Published online Dec 21, 2020. doi: 10.3748/wjg.v26.i47.7497
Peer-review started: July 21, 2020
First decision: September 30, 2020
Revised: October 13, 2020
Accepted: November 2, 2020
Article in press: November 2, 2020
Published online: December 21, 2020
Processing time: 151 Days and 6.3 Hours
Gastric cancer (GC) is one of the most common and deadliest types of cancer worldwide due to its delayed diagnosis and high metastatic frequency, but its exact pathogenesis has not been fully elucidated. ETS homologous factor (EHF) is an important member of the ETS family and contributes to the pathogenesis of multiple malignant tumors. To date, whether EHF participates in the development of GC via the c-Met signaling pathway remains unclear.
To investigate the role and mechanism of EHF in the occurrence and development of GC.
The expression of EHF mRNA in GC tissues and cell lines was measured by quantitative PCR. Western blotting was performed to determine the protein expression of EHF, c-Met, and its downstream signal molecules. The EHF expression in GC tissues was further detected by immunohistochemical staining. To investigate the role of EHF in GC oncogenesis, small interfering RNA (siRNA) against EHF was transfected into GC cells. The cell proliferation of GC cells was determined by Cell Counting Kit-8 and colony formation assays. Flow cytometry was performed following Annexin V/propidium iodide (PI) to identify apoptotic cells and PI staining to analyze the cell cycle. Cell migration and invasion were assessed by transwell assays.
The data showed that EHF was upregulated in GC tissues and cell lines in which increased expression of c-Met was also observed. Silencing of EHF by siRNA reduced the proliferation of GC cells. Inhibition of EHF induced significant apoptosis and cell cycle arrest in GC cells. Cell migration and invasion were significantly inhibited. EHF silencing led to c-Met downregulation and further blocked the Ras/c-Raf/extracellular signal-related kinase 1/2 (Erk1/2) pathway. Additionally, phosphatase and tensin homolog was upregulated and glycogen synthase kinase 3 beta was deactivated. Moreover, inactivation of signal transducer and activator of transcription 3 was detected following EHF inhibition, leading to inhibition of the epithelial-to-mesenchymal transition (EMT).
These results suggest that EHF plays a key role in cell proliferation, invasion, apoptosis, the cell cycle and EMT via the c-Met pathway. Therefore, EHF may serve as an antineoplastic target for the diagnosis and treatment of GC.
Core Tip: The purpose of this study was to investigate the role and mechanism of ETS homologous factor (EHF) in the occurrence and development of gastric cancer (GC). The results showed that EHF plays a key role in cell proliferation, invasion, apoptosis, the cell cycle and epithelial-to-mesenchymal transition. EHF may contribute to the tumorigenesis and progression of GC via the c-Met pathway. Therefore, EHF is a promising target for the diagnosis and treatment of GC.
- Citation: Gu ML, Zhou XX, Ren MT, Shi KD, Yu MS, Jiao WR, Wang YM, Zhong WX, Ji F. Blockage of ETS homologous factor inhibits the proliferation and invasion of gastric cancer cells through the c-Met pathway. World J Gastroenterol 2020; 26(47): 7497-7512
- URL: https://www.wjgnet.com/1007-9327/full/v26/i47/7497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i47.7497
Gastric cancer (GC) is a common malignant tumor in the gastrointestinal tract and the third leading cause of cancer-related mortality worldwide[1]. Risk factors such as Helicobacter pylori infection, atrophic gastritis, a high-salt diet and heavy alcohol drinking contribute to the tumorigenesis and progression of GC, but its exact pathogenesis has not been fully elucidated[2,3]. Currently, surgical resection represents the best curative option for GC. On the other hand, advanced novel targets for GC diagnosis and treatment have resulted in decreased incidence and mortality rates in recent years[4]. However, the 5-year overall survival rate of patients with advanced GC is less than 30% due to delayed diagnosis and recurrence[5]. Therefore, revealing the oncogenic mechanisms of GC and further exploring the strategies for GC diagnosis and treatment are urgent.
The ETS family consists of various transcriptional factors that contain a conservative ETS structure domain, which specifically identifies and binds to the GGAA/T sequence within the promoter or enhancer region of target genes to regulate their transcription[6]. ETS homologous factor (EHF) is a member of the ETS family of transcription factors expressed in multiple epithelial cell types, which participates in the oncogenesis of various malignant tumors via regulating cell proliferation and differentiation[7,8]. The aberrant expression of EHF is involved in the epithelial-to-mesenchymal transition (EMT) and is a risk factor for the increased recurrence and reduced overall survival of prostate cancer[9]. A recent study demonstrated that upregulated EHF expression is correlated with the poor prognosis of ovarian cancer patients and that the inhibition of EHF exerts an antineoplastic effect on ovarian cancer cells[10]. Additionally, EHF is highly expressed in colon cancers, and the down-regulation of EHF leads to apoptosis of colon cancer cells[11,12]. However, the roles of EHF in the tumorigenesis and progression of GC need further investigation.
A previous study indicated that EHF regulates the expression of c-Met by directly binding to the promoter region of the c-Met coding sequence[7]. c-Met is a receptor tyrosine kinase (RTK), and its dysregulation is closely related to the pathogenesis and development of GC[13]. In advanced GC patients, c-Met-positive status is an independent prognostic factor for poor outcomes[14]. To date, whether EHF participates in the development of GC through the c-Met pathway remains unclear. Herein, we investigated EHF expression in GC and elucidated its potential function and regulatory mechanisms involving the oncogenesis of GC, providing important evidence for the exploration of novel therapeutic targets for GC.
A total of 10 paired GC tissues and adjacent normal tissues were obtained from The First Affiliated Hospital of Zhejiang University (Zhejiang, China). The patients did not receive radiotherapy or chemotherapy prior to collection. The tissues were immediately stored in liquid nitrogen postoperatively. This study was performed according to the Declaration of Helsinki and approved by The Research Ethics Committee of The First Affiliated Hospital, College of Medicine, Zhejiang University, No. 20191218. Informed consent was obtained from all subjects involved in the study.
The human normal gastric epithelial cell line (GES-1) and human GC cell lines (BGC-823, KATO III and SGC-7901) were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China). The cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (FBS; Gibco) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
Small interfering RNA (siRNA) transfections were performed using Lipofectamine™ 3000 reagent (Invitrogen, San Diego, CA, United States) in antibiotic-free RPMI 1640 according to the manufacturer’s instructions. The GC cell lines were transfected with 10 nmol/L siRNA. The control siRNA and siRNA targeting EHF were purchased from Invitrogen.
The total RNA of each cell line was extracted using RNAiso Plus reagent (Takara, Dalian, China) following the manufacturer's protocol. The quantitative polymerase chain reaction (qPCR) assay was performed as previously described[15]. The primers were obtained from Sangon Biotech (Shanghai, China). Primer sequences were listed as follows: EHF, forward 5'-GAAGAACAACAGCAGCATGACC-3' and reverse 5'-TCAGGTTTCGGTGTATGAGTTG-3'; GAPDH, forward 5'-AGAAGGCT GGGGCTCATTTG-3' and reverse 5'-AGGGGCCATCCACAGTCTT-3'. The relative gene expression levels of the tested genes were calculated using the 2-ΔΔCt method[16].
Cell proliferation was determined using the Cell Counting Kit-8 assay according to our previous study[15].
After transfection for 48 h, BGC-823, KATO III, and SGC-7901 cells were seeded into 6-well plates at a density of 2 × 103 cells per well and incubated for 10 d at 37 °C. Then, the culture medium was removed, and cells were washed with phosphate-buffered saline (referred to as PBS). The colonies were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet. The colony numbers were ultimately counted.
Cells were inoculated into 6-well plates at a density of 4 × 105 cells per well and incubated for 24 h. Then the cells were transfected with negative control and EHF-targeted siRNA for 72 h. Finally, cell cycle assays and cell apoptosis assays were performed as previously described[15].
Following the transfection of EHF-targeted siRNA for 24 h, 2.5 × 104 cells were plated into 24-well chambers (Corning, NY, United States) with medium containing 1% FBS. Medium containing 10% FBS was added into the lower wells. For the invasion assays, Matrigel was added to each transwell chamber and incubated for 5 h before the cells were seeded. After 48 h of incubation, the cells remaining in the upper chamber were carefully removed with cotton swabs. The membrane-penetrating cells were stained with crystal violet for 15 min. The cells that migrated and invaded were counted from at least five randomly selected fields.
The expression of EHF was evaluated by immunohistochemical staining. Paraffin blocks that contained formalin-fixed GC and adjacent normal tissues were sectioned at 4 μm. The sections were deparaffinized in 100% xylene and re-hydrated at graded ethanol concentrations. Endogenous peroxidase activity was blocked using fresh 3% hydrogen peroxide for 10 min at room temperature. Antigen retrieval was done by boiling the slides in sodium citrate buffer (pH 6.0). After blocking in 3% bovine serum albumin, sections were incubated with anti-EHF antibody (1:100 dilution in PBS; Abcam, Cambridge, MA, United States) overnight at 4 °C. Subsequently, the sections were incubated for 30 min at room temperature with anti-rabbit horseradish peroxidase-conjugated secondary antibody. The immunostaining was performed using diaminobenzidine solution. The sections were then counterstained with hematoxylin. dehydrated and mounted. Blinded evaluations of EHF immunostaining were carried out independently by two experienced pathologists.
According to the manufacturer's instructions, the total protein was extracted from cells by using cell lysis buffer (Cell Signaling, Beverly, MA, United States). Western blotting analysis was performed as previously described[15]. Rabbit antibodies corresponding to EHF were purchased from Abcam. Antibodies against c-Met, Ras, total c-Raf, phospho-c-Raf (p-c-Raf), total Erk1/2, p-Erk1/2, phosphatase and tensin homolog (PTEN), total glycogen synthase kinase 3 beta (GSK-3β), phospho-GSK-3β, Bcl-2-associated X protein (Bax), survivin, total signal transducer and activator of transcription 3 (STAT3), p-STAT3, Twist, Snail, E-cadherin, and GAPDH were purchased from Cell Signaling Technology, Inc.
Statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, United States). Results are presented as the mean ± standard deviation. The statistical differences between two groups was assessed by the Student’s t-test. One-way analysis of variance followed by Tukey's multiple comparison test was used to determine the statistical difference among multiple groups. The significance level was set at P < 0.05. All experiments were performed in triplicate.
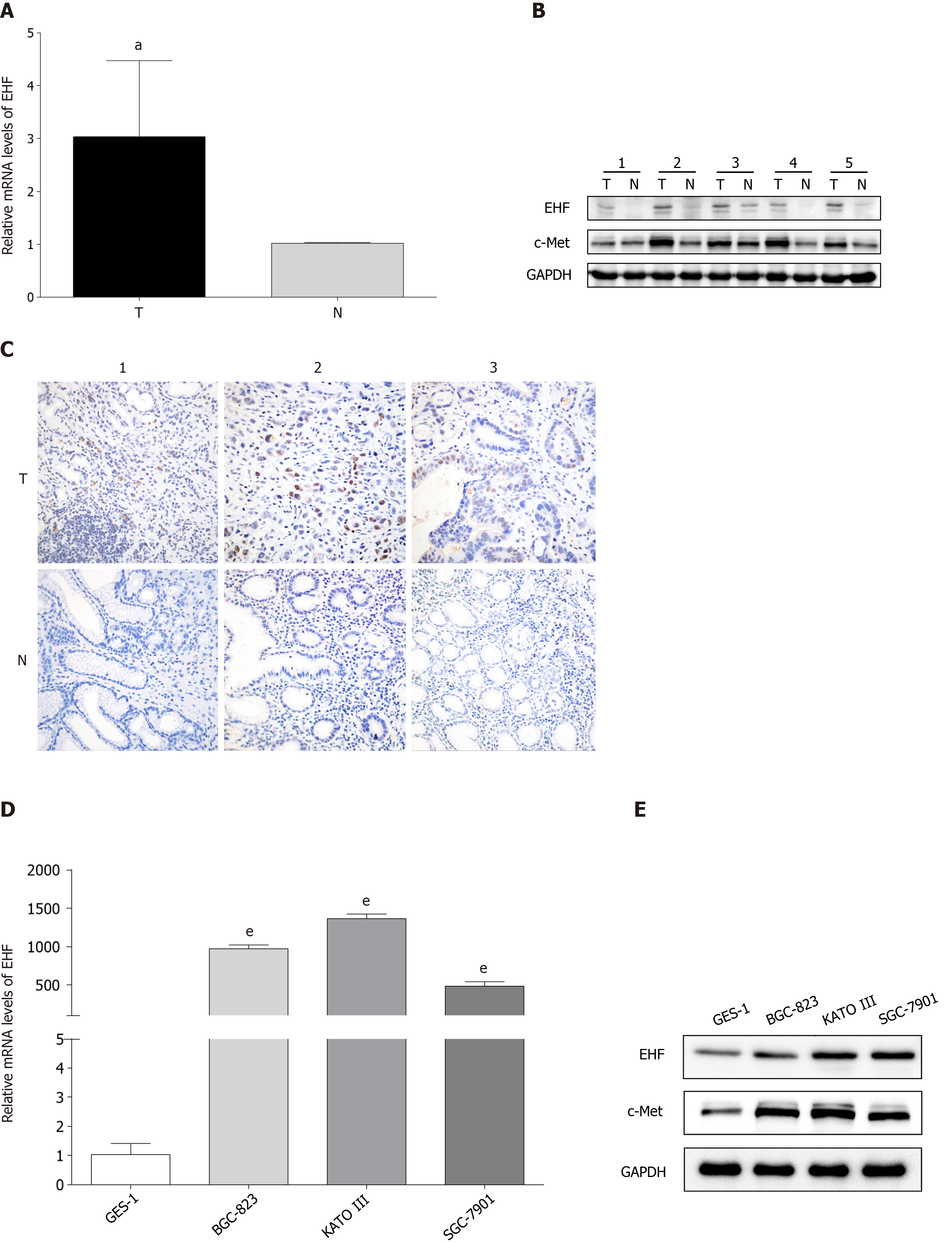
The expression of EHF mRNA in paired adjacent normal tissues and GC tissues were first measured via qPCR and Western blotting. The mRNA expression level of EHF was significantly upregulated in GC tissues compared with adjacent control tissues (Figure 1A). The protein expression of EHF was increased in GC tissues overexpressing c-Met (Figure 1B). As shown in Figure 1C, increased expression of EHF was also detected in GC tissues by immunohistochemistry. Second, the gene and protein expression of EHF in human GES-1 and GC cell lines (BGC-823, KATO III and SGC-7901) was detected. The expression of EHF mRNA was increased in these GC cell lines compared with GES-1 (Figure 1D). In GC cell lines with upregulated EHF, the protein expression of c-Met was also increased in a similar pattern (Figure 1E).
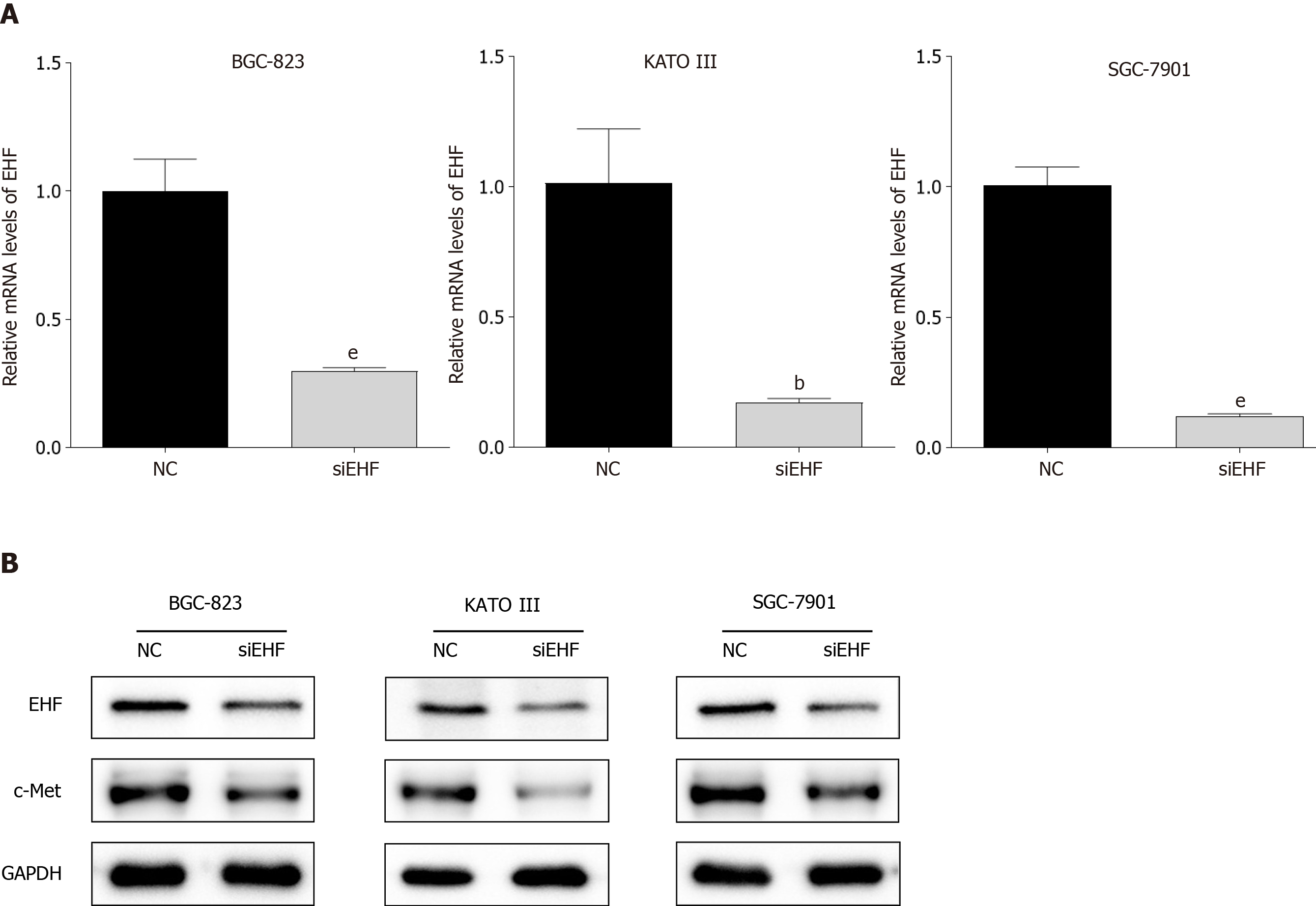
To clarify the potential function of EHF in the tumorigenesis of GC, EHF silencing was performed by transfecting EHF-targeted siRNA into human GC cell lines (BGC-823, KATO III and SGC-7901). As shown in Figure 2, the mRNA and protein expression levels of EHF significantly decreased in the GC cell lines compared with the negative control group after transfection for 72 h (P < 0.05). In addition, the inhibition of EHF also led to the downregulation of c-Met in these GC cell lines.
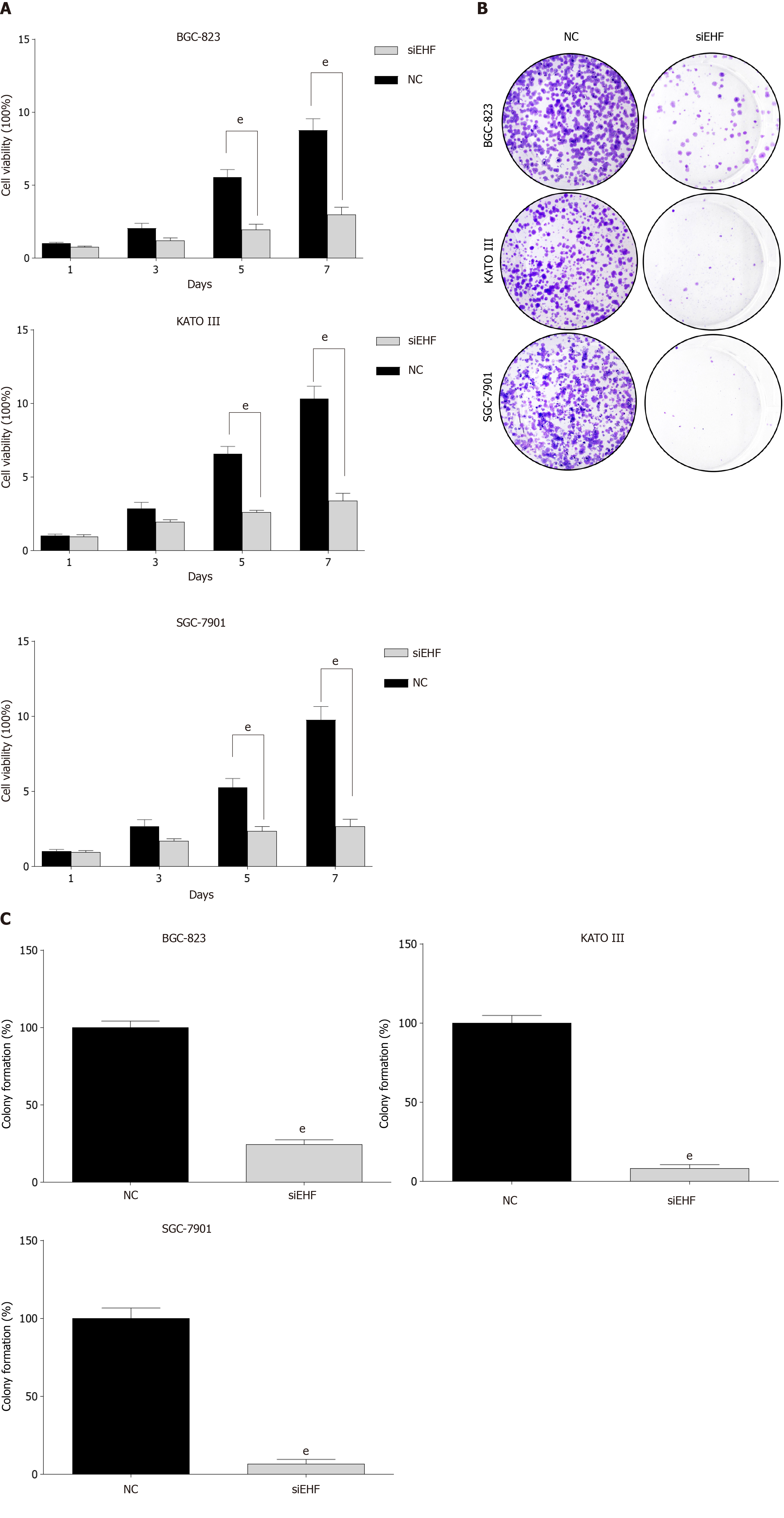
The aforementioned results demonstrated that EHF expression was higher in GC cell lines and GC tissues. The potential functional roles of EHF in GC cells were further explored. Knockdown of EHF significantly reduced the cell viability of GC cell lines after transfection for 5 d (Figure 3A). In addition, EHF silencing also significantly inhibited the colony formation of GC cell lines compared with the negative control group (Figure 3B and C). Thus, EHF plays a crucial role in the regulation of proliferation in GC.
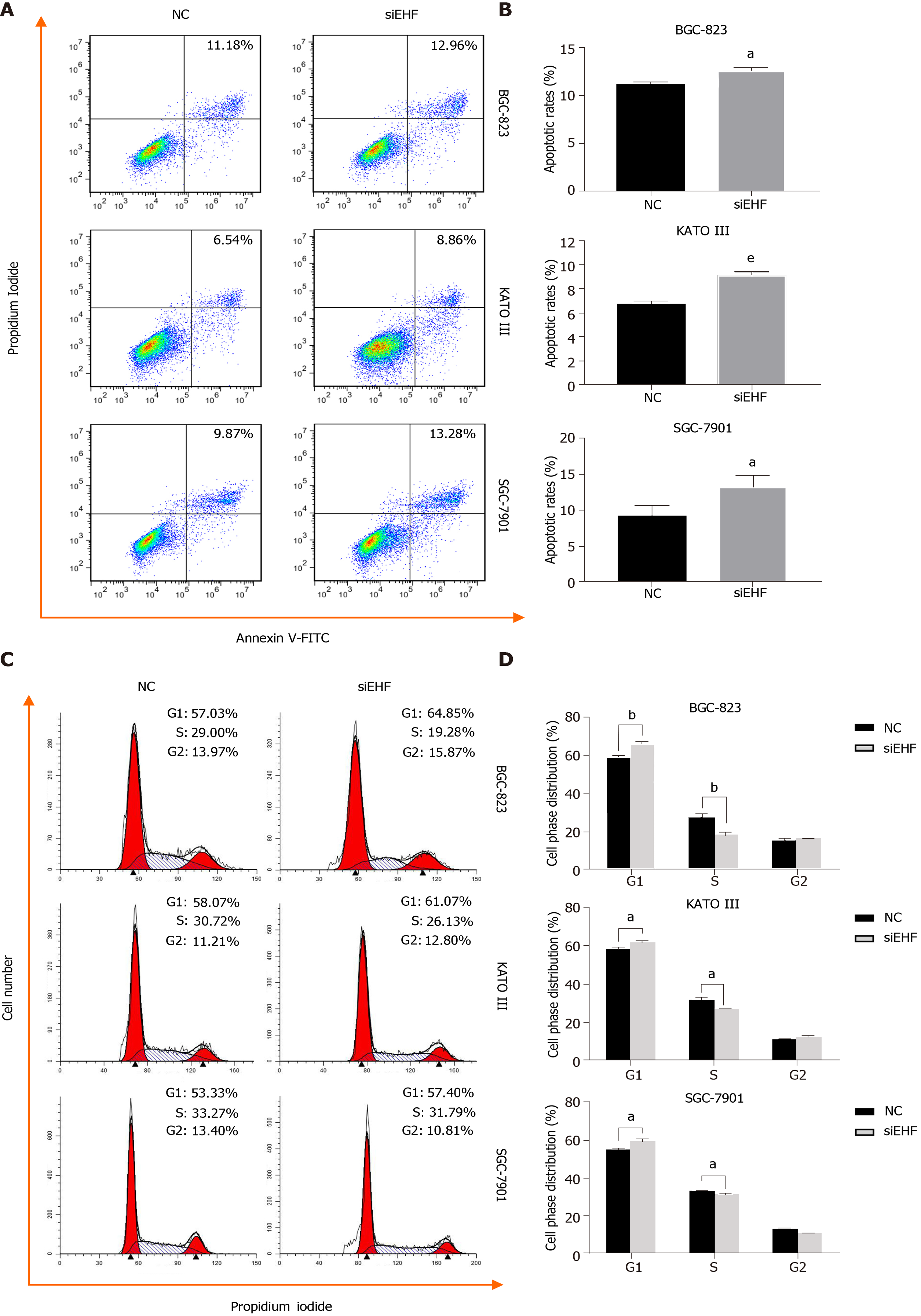
To investigate whether EHF can affect apoptotic cell death in GC, Annexin V-FITC/propidium iodide (PI) staining and flow cytometry were performed. As shown in Figure 4A and B, EHF downregulation induced significant apoptosis in the GC cell lines (P < 0.05), suggesting that EHF exerts an antiapoptotic effect in these GC cell lines. To further assess the cell cycle status following EHF silencing, PI staining, and flow cytometry were performed. As shown in Figure 4C and D, the inhibition of EHF caused an increase in GC cells in the G1 phase, but a significant decrease in the S phase (P < 0.05). These data indicate that EHF participates in regulating cell cycle progression in GC cell lines by promoting the transition from the G1 phase to the S phase.
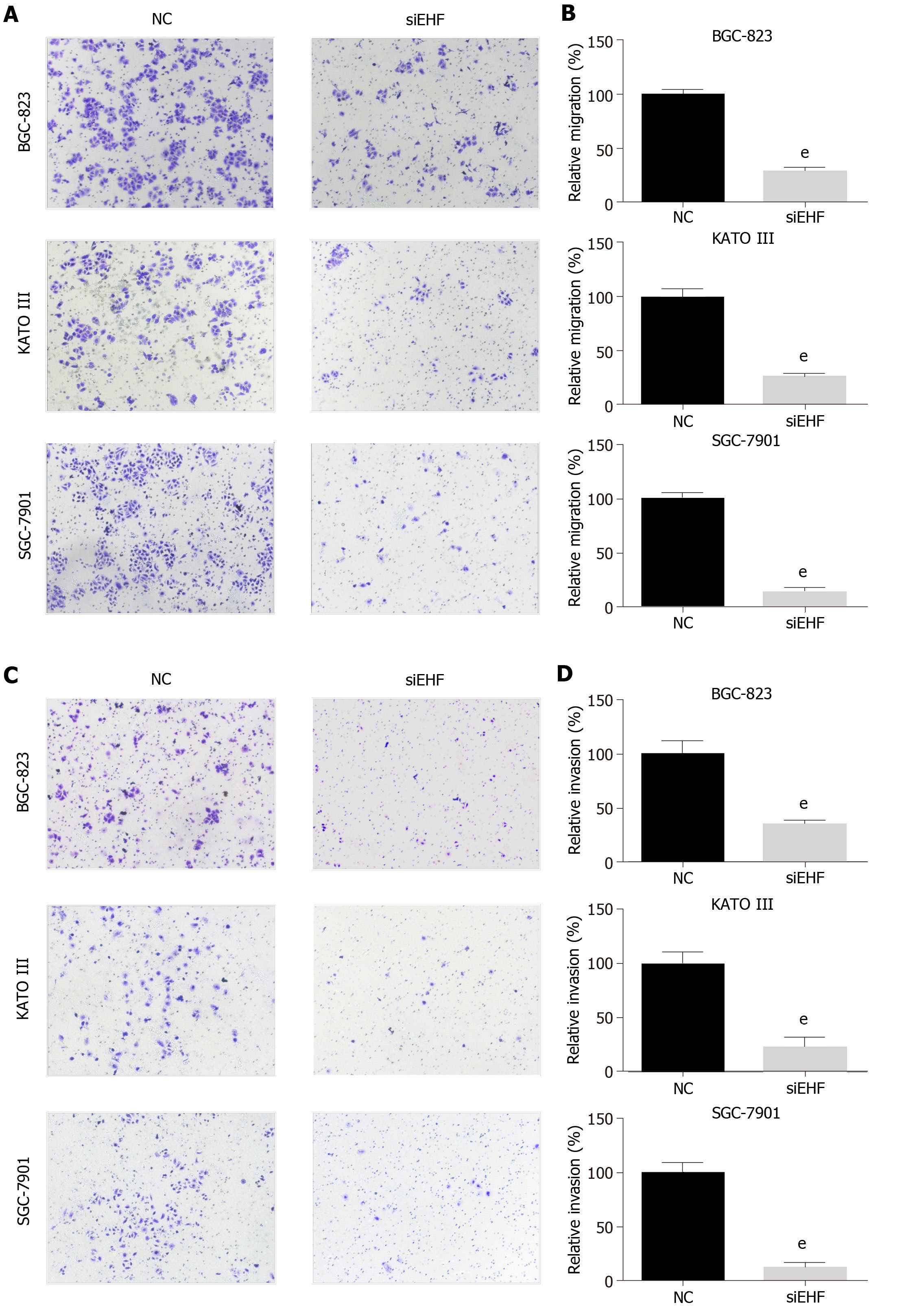
Transwell assays were used to detect cell migration after transfection for 24 h. As shown in Figure 5A and B, the migration of GC cell lines significantly decreased following EHF silencing (P < 0.01). The effect of EHF on GC cell invasion ability was further investigated. As shown in Figure 5C and D, the number of cells that invaded through the filters significantly decreased in the EHF silencing group compared with the negative control group in these GC cell lines (P < 0.01). These results suggested that EHF promotes the migration and invasion of BGC-823, KATO III and SGC-7901 cells, contributing to the high metastatic potential of GC.
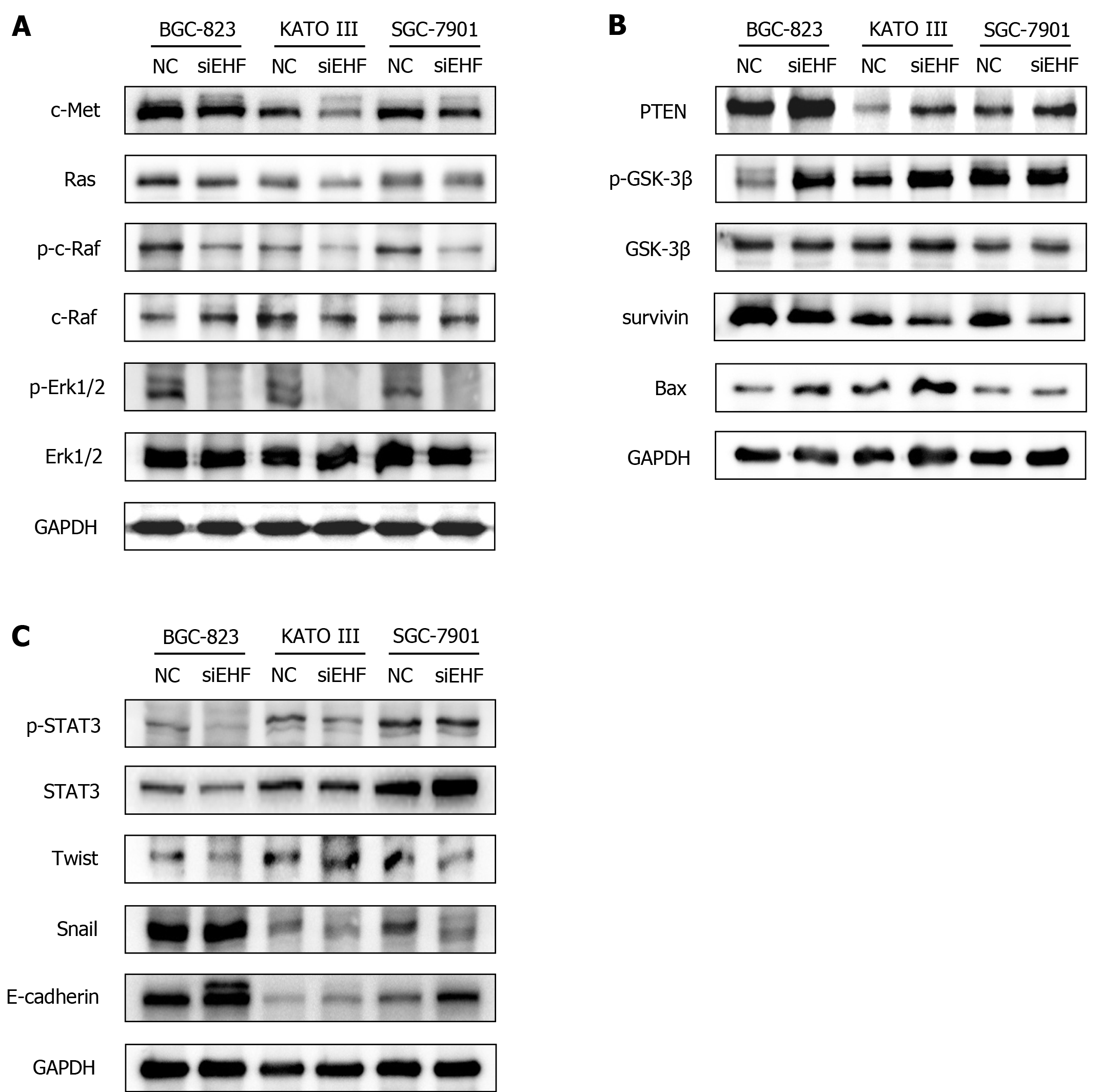
Previous findings have demonstrated that c-Met has a pivotal role in the development and progression of GC[17]. Aberrantly activated c-Met triggers tumor growth, survival, metastasis, and EMT via mitogen-activated protein kinases (MAPK), PTEN, and STAT3 signaling pathways[18-20]. Consequently, the expression of c-Met protein and its downstream molecules in the GC cell lines following EHF downregulation were detected. As shown in Figure 6A, the results suggested that EHF silencing led to the considerable inhibition of c-Met and Ras expression in BGC-823, KATO III and SGC-7901 cells. Moreover, the inactivation of c-Raf and Erk1/2 were both observed after EHF downregulation. In addition to the MAPK pathway, expression of the tumor suppressor molecule PTEN in these GC cells was significantly upregulated (Figure 6B). The decreased phosphorylation of GSK-3β, the downstream target of PTEN, was observed in BGC-823 and KATO III cells. Moreover, the expression of antiapoptotic survivin was remarkably inhibited in these GC cells, whereas the expression of proapoptotic Bax was significantly upregulated in BGC-823 and KATO III cells. Furthermore, the data indicated that STAT3, a signal transducer of the c-Met pathway, was inactivated in BGC-823, KATO III, and SGC-7901 cells following the downregulation of EHF (Figure 6C). The protein expression of the downstream transcriptional factors of STAT3 was also examined. Interestingly, the expression of Twist decreased in BGC-823 and SGC-7901 cells, while the inhibitory effect of Snail expression was considerable in KATO III and SGC-7901 cells. E-cadherin is a target of Twist and Snail, while it is also known as a biomarker for the EMT. Herein, we found that E-cadherin was significantly increased in the EHF silencing group, indicating an inhibitory effect on EMT in GC cells.
The ETS family is a transcription factor family consisting of various members, which can be divided into 11 subfamilies according to their structural characteristics[21]. EHF belongs to the epithelium-specific ETS (ESE) subfamily, and consequently is also known as ESE-3. According to previous studies, EHF is mainly expressed in multiple types of epithelial cells; its dysregulation is often detected in multiple malignant tumors, and it is associated with poor clinical outcomes[9,22-25]. However, the biological roles and potential regulatory mechanisms of EHF in GC is not completely clear.
In this study, qPCR was performed to detect the expression of EHF mRNA in GC cell lines and tissues compared with that in the gastric mucosal epithelial cell line and the adjacent tissues. The data demonstrated that EHF is overexpressed in GC tissues and three GC cell lines (BGC-823, KATO III, and SGC-7901), which is consistent with the alterations in protein expression. As a potential downstream target of EHF, the expression of c-Met was also increased in GC tissues and cell lines with high EHF expression. c-Met is an RTK encoded by the proto-oncogene MET, and hepatocyte growth factor is its only known ligand, whose aberrant expression and functional changes are closely related to the occurrence and development of GC[26]. The expression of c-Met in GC tissues is positively associated with distant metastasis and poor patient prognosis[14]. Several in vitro experiments have indicated that activated c-Met triggers its downstream MAPK, Akt/mTOR and other signaling pathways, thereby inhibiting the apoptosis of tumor cells and promoting their proliferation and migration[27]. Additionally, the inhibition of c-Met leads to the apoptosis of GC cells and promotes their sensitivity to chemotherapeutic agents[28].
The results demonstrated that EHF silencing can significantly reduce cell proliferation, promote apoptosis and induce cell cycle arrest in GC cell lines (BGC-823, KATO III and SGC-7901). On the other hand, both the migration and invasion abilities of these GC cell lines were significantly inhibited following the interference of EHF. These data indicate that EHF positively regulates the malignant biological behaviors of GC. Moreover, knockdown of EHF by specific siRNA led to the downregulation of c-Met in both GC tissues and cell lines. Currently, whether EHF participates in the tumorigenesis and progression of GC via the c-Met pathway remains unknown. Previous investigations have shown that the inhibition of c-Met in GC results in the suppression of cell proliferation both in vitro and in vivo[29]. Selective c-Met inhibitors block cell DNA synthesis and induce cell cycle arrest at the G1-S phase transition in GC cells[30]. The inactivation of c-Met can also reduce migration and invasion in vitro and ultimately block the malignant progression of GC[31]. EHF directly regulates c-Met expression via binding to its promoter region[7]. A recent study showed that EHF contributes to the metastasis and chemoresistance of oral squamous cell carcinoma through the positive regulation of the c-Met pathway[32]. Thus, EHF may be involved in the regulation of the biological behaviors of GC via promoting c-Met expression.
Recently, it has been verified that the abnormally upregulated expression of at least one RTK (human epidermal growth factor receptor 2, epidermal growth factor receptor, c-Met or fibroblast growth factor receptor 2) was found in approximately two-thirds of GC patients, indicating that the detection of RTK expression levels or their gene mutations can provide important evidence for the diagnosis and treatment of GC[33]. However, the identification of one single type of RTK expression or mutation is inadequate to accurately predict chemotherapy sensitivity or overall prognosis[34]. In recent years, the detection of multiple RTKs has improved the efficiency of predicting the prognosis to some extent, whereas the accuracy of RTK pathway analysis is still limited by the variable mutation sites and complex mutation types of these coding genes. It is possible to determine the abnormal expression or activation of RTKs by examining the expression level of the upstream regulators that affect RTK transcription regardless of their mutation status. Consequently, the combined detection of EHF and c-Met may serve as potential markers for the diagnosis and prognosis of GC.
Activation of c-Met and its key downstream MAPK cascades are responsible for maintaining the abnormal biological function of tumor cells[35]. Erk1/2 is a subtype of MAPK that is closely associated with cell proliferation, differentiation and migration[36]. The current study demonstrated that the inhibition of EHF leads to the impaired biological function of GC cell lines via the blockage of Ras-Erk1/2 cascades.
PTEN is a tumor suppressor, and its expression level is negatively correlated with the tumor-node-metastasis stage of GC. In vivo experiments have confirmed that the absence of PTEN promotes the tumorigenesis of GC[37]. It has been reported that PTEN interacts with the c-Met signaling pathway[38], and a high expression level of c-Met is associated with the downregulation of PTEN[39]. The inhibition of PTEN expression leads to the increased proliferation activity of GC cells via GSK-3β inactivation[40]. The present study demonstrated that EHF may affect the apoptosis and cell cycle progression of GC via the c-Met/PTEN/GSK-3β cascade. Interestingly, the upregulation of PTEN was detected in these GC cell lines, whereas considerable inactivation of GSK-3β was not observed in SGC-7901 cells. Previous findings have suggested that survivin is a direct target of GSK-3β, which inhibits survivin expression, leading to apoptosis in GC cells[41]. Herein, the expression of survivin increased in all the GC cells, indicating that survivin may not be completely controlled by GSK-3β in SGC-7901 cells.
In this study, STAT3 was deactivated following EHF silencing. In addition, as a biomarker for EMT, E-cadherin expression was upregulated in the GC cell lines. The activation of STAT3 promotes the expression of Twist and Snail that reduces E-cadherin expression, resulting in the EMT of malignant tumor cells[42-44]. EMT causes significant epithelial phenotypic changes and is associated with the progression, metastasis, and drug resistance of GC[45]. A previous study indicated that abnormally activated STAT3 plays an important role in c-Met-mediated EMT in GC[46]. Accordingly, the results suggested that EHF may regulate EMT in GC cell lines via the c-Met/STAT3 pathway. However, the downregulation of Twist was not observed in KATO III cells, and Snail expression was not significantly affected in BGC-823 cells, indicating that STAT3 may affect the EMT of BGC-823 and KATO III cells through Twist and Snail, respectively.
This study had some limitations. Further investigations with large sample sizes are needed to clarify the relationship between EHF and c-Met expression in GC. In the present study, the potential role of EHF in GC tumorigenesis was demonstrated and the possible mechanisms underlying the antineoplastic effects induced by EHF downregulation were further sought. Nevertheless, the advantage of EHF as an antineoplastic target for GC diagnosis and treatment has not yet been fully elucidated and remains to be validated both in vitro and in vivo.
In summary, EHF may promote cell proliferation and cell cycle progression and inhibit the apoptosis of GC cells via regulating the c-Met pathway. Furthermore, EHF may contribute to the migration and invasion of GC cells by inducing EMT via the STAT3 pathway. Therefore, by targeting the c-Met pathway, EHF can be regarded as a promising candidate target for the development of a novel antineoplastic strategy for GC treatment.
Gastric cancer (GC) is one of the most common and deadliest types of cancer worldwide due to its delayed diagnosis and high metastatic frequency, and its exact pathogenesis has not been fully elucidated. ETS homologous factor (EHF) is an important member of the ETS family and contributes to the pathogenesis of multiple malignant tumors. To date, whether EHF participates in the development of GC via the c-Met signaling pathway remains unclear.
Currently, surgical resection represents the best curative option for GC. On the other hand, advanced novel targets for GC diagnosis and treatment have resulted in decreased incidence and mortality rates in recent years. However, the 5-year overall survival rate of patients with advanced GC is less than 30% due to delayed diagnosis and recurrence. Therefore, revealing the oncogenic mechanisms of GC and further exploring the strategies for GC diagnosis and treatment are urgent.
To investigate the role and mechanism of EHF in the occurrence and development of GC.
The expression of EHF mRNA in GC tissues and cell lines was measured by qPCR. Western blotting was performed to determine the protein expression of EHF, c-Met and its downstream signal molecules. The EHF expression in GC tissues was further checked by immunohistochemical staining. To investigate the role of EHF in GC oncogenesis, small interfering RNAs (siRNAs) against EHF were transfected into GC cells. The cell proliferation of GC cells was determined by cell counting kit-8 and colony formation assays. Flow cytometry was performed following annexin V/PI and PI staining to determine apoptosis and the cell cycle, respectively. Cell migration and invasion were assessed by transwell assays.
The data indicated that EHF was upregulated in GC tissues and cell lines in which increased expression of c-Met was also observed. Silencing of EHF by siRNA reduced cell proliferation in GC cells. Inhibition of EHF induced significant apoptosis and cell cycle arrest in GC cells. Cell migration and invasion were significantly inhibited. EHF silencing led to the c-Met downregulation and further blocked Ras/c-Raf/Erk1/2. Additionally, PTEN was upregulated and GSK-3β was deactivated. Moreover, inactivation of STAT3 was detected following EHF inhibition, leading to the blockage of the epithelial-to-mesenchymal transition (EMT).
These results suggested that EHF plays a key role in cell proliferation, invasion, apoptosis, the cell cycle and EMT via the c-Met pathway.
EHF may serve as an antineoplastic target for the diagnosis and treatment of GC.
We are very grateful to The State Key Laboratory for Diagnosis and Treatment of Infectious Diseases of The First Affiliated Hospital of Zhejiang University for providing excellent technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li Y, Neninger E S-Editor: Huang P L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55500] [Article Influence: 7928.6] [Reference Citation Analysis (131)] |
| 2. | Doorakkers E, Lagergren J, Engstrand L, Brusselaers N. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut. 2018;67:2092-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Fang X, Wei J, He X, An P, Wang H, Jiang L, Shao D, Liang H, Li Y, Wang F, Min J. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C, Cervantes A. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. 2014;40:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP; CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385:977-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1728] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 6. | Feng FY, Brenner JC, Hussain M, Chinnaiyan AM. Molecular pathways: targeting ETS gene fusions in cancer. Clin Cancer Res. 2014;20:4442-4448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Kas K, Finger E, Grall F, Gu X, Akbarali Y, Boltax J, Weiss A, Oettgen P, Kapeller R, Libermann TA. ESE-3, a novel member of an epithelium-specific ets transcription factor subfamily, demonstrates different target gene specificity from ESE-1. J Biol Chem. 2000;275:2986-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Tugores A, Le J, Sorokina I, Snijders AJ, Duyao M, Reddy PS, Carlee L, Ronshaugen M, Mushegian A, Watanaskul T, Chu S, Buckler A, Emtage S, McCormick MK. The epithelium-specific ETS protein EHF/ESE-3 is a context-dependent transcriptional repressor downstream of MAPK signaling cascades. J Biol Chem. 2001;276:20397-20406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Albino D, Longoni N, Curti L, Mello-Grand M, Pinton S, Civenni G, Thalmann G, D'Ambrosio G, Sarti M, Sessa F, Chiorino G, Catapano CV, Carbone GM. ESE3/EHF controls epithelial cell differentiation and its loss leads to prostate tumors with mesenchymal and stem-like features. Cancer Res. 2012;72:2889-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Cheng Z, Guo J, Chen L, Luo N, Yang W, Qu X. Knockdown of EHF inhibited the proliferation, invasion and tumorigenesis of ovarian cancer cells. Mol Carcinog. 2016;55:1048-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Hufton SE, Moerkerk PT, Brandwijk R, de Bruïne AP, Arends JW, Hoogenboom HR. A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett. 1999;463:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Taniue K, Oda T, Hayashi T, Okuno M, Akiyama T. A member of the ETS family, EHF, and the ATPase RUVBL1 inhibit p53-mediated apoptosis. EMBO Rep. 2011;12:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kawakami H, Okamoto I. MET-targeted therapy for gastric cancer: the importance of a biomarker-based strategy. Gastric Cancer. 2016;19:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Wang YM, Gu ML, Meng FS, Jiao WR, Zhou XX, Yao HP, Ji F. Histone acetyltransferase p300/CBP inhibitor C646 blocks the survival and invasion pathways of gastric cancer cell lines. Int J Oncol. 2017;51:1860-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117419] [Cited by in RCA: 132850] [Article Influence: 5535.4] [Reference Citation Analysis (1)] |
| 17. | Matsumoto K, Umitsu M, De Silva DM, Roy A, Bottaro DP. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017;108:296-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 18. | Choi KM, Cho E, Kim E, Shin JH, Kang M, Kim B, Han EH, Chung YH, Kim JY. Prolonged MEK inhibition leads to acquired resistance and increased invasiveness in KRAS mutant gastric cancer. Biochem Biophys Res Commun. 2018;507:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 20. | Lai AZ, Cory S, Zhao H, Gigoux M, Monast A, Guiot MC, Huang S, Tofigh A, Thompson C, Naujokas M, Marcus VA, Bertos N, Sehat B, Perera RM, Bell ES, Page BD, Gunning PT, Ferri LE, Hallett M, Park M. Dynamic reprogramming of signaling upon met inhibition reveals a mechanism of drug resistance in gastric cancer. Sci Signal. 2014;7:ra38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kar A, Gutierrez-Hartmann A. Molecular mechanisms of ETS transcription factor-mediated tumorigenesis. Crit Rev Biochem Mol Biol. 2013;48:522-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Gutierrez-Hartmann A, Duval DL, Bradford AP. ETS transcription factors in endocrine systems. Trends Endocrinol Metab. 2007;18:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Brenne K, Nymoen DA, Hetland TE, Trope' CG, Davidson B. Expression of the Ets transcription factor EHF in serous ovarian carcinoma effusions is a marker of poor survival. Hum Pathol. 2012;43:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Zhao T, Jiang W, Wang X, Wang H, Zheng C, Li Y, Sun Y, Huang C, Han ZB, Yang S, Jia Z, Xie K, Ren H, Hao J. ESE3 Inhibits Pancreatic Cancer Metastasis by Upregulating E-Cadherin. Cancer Res. 2017;77:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | He J, Pan Y, Hu J, Albarracin C, Wu Y, Dai JL. Profile of Ets gene expression in human breast carcinoma. Cancer Biol Ther. 2007;6:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Ahn SY, Kim J, Kim MA, Choi J, Kim WH. Increased HGF Expression Induces Resistance to c-MET Tyrosine Kinase Inhibitors in Gastric Cancer. Anticancer Res. 2017;37:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Wu Y, Yao X, Zhu M, Qian H, Jiang L, Lan T, Wu M, Pang J, Chen Y. PKG II reverses HGF-triggered cellular activities by phosphorylating serine 985 of c-Met in gastric cancer cells. Oncotarget. 2016;7:34190-34200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Zhang Z, Kong Y, Yang W, Ma F, Zhang Y, Ji S, Ma EM, Liu H, Chen Y, Hua Y. Upregulation of microRNA-34a enhances the DDP sensitivity of gastric cancer cells by modulating proliferation and apoptosis via targeting MET. Oncol Rep. 2016;36:2391-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Ohashi Y, Okamura M, Hirosawa A, Tamaki N, Akatsuka A, Wu KM, Choi HW, Yoshimatsu K, Shiina I, Yamori T, Dan S. M-COPA, a Golgi Disruptor, Inhibits Cell Surface Expression of MET Protein and Exhibits Antitumor Activity against MET-Addicted Gastric Cancers. Cancer Res. 2016;76:3895-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Park CH, Cho SY, Ha JD, Jung H, Kim HR, Lee CO, Jang IY, Chae CH, Lee HK, Choi SU. Novel c-Met inhibitor suppresses the growth of c-Met-addicted gastric cancer cells. BMC Cancer. 2016;16:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Toiyama Y, Yasuda H, Saigusa S, Matushita K, Fujikawa H, Tanaka K, Mohri Y, Inoue Y, Goel A, Kusunoki M. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012;130:2912-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Huang WC, Jang TH, Tung SL, Yen TC, Chan SH, Wang LH. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing β5-integrin/c-met signaling pathway. J Exp Clin Cancer Res. 2019;38:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Nagatsuma AK, Aizawa M, Kuwata T, Doi T, Ohtsu A, Fujii H, Ochiai A. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer. 2015;18:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 34. | Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, Miwa H, Qin SK, Chung IJ, Yeh KH, Feng JF, Mukaiyama A, Kobayashi M, Ohtsu A, Bang YJ. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 486] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 35. | Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 432] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 36. | Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1960] [Cited by in RCA: 2221] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 37. | Kim SJ, Lee HW, Baek JH, Cho YH, Kang HG, Jeong JS, Song J, Park HS, Chun KH. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene. 2016;35:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Abounader R, Reznik T, Colantuoni C, Martinez-Murillo F, Rosen EM, Laterra J. Regulation of c-Met-dependent gene expression by PTEN. Oncogene. 2004;23:9173-9182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Ach T, Zeitler K, Schwarz-Furlan S, Baader K, Agaimy A, Rohrmeier C, Zenk J, Gosau M, Reichert TE, Brockhoff G, Ettl T. Aberrations of MET are associated with copy number gain of EGFR and loss of PTEN and predict poor outcome in patients with salivary gland cancer. Virchows Arch. 2013;462:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Tseng PC, Huang WC, Chen CL, Sheu BS, Shan YS, Tsai CC, Wang CY, Chen SO, Hsieh CY, Lin CF. Regulation of SHP2 by PTEN/AKT/GSK-3β signaling facilitates IFN-γ resistance in hyperproliferating gastric cancer. Immunobiology. 2012;217:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Li HL, Liang S, Cui JH, Han GY. Targeting of GSK-3β by miR-214 to facilitate gastric cancer cell proliferation and decrease of cell apoptosis. Eur Rev Med Pharmacol Sci. 2018;22:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Wang BJ, Zhang ZQ, Ke Y. Conversion of cadherin isoforms in cultured human gastric carcinoma cells. World J Gastroenterol. 2006;12:966-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Hsu KW, Hsieh RH, Huang KH, Fen-Yau Li A, Chi CW, Wang TY, Tseng MJ, Wu KJ, Yeh TS. Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression. Carcinogenesis. 2012;33:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1:a003129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 488] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 45. | Peng Z, Wang CX, Fang EH, Wang GB, Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20:5403-5410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 139] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 46. | Okamoto W, Okamoto I, Arao T, Yanagihara K, Nishio K, Nakagawa K. Differential roles of STAT3 depending on the mechanism of STAT3 activation in gastric cancer cells. Br J Cancer. 2011;105:407-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |