochondrial dysfunction[41].
Hepatic steatosis is commonly reported among patients with HCV genotype 1 and genotype 3. Its occurrence in the latter has been correlated mainly to the previously mentioned mechanisms. Therefore, resolution of steatosis observed after successful viral eradication suggests a direct steatogenic pathway for HCV genotype 3[42]. This hypothesis was backed in a study of patients treated with interferon-based regimen, in which 91% of genotype 3 patients and only 43% of other genotypes have had their steatosis improved after viral cure[43]. Kumar et al[44], have also observed similar findings when steatosis was profoundly reduced in genotype 3 patients post-SVR, while no change irrespective of the treatment response occurred in genotype 1. Although development of fatty liver was associated with viral characteristics in genotype 3 (viral steatosis), the condition in genotype 1 corresponded to metabolic features such as glucose level and IR (metabolic steatosis). This observation suggests that in patients with genotype 1, factors other than the viral features play an essential role in the development of hepatic steatosis[28].
After achieving SVR with antiviral therapy, reversal of steatosis is the most common reported outcome, which was seen in several studies[17,27,28,39]. However, recent reviews showed contradictory findings[45,46]. In a prospective study that investigated the prevalence of hepatic steatosis and fibrosis in patients with CHC post-SVR, steatosis prevalence found to be 47.5%, almost as same as the pre-treatment figure (50%). Besides, overall average fibrosis score was reduced after viral clearance. Nevertheless, patients who had steatosis have maintained clinically significant fibrosis scores, compared to those without fatty liver[46]. In another study included 49 patients aimed to evaluate the impact of DAAs on glucose and lipid homeostasis, controlled attenuation parameter values were markedly increased at the end of follow up compared to baseline. More importantly, this finding was independent of weight gain, since no change in body mass index (BMI) was observed over time[45].
Patients with CHC and viral-induced hepatic steatosis have been shown to have worse hepatic outcomes in pre-treatment setting[47]. Nonetheless, a recent study depicts that presence of post-SVR steatosis does not carry a better risk profile[48]. In this study, which aimed to assess the effect of steatosis on HCC and all-cause mortality in CHC patients post-SVR, presence of fatty liver was associated with a considerable 7.5-fold increase in both primary endpoints[48]. Furthermore, there is also a substantially higher risk of EHMs, particularly CVD, after amelioration of steatosis post-SVR[17]. These findings combined highlight the importance of hepatic steatosis as a major risk factor for poor outcome and warrant a special consideration of screening and follow-up in this population.
Based on multiple epidemiological studies, metabolic alterations such as IR, DM, and metabolic syndrome are frequent comorbidities in patients with CHC, as opposed to controls[19,49]. The rationale behind this association is still not completely understood but it could be attributed to the presence of liver disease, metabolic characteristics such as obesity, or the inflammatory process induced by HCV infection. HCV has been found to modulate insulin signaling pathways although the precise molecular mechanism of HCV-mediated IR is not fully understood. In two mouse-model experimental studies, HCV core protein was found to play a major role in the development of IR[50,51] particularly through PA28γ gene-dependent pathway[50]. HCV genotypes 1, 2 and 4[52] and genotypes 1 and 4[53] were noticed to have higher IR compared to genotype 3[52] and genotypes 2 and 3[53], respectively. Oxidative stress and proinflammatory cytokines were also found to play a role in de novo IR[54,55]. The disruption in glucose and lipid metabolism associated with IR[56] leads to evolution of hepatic steatosis and development of DM. Among subjects with chronic liver disease, the prevalence of DM in CHC patients prior to treatment varies from 13.6% to 67.4%, which is higher than that reported in individuals with other etiologies, such as chronic hepatitis B[57]. Furthermore, a case-control study demonstrated that the presence of CHC was associated with an over 11-fold increase in risk of developing DM over a follow-up period of 9 years[58]. DM seems to have a bidirectional relationship with HCV, in which the latter causes IR while DM is linked with more aggressive course of HCV-related outcomes such as progressive fibrosis[49,59,60], and increased risk of cirrhosis and HCC[61,62]. All the above conditions make patients with CHC more susceptible to have metabolic syndrome[63]. However, due to the hypolipidemia caused by HCV infection[64], which does not fit the traditional diagnostic criteria, a peculiar type of metabolic syndrome known as hepatitis C-associated dysmetabolic syndrome has been defined[63,65].
There is frequent evidence that have showed a beneficial effect of antiviral therapy using interferon-based regimens on IR in long-term HCV responders. Thompson et al[66], who studied 1038 non-diabetic patients, concluded that IR was substantially decreased in HCV-genotype 1 responders but not in genotype 1 non-responders or those with genotype 2 or 3 irrespective of treatment outcome. This finding was independent of any changes in BMI. Similar findings were also reported in a prospective study[29]. In the Virahep-C, a prospective multicenter study, an improvement in the homeostatic model assessment for IR (HOMA-IR) was observed 24 wk after treatment completion among HCV genotype 1 patients who had IR prior to therapy[67]. Nonetheless, Aghemo et al[68], who enrolled 384 non-diabetic patients with HCV genotypes 1 and 4 failed to display any differences in HOMA-IR values between baseline and 24 wk post-SVR. All the above findings indicate that longer follow-up may be needed to better assess glucose metabolism disturbances after HCV viral clearance with interferon-based regimens, especially in HCV genotype 1 patients. Paradoxically, in a head-to-head comparison of 178 subjects with HCV genotype 1 and 4 between interferon-based antiviral therapy and DAAs to assess metabolic outcomes, there was a significant elevation in HOMA-IR in those who have taken interferon-based regimen[11].
In addition to its effect on IR, antiviral therapy has been thought to decrease incidence of post-SVR hyperglycemia and DM. Interferon-based regimens have been studied extensively and they are usually associated with a decreased incidence of DM in non-diabetic patients with CHC after elimination of HCV[69]. However, several studies have emphasized the beneficial role of attaining SVR, which lessens glucose metabolism abnormalities induced by HCV infection[10,39,70]. Other studies could not detect any significant differences between treatment responders and non-responders[71,72]. Despite these conflicting results, a meta-analysis that included seven studies aiming to investigate the correlation between HCV clearance via interferon-based regimens and the incidence of hyperglycemia demonstrated that SVR is associated with lower risk of hyperglycemia [odds ratio 0.49, 95% confidence interval (CI): 0.42-0.58]. Heterogeneity between studies was minimal, indicating a reliable result. On the other hand, the use of DAAs for viral eradication was not investigated thoroughly. Studies on incidence of DM in non-diabetic patients demonstrated less glucose disturbance in long-term responders[10,73]. In a retrospective study conducted in United States, 5127 non-diabetic subjects with HCV were enrolled to investigate how the response to HCV treatment impacted the risk of subsequent DM. The authors found that those who achieved SVR had markedly lower risk of developing DM, compared to those with treatment failure[10]. Two studies investigated the effect of DAAs on previously known diabetic patients[74,75]. One illustrated an improvement in glycemic status after viral cure while in the other there was no difference in glycosylated hemoglobin between pretreatment and post-treatment values. Importantly, one-third of patients in the latter study required escalation of anti-diabetic therapy during antiviral treatment. Further long-term prospective studies are still needed to resolve the current dilemma of changes on IR related to antiviral treatment.
CHC infection has been linked to an array of EHMs, including an increased risk of CVD[76-79]. Several direct and indirect HCV pro-atherogenic mechanisms have been postulated. HCV is assumed to play a direct role in the development of arterial atherosclerosis by inducing endothelial dysfunction, likely through interleukin 1β[80], a pro-inflammatory cytokine. Likewise, it has been observed that HCV has the ability to live and replicate inside carotid plaques[81], which further supports an immediate pro-atherogenic effect. Moreover, chronic inflammation and oxidative stress that are caused by structural and non-structural viral proteins have also been shown to trigger plaque formation[80]. In a multicenter Italian study that evaluated the effect of attaining SVR using DAAs on subclinical carotid arteriosclerosis compared to an untreated cohort, ultrasonographic carotid measurements showed a significant reduction in mean carotid intima-media thickness in treatment group at the end of follow-up compared to baseline (from 0.94 mm to 0.81 mm, P < 0.001). No significant changes in the intima-media thickness were found in the control group. The BMI of these patients did not change during follow-up, while a significant increase in serum cholesterol levels was observed. The study concluded that eradication of HCV by DAAs led to an amelioration in carotid atherosclerosis, particularly intima-media thickness. Furthermore, HCV can also induce atherosclerosis indirectly since it is associated with an increased risk of metabolic syndrome components, including IR, DM, and hepatic steatosis, which are well-known predisposing factors for CVD[82-84]. On the other hand, some studies have failed to show any significant association between HCV and cardiovascular events[85,86].
Several studies have shown that either antiviral therapy or the attainment of SVR minimize CVD risk[87-90]. However, the results are rather controversial. Butt et al[87], who studied the effect of antiviral therapy, interferon- or DAAs-based regimens, on CVD risk found that the incidence of CVD in treatment arm was 7.2% in comparison with 13% in control group, regardless of the antiviral regimen. Treatment with DAAs was superior to interferon-based regimen, with a hazard ratio (HR) of 0.57 (95%CI: 0.51-0.65) and HR 0.78 (95%CI: 0.71-0.85), respectively. SVR was also associated with lower risk of incident CVD events HR 0.87 (95%CI: 0.77-0.98). In a nation-wide cohort study on Taiwanese residents with HCV who had received interferon-based regimens compared to an untreated cohort, antiviral therapy was associated with lower risks of acute coronary syndrome and ischemic stroke, with HR 0.77 (95%CI: 0.62-0.97) and HR 0.62 (95%CI: 0.46-0.83), respectively. This risk reduction was not observed in subject who had insufficient treatment course (< 16 wk)[88]. Further supporting data was observed in a study comprising 3385 HCV patients, which found that SVR was associated with a lower relative hazard reduction and absolute risk reduction for CVD[89]. However, some epidemiological studies have found contradictory findings. A large retrospective cohort study which enrolled 160875 subjects was aimed to investigate the impact of successful viral eradication on a variety of EHMs. In terms of CVD risk, the study concluded that SVR was associated with a diminished risk for stroke HR 0.84 (95%CI: 0.74-0.94), but not for CVD aHR 1.12 (95%CI: 0.81-1.56), when compared to the untreated cohort[69]. From the same perspective, a negative result was also reported by Leone et al[72], who studied the influence of SVR on EHMs. The researchers did not find any significant cardiovascular risk reduction in SVR group compared to non-SVR, with HR 1.14 (95%CI: 0.57-2.3). Despite disparities in the findings across individual studies, a meta-analysis including 53841 patients demonstrated that SVR significantly reduces CVD risk, with a pooled of HR 0.76 (95%CI: 0.61-0.94)[91].
Apart from the direct treatment effect on CVD risk, therapeutic changes on other EHMs may also play role in the development of atherosclerotic events. Deteriorated lipid profile after HCV clearance has been shown to predispose patients to an elevated risk of CVD[31]. In a study of 617 patients with a mean follow-up of 26.8 mo, Huang et al[31] investigated whether deterioration of lipid profile post-SVR increased the risk of cardio-cerebral disease. Five patients developed cardio-cerebrovascular events (3 CVD and 2 cerebrovascular disease) over 1376 person-years. An LDL surge >40% was found to be the only predictor of these vascular events, with a HR of 15.44 (95%CI: 1.73-138.20)[31]. Evidence on risk of CVD in CHC pre- or post-treatment remains contro-versial. Nonetheless, most of the literature indicates that achieving SVR via antiviral therapy is associated with a significant risk reduction.
EHMs including cardiometabolic conditions are commonly seen among patients with CHC infection. Data on these conditions after elimination of HCV is inconsistent. However, the predominant evidence in the literature suggests that viral clearance using antiviral therapy leads to deterioration in lipid profile, reduction in the incidence of metabolic alteration such as IR, DM, and hepatic steatosis, and improvement in CVD risk. To determine more robust level of association between SVR and EHMs and to understand the exact mechanisms of how antiviral therapies act on these EHMs, large prospective studies with long-term follow-up are needed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masaki N S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824-7840. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742-748. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Osinubi A, Harris AM, Vellozzi C, Lom J, Miller L, Millman AJ. Evaluation of the Performance of Algorithms That Use Serial Hepatitis C RNA Tests to Predict Treatment Initiation and Sustained Virological Response Among Patients Infected With Hepatitis C Virus. Am J Epidemiol. 2019;188:555-561. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, Abergel A, Pessôa MG, Lin A, Tietz A, Connell EV, Diago M. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Sáez-González E, Vinaixa C, San Juan F, Hontangas V, Benlloch S, Aguilera V, Rubín A, García M, Prieto M, López-Andujar R, Berenguer M. Impact of hepatitis C virus (HCV) antiviral treatment on the need for liver transplantation (LT). Liver Int. 2018;38:1022-1027. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Durand F, Francoz C. The future of liver transplantation for viral hepatitis. Liver Int. 2017;37 Suppl 1:130-135. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Sherman AC, Sherman KE. Extrahepatic manifestations of hepatitis C infection: navigating CHASM. Curr HIV/AIDS Rep. 2015;12:353-361. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Wu ZY, Li JR, Huang MH, Cheng JJ, Li H, Chen JH, Lv XQ, Peng ZG, Jiang JD. Internal driving factors leading to extrahepatic manifestation of the hepatitis C virus infection. Int J Mol Med. 2017;40:1792-1802. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Li Y, Wang X, Yu G, Sun H, Lv J, Chi X, Wu R, Gao X, Niu J. The association of hepatitis c virus infection status with serum glucose levels. BMC Gastroenterol. 2019;19:86. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Carvalho JR, Velosa J, Serejo F. Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication - comparison of the new direct-acting antiviral agents with the old regimens. Scand J Gastroenterol. 2018;53:857-863. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Flores-Chávez A, Carrion JA, Forns X, Ramos-Casals M. Extrahepatic manifestations associated with Chronic Hepatitis C Virus Infection. Rev Esp Sanid Penit. 2017;19:87-97. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Chang ML. Metabolic alterations and hepatitis C: From bench to bedside. World J Gastroenterol. 2016;22:1461-1476. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Chang ML, Hsu CM, Lin CH, Lin CY, Kuo CJ, Huang SW, Chen CW, Cheng HT, Yeh CT, Chiu CT. The Evolving Interplay among Abundant Adipokines in Patients with Hepatitis C during Viral Clearance. Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Forde KA, Haynes K, Troxel AB, Trooskin S, Osterman MT, Kimmel SE, Lewis JD, Lo Re V 3rd. Risk of myocardial infarction associated with chronic hepatitis C virus infection: a population-based cohort study. J Viral Hepat. 2012;19:271-277. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Chang ML, Lin YS, Pao LH, Huang HC, Chiu CT. Link between plasminogen activator inhibitor-1 and cardiovascular risk in chronic hepatitis C after viral clearance. Sci Rep. 2017;7:42503. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Kawagishi N, Suda G, Nakamura A, Kimura M, Maehara O, Suzuki K, Ohara M, Izumi T, Umemura M, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Kudo Y, Nishida M, Miyoshi H, Sakamoto N. Liver steatosis and dyslipidemia after HCV eradication by direct acting antiviral agents are synergistic risks of atherosclerosis. PLoS One. 2018;13:e0209615. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030-1037. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Mostafa A, Mohamed MK, Saeed M, Hasan A, Fontanet A, Godsland I, Coady E, Esmat G, El-Hoseiny M, Abdul-Hamid M, Hughes A, Chaturvedi N. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135-1140. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791-796. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Lindenbach BD. Measuring HCV infectivity produced in cell culture and in vivo. Methods Mol Biol. 2009;510:329-336. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Aizawa Y, Seki N, Nagano T, Abe H. Chronic hepatitis C virus infection and lipoprotein metabolism. World J Gastroenterol. 2015;21:10299-10313. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Ploss A, Dubuisson J. New advances in the molecular biology of hepatitis C virus infection: towards the identification of new treatment targets. Gut. 2012;61 Suppl 1:i25-i35. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Meissner EG, Lee YJ, Osinusi A, Sims Z, Qin J, Sturdevant D, McHutchison J, Subramanian M, Sampson M, Naggie S, Patel K, Remaley AT, Masur H, Kottilil S. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790-801. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Clark PJ, Thompson AJ, Vock DM, Kratz LE, Tolun AA, Muir AJ, McHutchison JG, Subramanian M, Millington DM, Kelley RI, Patel K. Hepatitis C virus selectively perturbs the distal cholesterol synthesis pathway in a genotype-specific manner. Hepatology. 2012;56:49-56. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Hashimoto S, Yatsuhashi H, Abiru S, Yamasaki K, Komori A, Nagaoka S, Saeki A, Uchida S, Bekki S, Kugiyama Y, Nagata K, Nakamura M, Migita K, Nakao K. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS One. 2016;11:e0163644. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Kuo YH, Chuang TW, Hung CH, Chen CH, Wang JH, Hu TH, Lu SN, Lee CM. Reversal of hypolipidemia in chronic hepatitis C patients after successful antiviral therapy. J Formos Med Assoc. 2011;110:363-371. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Fernández-Rodríguez CM, López-Serrano P, Alonso S, Gutiérrez ML, Lledó JL, Pérez-Calle JL, Temiño R, Cacho G, Nevado M, Casas ML, Gasalla JM, Bonet B. Long-term reversal of hypocholesterolaemia in patients with chronic hepatitis C is related to sustained viral response and viral genotype. Aliment Pharmacol Ther. 2006;24:507-512. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Chang ML, Tsou YK, Hu TH, Lin CH, Lin WR, Sung CM, Chen TH, Cheng ML, Chang KC, Chiu CT, Yeh CT, Pang JH, Shiao MS. Distinct patterns of the lipid alterations between genotype 1 and 2 chronic hepatitis C patients after viral clearance. PLoS One. 2014;9:e104783. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Ramcharran D, Wahed AS, Conjeevaram HS, Evans RW, Wang T, Belle SH, Yee LJ; Virahep-C Study Group. Associations between serum lipids and hepatitis C antiviral treatment efficacy. Hepatology. 2010;52:854-863. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Huang CF, Dai CY, Yeh ML, Huang CI, Lee HC, Lai WT, Liang PC, Lin YH, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Huang JF, Chuang WL, Yu ML. Cure or curd: Modification of lipid profiles and cardio-cerebrovascular events after hepatitis C virus eradication. Kaohsiung J Med Sci. 2020;36:920-928. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34:1069-1077. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ; National Heart; Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Host- and disease-specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198-206. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Syed GH, Tang H, Khan M, Hassanein T, Liu J, Siddiqui A. Hepatitis C virus stimulates low-density lipoprotein receptor expression to facilitate viral propagation. J Virol. 2014;88:2519-2529. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481-37488. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Cheng Y, Dharancy S, Malapel M, Desreumaux P. Hepatitis C virus infection down-regulates the expression of peroxisome proliferator-activated receptor alpha and carnitine palmitoyl acyl-CoA transferase 1A. World J Gastroenterol. 2005;11:7591-7596. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Tsukuda Y, Suda G, Tsunematsu S, Ito J, Sato F, Terashita K, Nakai M, Sho T, Maehara O, Shimazaki T, Kimura M, Morikawa K, Natsuizaka M, Ogawa K, Ohnishi S, Chuma M, Sakamoto N. Anti-adipogenic and antiviral effects of l-carnitine on hepatitis C virus infection. J Med Virol. 2017;89:857-866. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Yair-Sabag S, Nussinson E, Ben-Assuli O, Shibli F, Shahbari A, Zelber-Sagi S. Retrospective study of the associations between hepatitis C virus infection and metabolic factors. World J Hepatol. 2016;8:1269-1278. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, Bréchot C. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Miyoshi H, Moriya K, Tsutsumi T, Shinzawa S, Fujie H, Shintani Y, Fujinaga H, Goto K, Todoroki T, Suzuki T, Miyamura T, Matsuura Y, Yotsuyanagi H, Koike K. Pathogenesis of lipid metabolism disorder in hepatitis C: polyunsaturated fatty acids counteract lipid alterations induced by the core protein. J Hepatol. 2011;54:432-438. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Castéra L, Hézode C, Roudot-Thoraval F, Lonjon I, Zafrani ES, Pawlotsky JM, Dhumeaux D. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420-424. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266-1272. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Graf C, Welzel T, Bogdanou D, Vermehren J, Beckel A, Bojunga J, Friedrich-Rust M, Dietz J, Kubesch A, Mondorf A, Fischer S, Lutz T, Stoffers P, Herrmann E, Poynard T, Zeuzem S, Dultz G, Mihm U. Hepatitis C Clearance by Direct-Acting Antivirals Impacts Glucose and Lipid Homeostasis. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA. Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals. World J Gastroenterol. 2018;24:1269-1277. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, Terrault N, Pazienza V, Giordani MT, Giostra E, Sonzogni A, Ruggiero G, Marcellin P, Powell EE, George J, Negro F; HCV Meta-Analysis (on) Individual Patients' Data Study Group. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Harif Y, Oxtrud E, Braun M, Leshno M, Barsheshet A, Shlomai A. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepat. 2019;26:1257-1265. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, Paradis V, Vidaud M, Valla D, Bedossa P, Marcellin P. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416-423. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727-1735. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Mihm S. Hepatitis C virus, diabetes and steatosis: clinical evidence in favor of a linkage and role of genotypes. Dig Dis. 2010;28:280-284. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Sersté T, Nkuize M, Moucari R, Van Gossum M, Reynders M, Scheen R, Vertongen F, Buset M, Mulkay JP, Marcellin P. Metabolic disorders associated with chronic hepatitis C: impact of genotype and ethnicity. Liver Int. 2010;30:1131-1136. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, Baba S, Koga H, Kumashiro R, Ueno T, Ogata H, Yoshimura A, Sata M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Kawaguchi Y, Mizuta T. Interaction between hepatitis C virus and metabolic factors. World J Gastroenterol. 2014;20:2888-2901. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Serfaty L. Metabolic Manifestations of Hepatitis C Virus: Diabetes Mellitus, Dyslipidemia. Clin Liver Dis. 2017;21:475-486. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology. 2003;125:1695-1704. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, George J. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66-73. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, de Knegt RJ, Zeuzem S, Manns MP, Hansen BE, Schalm SW, Janssen HL. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856-1862. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Adinolfi LE, Restivo L, Zampino R, Lonardo A, Loria P. Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin Pharmacother. 2011;12:2215-2234. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Negro F. HCV infection and metabolic syndrome: which is the chicken and which is the egg? Gastroenterology. 2012;142:1288-1292. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Adinolfi LE, Restivo L, Marrone A. The predictive value of steatosis in hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2013;7:205-213. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Thompson AJ, Patel K, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Rustgi VK, Flisiak R, Pianko S, Diago M, Arora S, Foster GR, Torbenson M, Benhamou Y, Nelson DR, Sulkowski MS, Zeuzem S, Pulkstenis E, Subramanian GM, McHutchison JG; ACHIEVE-1 and ACHIEVE-2/3 Study Teams. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61:128-134. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Conjeevaram HS, Wahed AS, Afdhal N, Howell CD, Everhart JE, Hoofnagle JH; Virahep-C Study Group. Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology. 2011;140:469-477. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Aghemo A, Prati GM, Rumi MG, Soffredini R, D'Ambrosio R, Orsi E, De Nicola S, Degasperi E, Grancini V, Colombo M. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56:1681-1687. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Mahale P, Engels EA, Li R, Torres HA, Hwang LY, Brown EL, Kramer JR. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2018;67:553-561. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, Ikeda K, Kumada H. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739-744. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Giordanino C, Bugianesi E, Smedile A, Ciancio A, Abate ML, Olivero A, Pellicano R, Cassader M, Gambino R, Bo S, Ciccone G, Rizzetto M, Saracco G. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008;103:2481-2487. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Leone S, Prosperi M, Costarelli S, Nasta P, Maggiolo F, Di Giambenedetto S, Saracino A, Di Pietro M, Gori A. Incidence and predictors of cardiovascular disease, chronic kidney disease, and diabetes in HIV/HCV-coinfected patients who achieved sustained virological response. Eur J Clin Microbiol Infect Dis. 2016;35:1511-1520. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Li J, Zhang T, Gordon SC, Rupp LB, Trudeau S, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Boscarino JA, Schmidt MA, Daida YG, Lu M; CHeCS Investigators. Impact of sustained virologic response on risk of type 2 diabetes among hepatitis C patients in the United States. J Viral Hepat. 2018;25:952-958. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Stine JG, Wynter JA, Niccum B, Kelly V, Caldwell SH, Shah NL. Effect of Treatment with Direct Acting Antiviral on Glycemic Control in Patients with Diabetes Mellitus and Chronic Hepatitis C. Ann Hepatol. 2017;16:215-220. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Abdo M, Rabiee A, Abdellatif Z, Abdel Alem S, Moustafa A. Impact of sustained virological response on metabolic disorders in diabetic chronic hepatitis C virus patients after treatment with generic sofosbuvir and daclatasvir. Eur J Gastroenterol Hepatol. 2020;. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Ambrosino P, Lupoli R, Di Minno A, Tarantino L, Spadarella G, Tarantino P, Nasto A, Celentano A, Di Minno MN. The risk of coronary artery disease and cerebrovascular disease in patients with hepatitis C: A systematic review and meta-analysis. Int J Cardiol. 2016;221:746-754. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Petta S, Maida M, Macaluso FS, Barbara M, Licata A, Craxì A, Cammà C. Hepatitis C Virus Infection Is Associated With Increased Cardiovascular Mortality: A Meta-Analysis of Observational Studies. Gastroenterology 2016; 150: 145-155. quiz e15-6. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Vassalle C, Masini S, Bianchi F, Zucchelli GC. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565-566. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Sawayama Y, Okada K, Maeda S, Ohnishi H, Furusyo N, Hayashi J. Both hepatitis C virus and Chlamydia pneumoniae infection are related to the progression of carotid atherosclerosis in patients undergoing lipid lowering therapy. Fukuoka Igaku Zasshi. 2006;97:245-255. [PubMed] [Cited in This Article: ] |
| 80. | Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410-3417. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Rossi L, Pratesi C, Gensini GF, Paperetti L, Zignego AL. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47:72-75. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40:115-119. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Shaheen M, Echeverry D, Oblad MG, Montoya MI, Teklehaimanot S, Akhtar AJ. Hepatitis C, metabolic syndrome, and inflammatory markers: results from the Third National Health and Nutrition Examination Survey [NHANES III]. Diabetes Res Clin Pract. 2007;75:320-326. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Arcari CM, Nelson KE, Netski DM, Nieto FJ, Gaydos CA. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis. 2006;43:e53-e56. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Völzke H, Schwahn C, Wolff B, Mentel R, Robinson DM, Kleine V, Felix SB, John U. Hepatitis B and C virus infection and the risk of atherosclerosis in a general population. Atherosclerosis. 2004;174:99-103. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Butt AA, Yan P, Shuaib A, Abou-Samra AB, Shaikh OS, Freiberg MS. Direct-Acting Antiviral Therapy for HCV Infection Is Associated With a Reduced Risk of Cardiovascular Disease Events. Gastroenterology 2019; 156: 987-996. e8. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, Wu CY. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495-503. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Innes HA, McDonald SA, Dillon JF, Allen S, Hayes PC, Goldberg D, Mills PR, Barclay ST, Wilks D, Valerio H, Fox R, Bhattacharyya D, Kennedy N, Morris J, Fraser A, Stanley AJ, Bramley P, Hutchinson SJ. Toward a more complete understanding of the association between a hepatitis C sustained viral response and cause-specific outcomes. Hepatology. 2015;62:355-364. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, Fontaine H, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Leroy V, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Dharancy S, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Zucman D, Di Martino V, Thibaut V, Salmon D, Ziol M, Sutton A, Pol S, Roudot-Thoraval F; ANRS CO12 CirVir Group. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology 2017; 152: 142-156. e2. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Lapumnuaypol K, Thongprayoon C, Wijarnpreecha K, Cheungpasitporn W. Impact of hepatitis C sustained viral response on cardiovascular diseases: a meta-analysis. Hosp Pract (1995). 2019;47:105-110. [PubMed] [DOI] [Cited in This Article: ] |