Published online Oct 21, 2020. doi: 10.3748/wjg.v26.i39.6098
Peer-review started: May 19, 2020
First decision: May 29, 2020
Revised: June 2, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: October 21, 2020
Processing time: 154 Days and 11.1 Hours
Colonic transendoscopic enteral tubing (TET) requires double cecal intubation, raising a common concern of how to save cecal intubation time and make the tube stable. We hypothesized that cap-assisted colonoscopy (CC) might reduce the second cecal intubation time and bring potential benefits during the TET procedure.
To investigate if CC can decrease the second cecal intubation time compared with regular colonoscopy (RC).
This prospective multicenter, randomized controlled trial was performed at four centers. Subjects ≥ 7 years needing colonic TET were recruited from August 2018 to January 2020. All subjects were randomly assigned to two groups. The primary outcome was the second cecal intubation time. Secondary outcomes included success rate, insertion pain score, single clip fixation time, purpose and retention time of TET tube, length of TET tube inserted into the colon, and all procedure-related (serious) adverse events.
A total of 331 subjects were randomized to the RC (n = 165) or CC (n = 166) group. The median time of the second cecal intubation was significantly shorter for CC than RC (2.2 min vs 2.8 min, P < 0.001). In patients with constipation, the median time of second cecal intubation in the CC group (n = 50) was shorter than that in the RC group (n = 43) (2.6 min vs 3.8 min, P = 0.004). However, no difference was observed in the CC (n = 42) and RC (n = 46) groups of ulcerative colitis patients (2.0 min vs 2.5 min, P = 0.152). The insertion pain score during the procedure in CC (n = 14) was lower than that in RC (n = 19) in unsedated colonoscopy (3.8 ± 1.7 vs 5.4 ± 1.9; P = 0.015). Multivariate analysis revealed that only CC (odds ratio [OR]: 2.250, 95% confidence interval [CI]: 1.161-4.360; P = 0.016) was an independent factor affecting the second cecal intubation time in difficult colonoscopy. CC did not affect the colonic TET tube’s retention time and length of the tube inserted into the colon. Moreover, multivariate analysis found that only endoscopic clip number (OR: 2.201, 95%CI: 1.541-3.143; P < 0.001) was an independent factor affecting the retention time. Multiple regression analysis showed that height (OR: 1.144, 95%CI: 1.027-1.275; P = 0.014) was the only independent factor influencing the length of TET tube inserted into the colon in adults.
CC for colonic TET procedure is a safe and less painful technique, which can reduce cecal intubation time.
Core Tip: The design of colonic transendoscopic enteral tubing (TET) requires repeated colonoscopies, which increase procedure time and potential procedure-related risk. This multicenter, prospective, randomized controlled trial explored whether cap-assisted colonoscopy (CC) can decrease the second cecal intubation time and has potential benefits compared with regular colonoscopy during the TET procedure. Our findings show that CC can decrease the second cecal intubation time during the TET procedure, especially for difficult colonoscopy. Moreover, CC for colonic TET can reduce the insertion pain score in unsedated colonoscopy and does not affect the safety and stability of the TET tube.
- Citation: Wen Q, Liu KJ, Cui BT, Li P, Wu X, Zhong M, Wei L, Tu H, Yuan Y, Lin D, Hsu WH, Wu DC, Yin H, Zhang FM. Impact of cap-assisted colonoscopy during transendoscopic enteral tubing: A randomized controlled trial. World J Gastroenterol 2020; 26(39): 6098-6110
- URL: https://www.wjgnet.com/1007-9327/full/v26/i39/6098.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i39.6098
Fecal microbiota transplantation (FMT) as a promising novel therapeutic approach has shown superior effectiveness in many microbiota-related diseases such as Clostridium difficile infection (CDI) and inflammatory bowel disease (IBD)[1-4]. As reported in most studies, multiple fecal infusions are often necessary to obtain a higher remission rate. Ianiro et al[5] proved that multiple infusions of FMT were significantly more effective than a single infusion in curing severe refractory CDI via colonoscopy. Several randomized controlled trials have also confirmed the outstanding benefit of FMT in treating ulcerative colitis (UC) using the protocol of multiple fecal infusions by colonoscopy or enema[6,7]. Furthermore, previous studies have suggested that FMT via lower gut delivery is more effective than upper gut delivery[8,9]. The higher efficacy of FMT in CDI treatment via colonoscopy than duodenal delivery has also been proven by a meta-analysis[10]. However, delivering multiple FMTs by colonoscopy needs repeated bowel preparation over a short period of time, and it is challenging to retain the infused fecal suspensions on account of the residual effect of laxatives. Importantly, we have to consider the higher risk of complications in severe colitis via repeated colonoscopies. Although enema can meet the needs of multiple FMT treatments, the bacteria can only cover the rectum and sigmoid colon, which limits the input bacteria volume and is not suitable for patients who have difficulty in retaining the bacterial fluid. Thus, since 2014, our team have been exploring the placement of a tube through the anus into the cecum, a method called colonic transendoscopic enteral tubing (TET), capable of meeting the needs of patients receiving multiple fresh FMT treatments or whole-colon administration of drugs during a period of time[11-13]. The TET device (FMT Medical, Nanjing, China) has been approved by the China Food and Drug Agency for endoscopic use since 2017. At present, the colonic TET as a sought-after technique has been successfully used in many hospitals in Asia[3,11,14-17]. Allegretti et al[18] commented in the Lancet review that colonic TET is a promising method of FMT delivery. Recently, the consensus on the methodology of washed microbiota transplantation released a statement that washed microbiota suspensions can be delivered via colonic TET[19].
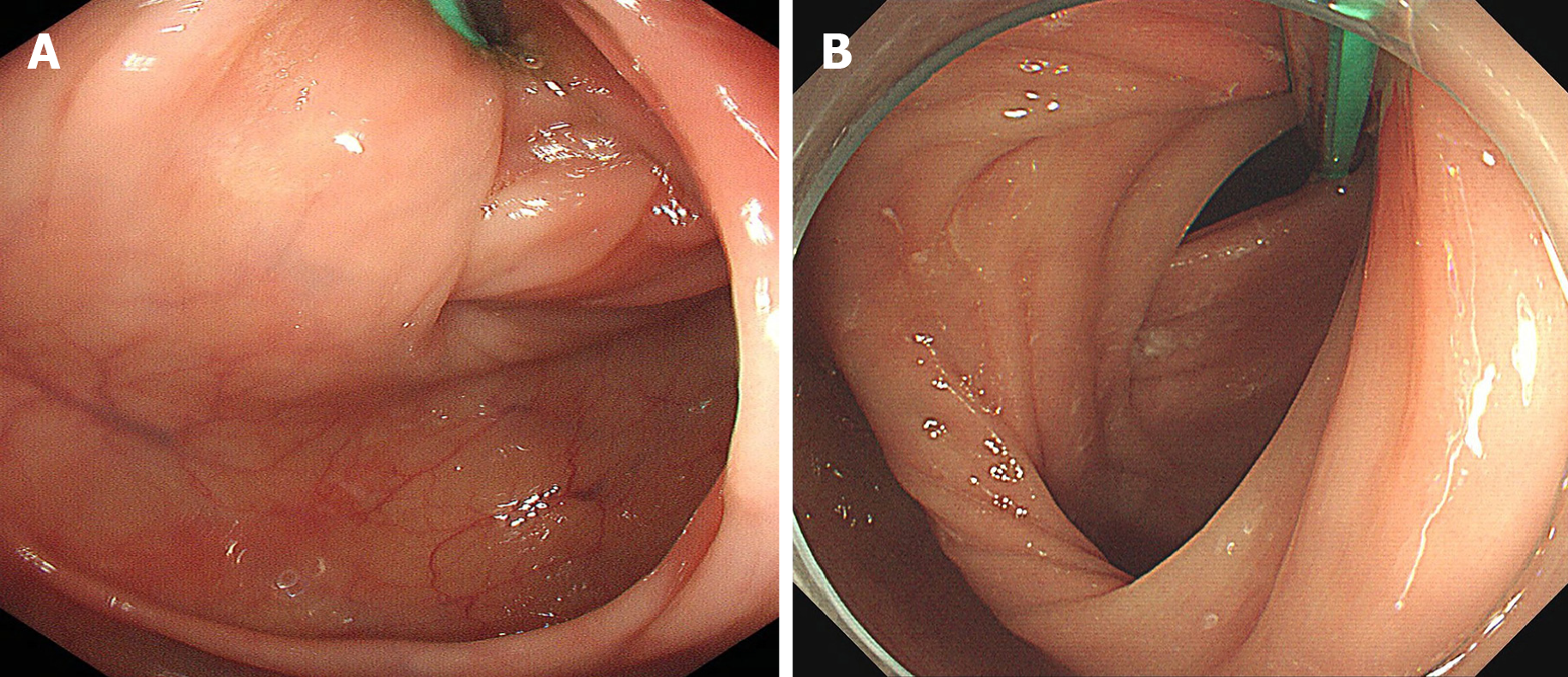
We first reported a colonic endoscopic procedure for TET in 2016 that requires cecal intubation twice, the first inserting the TET tube through the endoscopic channel, and the second affixing the TET tube to the intestinal wall using clips[11]. Generally, it is easier to insert the colonoscope for the second time with the TET tube’s guidance. However, for some patients, the intestinal lumen is difficult to find during the second insertion of colonoscope into the cecum because intestinal mucosa wrinkles and sharp corners could be caused by the TET tube pulling. This increases the difficulty of cecal intubation (Figure 1A). Thus, the TET technique raises a common concern on longer procedure time, increased medical cost and potential procedure-related risk. Cap-assisted colonoscopy (CC) is a well-known simple technique of attaching a transparent plastic cap to the tip of the colonoscope. In our early experience, we found that CC was beneficial to the decrease of cecal intubation time and difficulty of colonoscopy (Figure 1B). Cap method is a simple, practical, and inexpensive technique that serves several useful purposes in enhancing colonoscopy performance[20]. A Cochrane review indicated that CC had a faster cecal intubation time than the regular colonoscopy (RC)[21]. Reviewing studies individually would also seem to favor CC for cecal intubation rate and pain during the procedure[21]. Lee and colleagues found that CC shortened the cecal intubation time and performed better as a rescue method when the first attempt failed[22]. Kim et al[23] reported that CC had advantages in overcoming the problems associated with angulated and/or narrowed sigmoid and redundant colon. However, it is uncertain whether CC would facilitate the technical performance after the TET tube is inserted into the intestinal lumen during the TET procedure.
Therefore, this prospective randomized controlled trial compared CC with RC regarding second cecal intubation time among subjects undergoing colonic TET. We tested the hypothesis that CC might reduce the second cecal intubation time and bring potential benefits during the TET procedure.
This multicenter, prospective, subject-blinded, and randomized controlled trial was conducted at four tertiary hospitals from August 2018 to January 2020. The study protocol was approved by the Second Affiliated Hospital of Nanjing Medical University Institutional Review Board on October 10, 2017, and subsequently in all other participating centers. Written informed consent was obtained from all adult subjects or parents in pediatric cases. The study was registered at ClinicalTrials.gov (NCT03621033).
Inclusion criteria: Subjects aged ≥ 7 years who had suitability for endoscopy and needed colonic TET. Exclusion criteria were: (1) Severe bowel lesions with stenosis, fistula, or the risk of perforation; (2) Complex perianal lesions or serious lesions in the ileocecal junction or ascending colon; (3) No proper site for endoscopic clip fixation; (4) The distal of TET tube not placed in the cecum; (5) Use of the cap-assisted method in the first insertion of colonoscope; (6) Changed endoscopists during colonoscopy; (7) Poor bowel preparation affecting cecal intubation; (8) Allergy to TET tube material; and (9) Being unable to give informed consent.
Subjects were randomized (1:1) to CC and RC groups by computer-generated random numbers. Randomization was stratified by individual colonoscopists using permuted blocks of random sizes six. Each colonoscopist had his own set of randomization envelopes throughout the study at each center. The sealed opaque envelopes containing the codes were prepared by the clinical research coordinator who kept the randomization key under lock until the data collection was completed.
Subject blinding involved colonoscopists not informing the subjects of the methods and the equipment setup was the same for both groups. The endoscopic display screen was placed over the head of the subjects so they could not see the images. All data were collected by one investigator at each center who did not participate in data analysis.
All TET endoscopic procedures were performed by six colonoscopists with the experience of at least 500 colonoscopies and 10 colonic TET procedures before joining the study. All endoscopists were required to perform procedures with the same workflow.
The variable-stiffness colonoscope (CF-H260AI or CF-HQ290I; Olympus, Tokyo, Japan) was used. Some of the subjects received intravenous sedation with propofol and/or sufentanil and/or dezocine by anesthesiologists. Spasmolytic agents were not used before or during the procedure. The concept of colonic TET is to insert a tiny and soft tube into the deep colon and fix it onto the intestinal wall through the anus under endoscopy with endoscopic clips[11,24]. The endoscopic procedure of colonic TET was in accordance with our previous study[11,24].
During the first cecal intubation, when the scope reached the cecum, the TET tube (outer diameter of 2.7 mm and the inner diameter of 1.8 mm, FMT-DT-F-27/1350, FMT medical, Nanjing, China) was inserted into the endoscopic channel. Then, as the TET tube reached the cecum, the colonoscope was removed. For CC, before re-insertion of the colonoscope (the second cecal intubation), a transparent plastic cap (D-201-14304; Olympus) was attached to the tip of the colonoscope (diameter 15 mm). The edge of the cap protruded for approximately 4 mm beyond the tip of the colonoscope. For RC, the colonoscope was directly re-inserted until it reached the cecum. Then the disposable endoscopic clip (ROCC-D-26-195-C, 2.6 mm × 1950 mm; Nanjing Microtech Co., Ltd, Nanjing, China) was inserted through the endoscopic channel and the loop of the TET tube was fixed to the intestinal wall. The biopsy and polypectomy were performed after the TET procedure.
Baseline characteristics including age, sex, body mass index, previous abdominal or pelvic surgery, and disease category were collected before colonic TET. The diagnosis of constipation was based on Rome IV criteria[25]. The primary outcome was the second cecal intubation time which was recorded from the beginning of the second insertion (after inserting the TET tube) to reaching the cecum. The secondary outcomes included TET success rate, maximum insertion pain score, single clip fixation time, purpose and retaining time of TET tube, length of TET tube inserted into the colon, and all procedure-related (serious) adverse events (AEs). The first cecal intubation time was also recorded (from the anus to cecum). The cecal intubation time, maximum pain score and other outcomes of TET were recorded by one investigator at each center who was not involved in colonoscopy. Successful colonic TET was defined as inserting the TET tube into the cecum and closing the last clip to fix the tube onto the intestinal wall. The maximum real-time insertion pain in subjects undergoing unsedated colonoscopy was evaluated by visual rating scale. The assistant explained the pain scores (degree of abdominal pain) to the subjects. At the second insertion phase, the subjects were asked by the assistant to report the pain score by using an 11-point visual analog scale (0 no pain and 10 most severe pain imaginable) at regular intervals. Clip fixation time was defined as the time of insertion of the first clip into the endoscopic channel to the last clip closed. The number of endoscopic clips used was also recorded. The purposes of TET tube insertion included microbiota transplantation treatment, administration of medications, and collection of intestinal fluid samples to analyze the dynamic changes of healthy human intestinal microbiome at the ileocecal interface. The friction between the cap and the TET tube may potentially affect the safety and reliability of the procedure of TET. Thus, the retaining time and the length of TET tube inserted into the colon were recorded. The retaining time of TET tube was defined as the time from the implantation of the TET tube to its falling out naturally. The length of TET tube inserted into the colon was defined as the length of the tube from the distal of TET tube (close to mouth direction) to the anus. AEs with a potential relation to colonic TET during and after TET were also investigated.
Sample size calculation was carried out using Stata software system (version 14.0; Stata Corp., College Station, TX, United States). We assumed that the time of the second cecal intubation is 3.5 min by regular colonoscopic tube placement based on our previous experience and can be accelerated 0.4 min by the cap-assisted method. To detect the difference with a significance level (a) of 0.05 and a power of 90% with a two-tailed test, we calculated that at least 132 subjects in each group were needed. Considering possible dropout rate of 10% (unsuccessful intubation and inspection due to technical difficulty), 146 subjects were planned to be enrolled in each group.
Quantitative data were summarized as mean ± standard deviation or median (interquartile range [IQR]), and categorical variables were reported as percentages. Differences in continuous variables between both arms were tested using the t-test or Mann–Whitney nonparametric test, depending on which assumption was met. Categorical variables were analyzed using the χ2 test or Fisher’s exact test. The Kruskal-Wallis test was used to compare the relationship between the number of endoscopic clips and retention time of TET tube. Subjects were divided into two groups according to the median of the first cecal intubation time, and those with longer time than the median were defined as difficult colonoscopy. Univariate and multivariate logistic regression analyses were performed, and multivariate analysis was done using variables with P value < 0.20 in the univariate analysis. Analyses were performed with the SPSS software V.25.0 (IBM Corp., Armonk, NY, United States). A two-tailed P value < 0.05 was considered statistically significant.
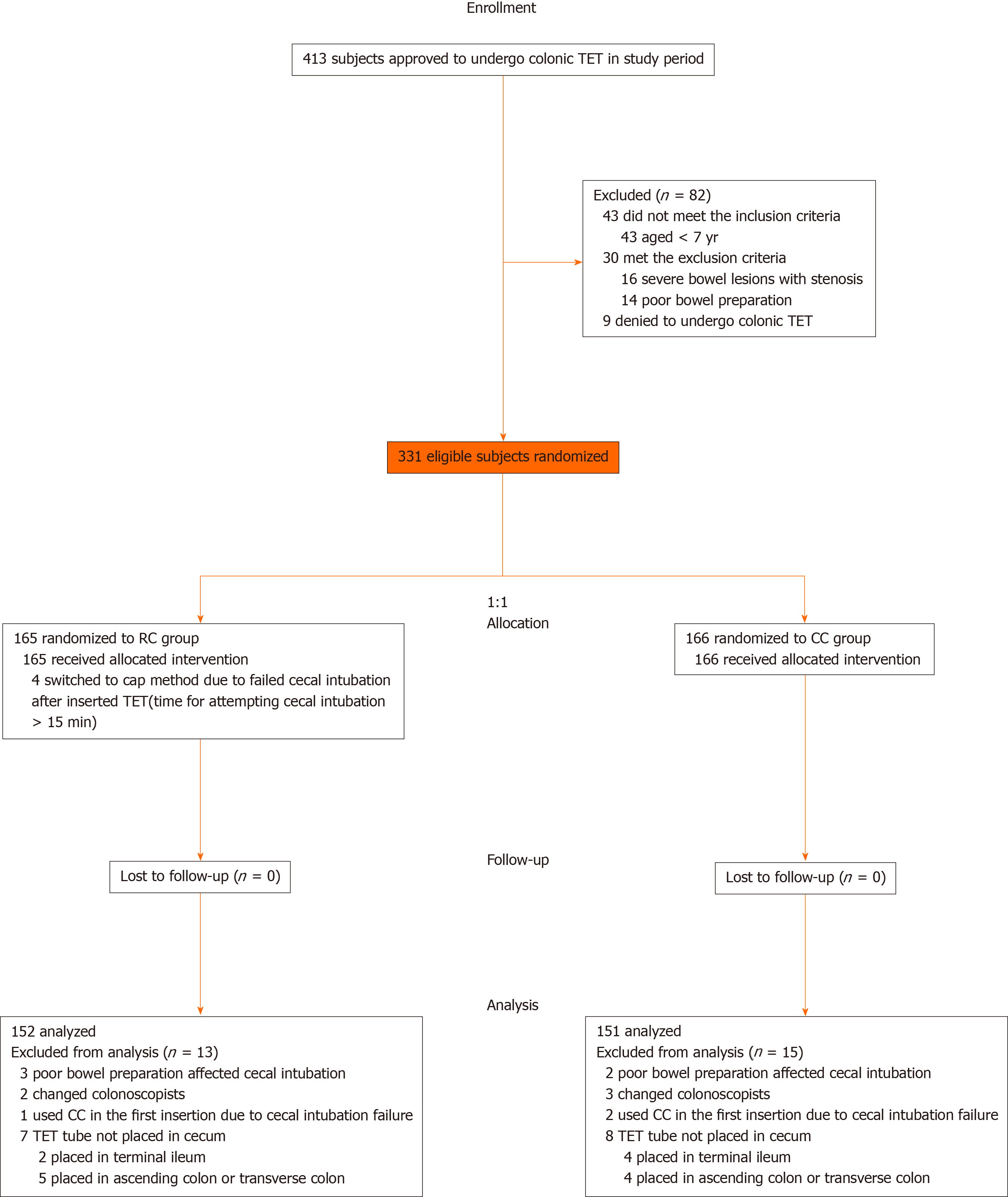
Between August 2018 and January 2020, 413 subjects referred to the four centers were considered for enrollment, with a total of 82 subjects excluded. Four subjects switched to the cap method successfully achieved cecal intubation. The tubes of six subjects were fixed at the terminal ileum for enteral administration, including two Crohn's disease (CD) patients with terminal ileum lesions (RC vs CC, 0 vs 2; P = 0.498) and four UC patients with extensive colitis (RC vs CC, 2 vs 2; P = 1.000), who had no proper site for endoscopic clip fixation. Moreover, the tubes were fixed at ascending colon or transverse colon in nine UC patients with left-side colitis for better coverage of intestinal lesions with medication (RC vs CC, 5 vs 4; P = 0.750). Finally, 152 subjects in the RC group and 151 in the CC group underwent analysis. The study flow is detailed in Figure 2. Baseline characteristics were well balanced between the two groups as shown in Table 1.
| RC group, n = 152 | CC group, n = 151 | P value | |
| Age in yr, mean ± SD | 44.4 ± 17.6 | 46.7 ± 17.0 | 0.248 |
| Children1, n (%) | 4 (2.6) | 4 (2.6) | 1.000 |
| Male, n (%) | 76 (50.0) | 79 (52.3) | 0.687 |
| Body mass index in kg/m2, mean ± SD | 21.1 ± 3.8 | 21.6 ± 3.3 | 0.243 |
| Disease category, n (%) | |||
| Ulcerative colitis | 46 (30.3) | 42 (27.8) | 0.639 |
| Constipation | 43 (28.3) | 50 (33.1) | 0.363 |
| Irritable bowel syndrome | 14 (9.2) | 18 (11.9) | 0.443 |
| Crohn's disease | 6 (3.9) | 3 (2.0) | 0.501 |
| Healthy volunteers | 3 (2.0) | 3 (2.0) | 1.000 |
| Others2 | 40 (26.3) | 35 (23.2) | 0.527 |
| Previous surgery, abdominal or pelvic, n (%) | 38 (25.0) | 33 (21.9) | 0.518 |
The study outcomes related to TET procedure are summarized in Table 2. The median second cecal intubation time in RC and CC groups was 2.8 (1.8-4.0) and 2.2 (1.6-3.2) min, respectively (P < 0.001). A subgroup analysis was done in the constipation patients, and the median time of the second cecal intubation in RC (n = 43) and CC (n = 50) groups was 3.8 (2.2-5.6) and 2.6 (1.9-3.7) min, respectively (P = 0.004). However, no statistical difference was observed in patients with UC, the median time of the second cecal intubation in RC (n = 46) and CC (n = 42) groups being 2.5 (1.6-3.5) and 2.0 (1.5-3.0) min, respectively (P = 0.152).
| RC group,n = 152 | CC group,n = 151 | P value | |
| Cecal intubation rate after inserting TET tube, n (%) (%) | 148 (97.4) | 151 (100) | - |
| The first cecal intubation time in min, median (IQR) | 5.9 (4.3-8.5) | 6.2 (4.1-8.3) | 0.921 |
| The second cecal intubation time in min, median (IQR) | 2.8 (1.8-4.0) | 2.2 (1.6-3.2) | 0.000 |
| The second cecal intubation time of constipation patients | 3.8 (2.2-5.6) | 2.6 (1.9-3.7) | 0.004 |
| The second cecal intubation time of ulcerative colitis patients | 2.5 (1.6-3.5) | 2.0 (1.5-3.0) | 0.152 |
| Average fixation time per endoscopic clip in min, mean ± SD | 1.1 ± 0.4 | 1.2 ± 0.6 | 0.238 |
| Number of endoscopic clips, mean ± SD | 2.7 ± 1.1 | 2.6 ± 1.1 | 0.339 |
| Colonoscopy type, n (%) | |||
| Sedated | 133 (87.5) | 137 (90.7) | 0.367 |
| Unsedated | 19 (12.5) | 14 (9.3) | 0.367 |
| Maximum pain score, mean ± SD | 5.4 ± 1.9 | 3.8 ± 1.7 | 0.015 |
| TET success rate, n (%) | 152 (100) | 151 (100) | - |
| Serious adverse event, n (%) | 0 | 0 | - |
| Adverse events, n (%) | 4 (2.6) | 5 (3.3) | 0.750 |
| Anal pain | 2 (1.3) | 1 (0.7) | 1.000 |
| Mild anal discomfort | 1 (0.7) | 3 (2.0) | 0.371 |
| Transient anal bleeding | 1 (0.7) | 1 (0.7) | 1.000 |
In total, 296 (97.7%) subjects used TET for single or multiple microbiota transplantations, 75 (24.8%) for intracolonic medication administrations, and 6 (2.0%) healthy volunteers for sampling. After the treatment was completed, 85 subjects (28.1%) actively pulled out the TET tube. The TET tube spontaneously fell out in 218 subjects (71.9%), and the median retention time was 8.0 (6.0-10.0) d. We analyzed possible factors contributing to the retention time of TET tube. These subjects were divided into the short retention time group (< 8 d) and the long retention time group (≥ 8 d). As shown in Table 3, multivariate analysis showed that only endoscopic clip number (OR = 2.201, 95%CI: 1.541-3.143, P < 0.001) was an independent factor affecting the retention time.
| Univariate analysis | Multivariate analysis | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age in yr | 1.008 (0.992-1.025) | 0.343 | - | - | |
| Sex, male vs female | 1.517 (0.804-2.862) | 0.198 | 1.930 (0.966-3.858) | 0.063 | |
| Disease type, IBD vs others | 0.589 (0.339-1.025) | 0.061 | 0.960 (0.499-1.846) | 0.903 | |
| Number of endoscopic clips | 2.137 (1.546-2.954) | 0.000 | 2.201 (1.541-3.143) | 0.000 | |
| Grouping, RC vs CC | 0.775 (0.455-1.320) | 0.348 | - | - | |
The maximum retention time of the TET tube was 28 d. As shown in Figure 3, the retention time of 1 (n = 37), 2 (n = 74), 3 (n = 78), and 4 (n = 29) clips used by the TET was 6.0 (4.5-7.0) d, 7.0 (6.0-10.0) d, 8.0 (7.0-11.0) d, and 10.0 (7.0-11.5) d, respectively.
The median of the first cecal intubation time was 6.1 min. The median of the second cecal intubation time was 3.1 min in the subjects with difficult colonoscopy. The subjects were divided into the fast cecal intubation group (< 3.1 min) and the slow cecal intubation group (≥ 3.1 min). As shown in Table 4, according to multivariate analysis, only CC (OR = 2.250, 95%CI: 1.161-4.360; P = 0.016) was an independent factor affecting the second cecal intubation time in subjects with difficult colonoscopy.
| Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age in yr | 1.002 (0.477-2.102) | 0.996 | - | - |
| Sex, male vs female | 0.930 (0.487-1.774) | 0.825 | - | - |
| Body mass index, < 21 vs ≥ 21 kg/m2 | 0.855 (0.452-1.616) | 0.629 | - | - |
| Previous surgery, abdominal or pelvic1 | 0.868 (0.414-1.822) | 0.709 | - | - |
| Colonoscopy, sedated vs unsedated | 1.062 (0.406-2.781) | 0.902 | - | - |
| Constipation1 | 0.559 (0.291-1.073) | 0.080 | 0.514 (0.263-1.005) | 0.052 |
| Grouping, RC vs CC | 2.103 (1.101-4.017) | 0.024 | 2.250 (1.161-4.360) | 0.016 |
During the late stage of the current procedure, we recorded the length of the tube at the anus in 63 adult subjects (aged ≥ 18). The mean length of TET tube inserted into the colon was 85.9 ± 10.0 cm. As shown in Table 5, in multiple regression analysis, height (OR = 1.144, 95%CI: 1.027-1.275; P = 0.014) was the only independent factor influencing the length of TET tube inserted into the colon in adults.
| Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age in yr | 0.996 (0.967-1.025) | 0.774 | - | - |
| Height, < 165 vs ≥ 165 cm) | 9.750 (2.523-37.675) | 0.001 | 1.144 (1.027-1.275) | 0.014 |
| Sex, male vs female | 0.352 (0.117-1.062) | 0.064 | 1.184 (0.246-5.693) | 0.833 |
| IBD1 | 1.161 (0.346-3.901) | 0.809 | - | - |
| Constipation1 | 1.212 (0.329-4.466) | 0.773 | - | - |
| Grouping, RC vs CC | 1.895 (0.645-5.569) | 0.245 | ||
There was no cap displacement during the TET procedure. No serious AE was observed during and after the procedure of TET in either group. Among all subjects with TET, merely 3.0% (9/303) complained of mild AEs as shown in Table 2. The anal pain in three cases was considered to be related to hemorrhoids. Four subjects reported abdominal discomfort during the TET tube retention period, and the abdominal discomfort disappeared after TET tube was removed.
In this randomized controlled study, we showed that CC shortened the second cecal intubation time during the TET procedure. Multiple studies have reported a significant decrease in cecal intubation time in CC compared with RC. The use of cap decreased the cecal intubation time by an average of 0.63 and 0.88 min, respectively, in two meta-analyses[26,27]. Among meta-analyses of randomized controlled trials, no study has shown a longer intubation time by the cap method[21]. Short cecal intubation time is vital for several reasons: Less anesthetic drug and sufficient withdrawal time for accurate examination and endoscopic treatment. A previous study reported that a longer cecal insertion time was associated with a decreased detection of adenomas and advanced adenomas[28]. Moreover, in the stratified subgroup analysis, the effect of cecal intubation time with a transparent cap was strong in the subgroups of constipation patients. However, the cap failed to show significant benefit in UC patients, probably because it is easier to cecal intubation due to chronic inflammation that results in the shortening of colon length, stiff colon and loss of haustral pattern. Most of the constipation patients with redundant colon, twisted colon, and mucosal prolapse receive typical difficult colonoscopy. As proven by multiple studies[22,23] the cap method showed significantly higher performance under challenging cases. Kim et al[23] found that CC helped shorten the cecal intubation time in difficult cases by an average of 1.43 min. An earlier study by Lee and colleagues also found that CC was effective as a back-up procedure in difficult cases[22]. Consistent with these previous results, we further demonstrated that CC was an independent factor affecting the insertion time in difficult cases during TET procedure. If the time of the endoscopy is prolonged during the procedure of TET, patients potentially have the risk of complications, including abdominal pain, respiratory depression, low oxygen saturation, hypotension, cardiac arrhythmia, aspiration, etc. Besides, rare serious complications related to barotraumas, such as mucosal tears, intestinal perforation and air embolism, might be observed[29]. Therefore, use of CC is recommended in TET procedure for difficult colonoscopy, except for UC patients, who may have more mucosal traumas, particularly in severe cases of colitis[30].
Several studies have explored patient discomfort and pain undergoing CC among unsedated patients. Both Tada et al[31] and Lee et al[22] showed that there was no difference in patient discomfort between the CC and RC groups. However, a significantly reduced pain level in the colonoscopy with cap was proved by several studies[32-34]. No study has shown a higher degree of patient discomfort or pain with CC compared with RC. The finding is consistent with this study, which found that the pain score was lower in the CC group than the RC during the TET procedure. This might be attributed to two factors: The cap method may prevent looping and there is also less need for air insufflation because the cap provides a better visual field[33,34].
Our current study showed that CC had a higher cecal intubation rate than RC, though no significant difference was found. It echoes the findings by two meta-analyses[26,35]. Although it seems that colonoscopy using the cap method does not confer any significant benefit for cecal intubation, there is evidence to suggest that it may be a back-up procedure when RC fails to intubate the cecum. Lee and colleagues reported that CC achieved a higher rescue rate in cecal intubation compared with RC[22].
In our experience, the cap method may help to stabilize the colonoscope which may be useful to the procedure of fixing endoscopic clips. However, the results of this study suggested that there was no difference in the TET tube’s single endoscopic clip fixation time between the two groups. This may be related to the fact that fecal matter may stick to the cap’s interior, thus impeding the view and needing a longer time to be cleaned by water insufflation or simple flushing.
Our results confirmed that the transparent cap did not affect the retention time of the colonic TET tube. The retention time of the tube was significantly correlated with the endoscopic clip number. Our previous study reported that two to four endoscopic clips are recommended to ensure the fixation of the TET tube onto the colonic wall and maintain it for 7-11 d[24]. We increased the sample size in using one endoscopic clip for the fixation of TET tube in the current study, suggesting that one clip could also meet short-term treatment needs within 6 d. Therefore, it is recommended to use one endoscopic clip to fix the TET tube in patients who only need 1 to 2 FMTs and do not need a long-term intracolonic administration of drugs, because the increased number may not bring more benefits to the patient and, conversely, may increase medical costs. Although the retention time of the TET tube using four endoscopic clips was longer than that of three, the absence of statistical significance in the three to four is likely a type II error due to the small sample size.
The length of the TET tube inserted into the colon is affected by many factors. On the one hand, it may be related to age and height in physiology. On the other hand, it may be associated with the type of disease. For example, constipation may cause redundant colon or twisted colon. Nevertheless, chronic inflammatory diseases such as IBD may shorten the colon. Multivariate analysis demonstrated that height was an independent factor affecting the length of the TET tube inserted into the colon in adults. This result of our current study may be useful to guide inexperienced endoscopists to perform the TET procedure in the future. Then, the more physicians would bring more opportunities to more patients with better education and endoscopic technique[36]. Further large sample studies are still warranted to confirm this opinion, due to the lack of sample size of children in current results. Also, evidence remains to be obtained from the Western population.
There were several limitations in the current study. First, the lack of blinding of the assistant who gathered the data on pain scores may have exposed individual bias outcomes. Second, as the endoscopists had a different personal preference for the cap assisted method before, we could not exclude the possibility that the cecal intubation time may have been affected by personal bias. However, we had used procedure randomization blocks in individual operators, and each colonoscopist would contribute equally in the two groups and the overall result should not be biased by the performance of individual colonoscopists. Third, due to the absence of withdrawal time, polyp and adenoma detection rate in this study, we still need further research to investigate the time for observation of the entire colon and the number of polyps and adenomas after placing a tube in the colon.
This study demonstrated for the first time that CC for the colonic TET procedure is a safe and less painful technique saving the cecal intubation time. However, further studies are needed in children aged 3-7 years and among the Western population.
The design of colonic transendoscopic enteral tubing (TET) requires repeated colonoscopies, which increase procedure time and potential procedure-related risks. It is uncertain whether cap-assisted colonoscopy (CC) would facilitate the technical performance after inserting the TET tube into the intestinal lumen during the TET procedure.
We conducted a multicenter, prospective, and randomized controlled trial to ascertain whether CC could decrease the second cecal intubation time and bring potential benefits compared with regular colonoscopy (RC) during TET.
The aim of this study was to compare CC with RC in the second cecal intubation time among subjects undergoing colonic TET.
This trial was performed at four centers. Subjects ≥ 7 years needing colonic TET were recruited from August 2018 to January 2020. All subjects were randomly assigned to the RC (n = 165) or CC (n = 166) group. Baseline characteristics including age, sex, body mass index, previous abdominal or pelvic surgery, and disease category were collected before colonic TET. The primary outcome was the second cecal intubation time. The secondary outcomes included TET success rate, maximum insertion pain score, single clip fixation time, purpose and retaining time of TET tube, length of TET tube inserted into the colon, and all procedure-related (serious) adverse events.
The median time of the second cecal intubation was significantly shorter for the CC group than RC (2.2 min vs 2.8 min; P < 0.001). In constipation patients, the median time of the second cecal intubation in group of CC (n = 50) was shorter than RC (n = 43) (2.6 min vs 3.8 min; P = 0.004). However, no difference was observed in the groups of CC (n = 42) and RC (n = 46) in ulcerative colitis patients (2.0 min vs 2.5 min; P = 0.152). The insertion pain score during the procedure in the group of CC (n = 14) was lower than that in RC (n = 19) in unsedated colonoscopies (3.8 ± 1.7 vs 5.4 ± 1.9; P = 0.015). Multivariate analysis revealed that only CC (OR = 2.250, 95%CI: 1.161-4.360; P = 0.016) was an independent factor affecting the second cecal intubation time in difficult colonoscopy. CC did not affect the colonic TET tube retention time and the length of the tube inserted into the colon. Moreover, multivariate analysis found that only endoscopic clip number (OR = 2.201, 95%CI: 1.541-3.143; P < 0.001) was an independent factor affecting the retention time. Height (OR = 1.144, 95%CI: 1.027-1.275; P = 0.014) was the only independent factor influencing the length of TET tube inserted into the colon in adults by multiple regression analysis.
CC for the colonic TET procedure is a safe and less painful technique which is able to save the cecal intubation time. Importantly, CC does not affect the safety and stability of the TET tube.
Further studies are needed in children aged 3-7 years and the Western population.
We appreciate the kind help from Jie Zhang by providing data from China Microbiota Transplantation System (http://www.fmtbank.org), and gratitude goes to Dr. Cicilia Marcella for polishing the language in the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lohsiriwat V S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 785] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 2. | Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, Rosewarne CP, Bickley C, Peters C, Schoeman MN, Conlon MA, Roberts-Thomson IC, Andrews JM. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019;321:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 605] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 3. | Ding X, Li Q, Li P, Zhang T, Cui B, Ji G, Lu X, Zhang F. Long-Term Safety and Efficacy of Fecal Microbiota Transplant in Active Ulcerative Colitis. Drug Saf. 2019;42:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Xiang L, Ding X, Li Q, Wu X, Dai M, Long C, He Z, Cui B, Zhang F. Efficacy of faecal microbiota transplantation in Crohn's disease: a new target treatment? MicrobBiotechnol. 2020;13:760-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Ianiro G, Masucci L, Quaranta G, Simonelli C, Lopetuso LR, Sanguinetti M, Gasbarrini A, Cammarota G. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment PharmacolTher. 2018;48:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102-109.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1074] [Article Influence: 107.4] [Reference Citation Analysis (1)] |
| 7. | Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 867] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 8. | Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, Iqbal TH. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment PharmacolTher. 2017;46:479-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 431] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 9. | Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 656] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 10. | Ianiro G, Maida M, Burisch J, Simonelli C, Hold G, Ventimiglia M, Gasbarrini A, Cammarota G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United European Gastroenterol J. 2018;6:1232-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 11. | Peng Z, Xiang J, He Z, Zhang T, Xu L, Cui B, Li P, Huang G, Ji G, Nie Y, Wu K, Fan D, Zhang F. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. EndoscInt Open. 2016;4:E610-E613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Zhang F, Cui B, He X, Nie Y, Wu K, Fan D; FMT-standardization Study Group. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9:462-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 13. | Zhang F, Zhang T, Zhu H, Borody TJ. Evolution of fecal microbiota transplantation in methodology and ethical issues. CurrOpinPharmacol. 2019;49:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Huang HL, Chen HT, Luo QL, Xu HM, He J, Li YQ, Zhou YL, Yao F, Nie YQ, Zhou YJ. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J Dig Dis. 2019;20:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Wang JW, Wang YK, Zhang F, Su YC, Wang JY, Wu DC, Hsu WH. Initial experience of fecal microbiota transplantation in gastrointestinal disease: A case series. Kaohsiung J Med Sci. 2019;35:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Xie WR, Yang XY, Xia HH, Wu LH, He XX. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: A case report and review of the literature. World J Clin Cases. 2019;7:3074-3081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Chen HT, Huang HL, Xu HM, Luo QL, He J, Li YQ, Zhou YL, Nie YQ, Zhou YJ. Fecal microbiota transplantation ameliorates active ulcerative colitis. ExpTher Med. 2020;19:2650-2660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Allegretti JR, Mullish BH, Kelly C, Fischer M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet. 2019;394:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 19. | Fecal Microbiota Transplantation-standardization Study Group. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J (Engl). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Rastogi A. Cap-assisted colonoscopy. GastroenterolClin North Am. 2013;42:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Morgan J, Thomas K, Lee-Robichaud H, Nelson RL, Braungart S. Transparent cap colonoscopy versus standard colonoscopy to improve caecal intubation. Cochrane Database Syst Rev. 2012;12:CD008211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, Wu JC, Chan HL, Leung WK, Lau JY, Sung JJ, Chan FK. Efficacy of cap-assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2009;104:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Kim HH, Park SJ, Park MI, Moon W, Kim SE. Transparent-cap-fitted colonoscopy shows higher performance with cecal intubation time in difficult cases. World J Gastroenterol. 2012;18:1953-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Zhang T, Long C, Cui B, Buch H, Wen Q, Li Q, Ding X, Ji G, Zhang F. Colonic transendoscopic tube-delivered enteral therapy (with video): a prospective study. BMC Gastroenterol. 2020;20:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1872] [Article Influence: 208.0] [Reference Citation Analysis (3)] |
| 26. | Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Nutalapati V, Kanakadandi V, Desai M, Olyaee M, Rastogi A. Cap-assisted colonoscopy: a meta-analysis of high-quality randomized controlled trials. EndoscInt Open. 2018;6:E1214-E1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | von Renteln D, Robertson DJ, Bensen S, Pohl H. Prolonged cecal insertion time is associated with decreased adenoma detection. GastrointestEndosc. 2017;85:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Park HJ, Hong JH, Kim HS, Kim BR, Park SY, Jo KW, Kim JW. Predictive factors affecting cecal intubation failure in colonoscopy trainees. BMC Med Educ. 2013;13:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Tseng CW, Koo M, Hsieh YH. Cecal intubation time between cap-assisted water exchange and water exchange colonoscopy: a randomized-controlled trial. Eur J GastroenterolHepatol. 2017;29:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Tada M, Inoue H, Yabata E, Okabe S, Endo M. Feasibility of the transparent cap-fitted colonoscope for screening and mucosal resection. Dis Colon Rectum. 1997;40:618-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | de Wijkerslooth TR, Stoop EM, Bossuyt PM, Mathus-Vliegen EM, Dees J, Tytgat KM, van Leerdam ME, Fockens P, Kuipers EJ, Dekker E. Adenoma detection with cap-assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Shida T, Katsuura Y, Teramoto O, Kaiho M, Takano S, Yoshidome H, Miyazaki M. Transparent hood attached to the colonoscope: does it really work for all types of colonoscopes? SurgEndosc. 2008;22:2654-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Harada Y, Hirasawa D, Fujita N, Noda Y, Kobayashi G, Ishida K, Yonechi M, Ito K, Suzuki T, Sugawara T, Horaguchi J, Takasawa O, Obana T, Oohira T, Onochi K, Kanno Y, Kuroha M, Iwai W. Impact of a transparent hood on the performance of total colonoscopy: a randomized controlled trial. GastrointestEndosc. 2009;69:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Morgan JL, Thomas K, Braungart S, Nelson RL. Transparent cap colonoscopy versus standard colonoscopy: a systematic review and meta-analysis. Tech Coloproctol. 2013;17:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Zhong M, Sun Y, Wang HG, Marcella C, Cui BT, Miao YL, Zhang FM. Awareness and attitude of fecal microbiota transplantation through transendoscopic enteral tubing among inflammatory bowel disease patients. World J Clin Cases. 2020;8:3786-3796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |