Published online Apr 21, 2020. doi: 10.3748/wjg.v26.i15.1792
Peer-review started: December 25, 2019
First decision: January 19, 2020
Revised: March 19, 2020
Accepted: March 27, 2020
Article in press: March 27, 2020
Published online: April 21, 2020
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic diseases in the world. Nowadays, the percentage of non-obese or lean patients with NAFLD is increasing. NAFLD in non-obese populations, especially the lean subgroup with a normal waist circumference (WC), might lead to more problems than obese individuals, as these individuals may not visit clinics for NAFLD diagnosis or ignore the diagnosis of NAFLD. If the precise characteristics of these populations, especially the lean subgroup, are identified, the clinicians would be able to provide more appropriate advice and treatment to these populations.
To investigate the prevalence, clinical characteristics, risk factors, and possible indicators for NAFLD in lean Chinese adults with a normal WC.
People without diabetes mellitus or significant alcohol consumption who underwent routine health examinations were included. Their fatty liver index (FLI), abdominal ultrasonography results, and controlled attenuation parameter were all assessed. Genotyping for single-nucleotide polymorphisms associated with NAFLD was performed in another small group consisting of biopsy-proven NAFLD subjects and healthy controls.
A total of 2715 subjects who underwent routine health examinations were included in the study. Among 810 lean participants with a normal WC, 142 (17.5%) fulfilled the diagnostic criteria for NAFLD. Waist-height ratio, hemoglobin, platelets, and triglycerides were significant factors associated with the presence of NAFLD in these participants. The appropriate cut-off value of the FLI score in screening for NAFLD in the lean subjects with a normal WC was 25.15, which had a 77.8% sensitivity and 75.9% specificity. There was no significant difference in the single-nucleotide polymorphisms in the SIRT1, APOC3, PNPLA3, AGTR1, and PPARGC1A genes between lean subjects with and without NAFLD (P < 0.05).
NAFLD is not uncommon in lean Chinese adults even with a normal WC. Metabolic factors, rather than genetic factors, may play important roles in the development of NAFLD in this population. A lower cut-off value of the FLI score in screening for NAFLD should be used for lean Chinese adults with a normal WC.
Core tip: The precise characteristics of nonalcoholic fatty liver disease (NAFLD) in lean Chinese adults with a normal waist circumference (WC) remain unclear. Therefore, we aimed to investigate the prevalence, clinical characteristics, risk factors, and possible indicators for NAFLD in this population. Among 810 lean participants with a normal WC, 142 (17.5%) fulfilled the diagnostic criteria for NAFLD. NAFLD is not uncommon in Chinese lean adults even with a normal WC. Metabolic factors, rather than genetic factors, may play important roles in the development of NAFLD in this population. A lower cut-off value of the fatty liver index score in screening for NAFLD should be used for lean Chinese adults with a normal WC.
- Citation: Zeng J, Yang RX, Sun C, Pan Q, Zhang RN, Chen GY, Hu Y, Fan JG. Prevalence, clinical characteristics, risk factors, and indicators for lean Chinese adults with nonalcoholic fatty liver disease. World J Gastroenterol 2020; 26(15): 1792-1804
- URL: https://www.wjgnet.com/1007-9327/full/v26/i15/1792.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i15.1792
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic diseases in the world[1,2]. It has been a serious public health problem worldwide, and its prevalence is rapidly increasing in Chinese individuals[3]. NAFLD encompasses a wide spectrum of liver damage, ranging from simple steatosis to steatohepatitis and fibrosis, and to cirrhosis and hepatocellular carcinoma[4]. Nowadays, NAFLD is considered the hepatic component of metabolic syndrome (MetS), which includes several metabolic alternations. Thus, metabolic profile and high energy intake may be both involved in the occurrence and progression of NAFLD[5]. Moreover, the presence of NAFLD significantly increases the risk of cardiovascular disease[6], type 2 diabetes mellitus[6,7], chronic kidney disease[6,8], and cancer[7]. Therefore, early detection of NAFLD and effective treatment are very important.
NAFLD is strongly associated with obesity. Considerable attention has been paid to be the prevalence of and risk factors for NAFLD in obese subjects[9]. However, not all subjects with NAFLD are obese. This condition is often referred to as lean or non-obese NAFLD[3,10]. The prevalence of non-obese NAFLD ranges from 12% to 16% among Korean adults[11]. An international study reported that non-obese NAFLD patients might have more severe histological necroinflammation and higher mortality than obese patients[12]. As non-obese individuals, especially the lean subgroup, may not visit clinics for NAFLD diagnosis or ignore the diagnosis of NAFLD, NAFLD in these populations might lead to more problems than obese individuals. Hence, it is critically important to understand its prevalence, characteristics, and risk factors to screen for the presence of NAFLD in lean subjects. At the same time, it is generally believed that genetic factors affect the phenotypes and progression of NAFLD. It has also been estimated that genetic factors influence the development of NAFLD in 26%-35% of cases, although whether genetic factors are involved in the development of lean NAFLD remains unclear.
Therefore, we aimed to investigate the prevalence and clinical and genetic characteristics of NAFLD in lean Chinese adults, and to identify the risk factors and possible indicators for lean NAFLD.
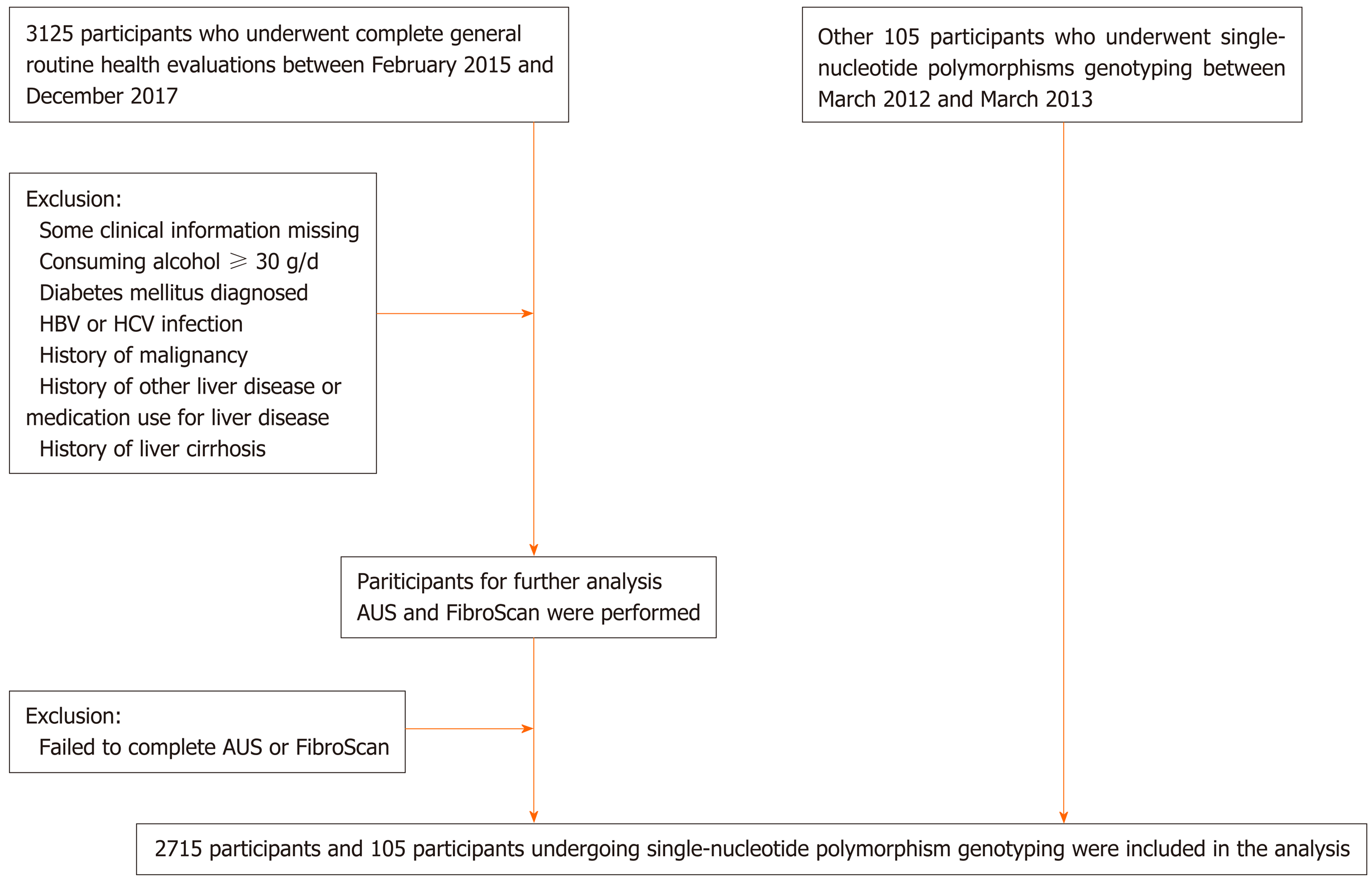
The individuals included in the study were consecutive Chinese Han adults who underwent routine health evaluations at Xinhua Hospital, Shanghai Jiao Tong University School of Medicine between February 2015 and December 2017. Another group of unrelated adult subjects (n = 105) were recruited between March 2012 and March 2013. The patients with NAFLD who underwent ultrasonically guided liver biopsy were also enrolled at Xinhua Hospital, Shanghai, China (n = 19); Tianjin Hospital of Infectious Diseases, Tianjin, China (n = 14); and Zhengxing Hospital, Zhangzhou, China (n = 26). The matched healthy controls (n = 46) were all recruited at Xinhua Hospital, Shanghai, China.
The exclusion criteria for the study population were as follows: (1) Excessive alcohol consumption of 20 g/d in men or 10 g/d in women; (2) Positivity for hepatitis B surface antigen or anti-hepatitis C virus antibody with detectable hepatitis C virus RNA, or the diagnosis of other types of liver diseases except NAFLD or any end-stage liver disease, including viral hepatitis, drug-induced liver injury, autoimmune liver disease, Wilson disease, primary biliary cholangitis, or any other chronic liver disease that could coexist with NAFLD; (3) Treatment with drugs known to cause hepatic steatosis or any hepatotoxic drugs (e.g., tamoxifen, amiodarone, sodium valproate, methotrexate, and glucocorticoid); and (4) Presence of malignancies, diabetes mellitus, or pregnancy. Ethical approval was obtained from the Ethics Committees of the hospitals involved. Informed consent was obtained from the individuals who participated in the study.
All participants underwent medical examinations at the Health Examination Centre of Xinhua Hospital. Participant characteristics and anthropometric indices, including age, sex, body weight, height, waist circumference (WC), hip circumference, body mass index (BMI), systolic blood pressure, and diastolic blood pressure (DBP), were obtained. BMI was defined as weight in kilograms divided by the square of height in metres (kg/m2). Waist-height ratio (WHtR) and waist-hip ratio (WHR) were calculated by WC (cm)/height (cm) and WC (cm)/hip circumference (cm), re-spectively.
Peripheral blood samples were collected from the participants in the morning after fasting for at least 12 h and were measured with a Hitachi 7600 series automatic analyser (Hitachi, Tokyo, Japan). The variables analyzed included hemoglobin (Hb), platelets (PLT), alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase (GGT), albumin, lactate dehydrogenase, glucose, total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), blood urea nitrogen (BUN), serum creatinine, and urea acid.
MetS was defined according to the ethnic specific criteria by the International Diabetes Federation, which were modified from the National Cholesterol Education Program, Adult Treatment Panel III Guidelines[13].
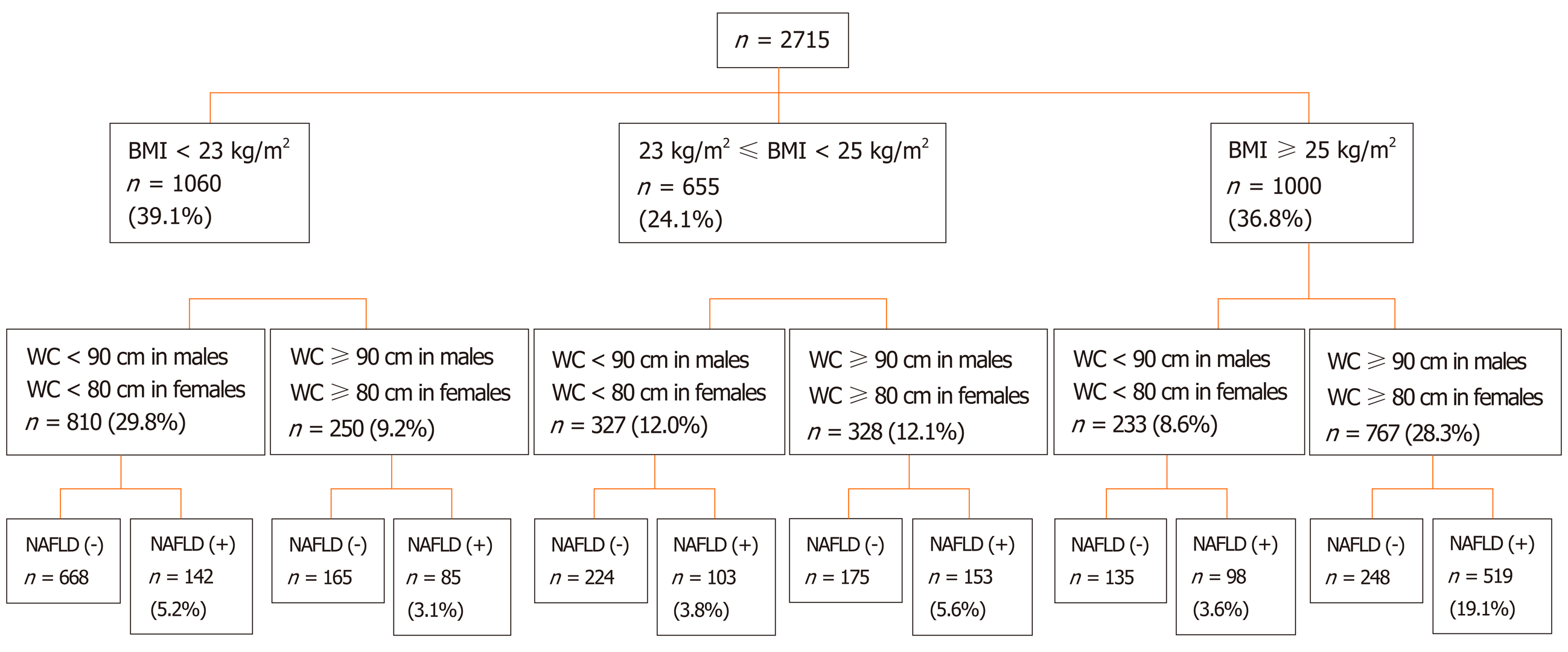
A BMI greater than 25 kg/m2 was used to define the obese population, and a BMI less than 25 kg/m2 was used to define the non-obese population. The non-obese population was further divided into lean (< 23 kg/m2) and overweight (23-25 kg/m2). A BMI less than 23 kg/m2 and a WC less than 80 cm in females or 90 cm in males were used to define the lean population with a normal WC.
The three non-invasive methods used to assess hepatic steatosis were abdominal ultrasound (AUS), controlled attenuation parameter (CAP), and the fatty liver index (FLI). AUS and CAP measurements were both performed by experienced operators who were blinded to the characteristics of the participants and the study. A diagnosis of NAFLD was made based on characteristic ultrasonographic features[14] and the exclusion of other causes of steatosis. The quantification of hepatic steatosis was performed based on CAP values measured using FibroScan-502 with an M probe (3.5 MHz) (Echosens, Paris, France) according to the previously described CAP measurement principle. The values of CAP ranged from 100-400 dB/m, and the representative values were the median values of all valid measurements. The degree of fatty liver was further classified as follows: S0 (no fatty liver), CAP < 238 dB/m; S1 (mild fatty liver), 238-258 dB/m; S2 (moderate fatty liver), 259-292 dB/m; and S3 (severe fatty liver), CAP > 292 dB/m.
Using clinical and biochemical data, the FLI was calculated according to the following formula: FLI = [e0.953 × ln(TG) + 0.139 × BMI + 0.718 × ln(γ-GT) + 0.053 × WC − 15.745]/[1 + e0.953 × ln(TG) + 0.139 × BMI + 0.718 × ln(γ-GT) + 0.053 × WC − 15.745] × 100. The FLI ranges from 0 to 100 and reflects the percentage of fatty liver. As previously mentioned, this score has been validated by a qualitative diagnosis of fatty liver by ultrasound[15].
The diagnosis of NAFLD in those participants who underwent routine health evaluations and the matched healthy controls was based on AUS. In the subjects who underwent liver biopsy, NAFLD and nonalcoholic steatohepatitis (NASH) were diagnosed and staged based on the liver biopsy specimen according to the current guidelines[16].
Genomic DNA was prepared from each blood sample using the QiAamp DNA Mini Kit (Qiagen, Hilden, Germany). Then, the single-nucleotide polymorphism (SNP) probes were customized, and real-time polymerase chain reaction was used to detect SNP in the SIRT1, APOC3, PNPLA3, AGTR1, and PPARGC1A genes. The emulation polymerase chain reaction of the template was performed using the Ion OneTouch 2 System (Life Technologies, MA, United States) according to the manufacturer’s instructions. The variants were genotyped by DNA sequencing using the Ion 318 Chip (Life Technologies, MA, United States) following the Ion PGM 200 Sequencing kit’s protocol. Negative controls were introduced for each run to ensure genotyping quality. Samples giving discordant results were reanalyzed.
Continuous variables are expressed as the mean ± SD for those with a normal distribution and median ± IQR for those with a skewed distribution. The t-test and χ2 test were used to test differences in continuous and categorical variables between groups with different BMIs. Based on the results of univariate analysis, a logistic regression model was used to identify the independent risk factors for incident NAFLD, with odds ratios (ORs) and 95% confidence intervals (CIs) calculated. The cutoff value for the possible predictor was determined using the receiver operating characteristic (ROC) curve. The Hardy-Weinberg equilibrium was tested using the De Finetti program. All SNPs were in the Hardy-Weinberg equilibrium. All analyses were performed under an additive mode of inheritance. Linear regression was used to quantify the effect of an SNP on the severity of histologically determined liver damage. The significance level was set at P < 0.05.
From February 2015 to December 2017, 3125 participants agreed to be included in the study. After excluding patients with missing height, weight, or WC data and those with diabetes mellitus or significant alcohol consumption, 2715 participants were included in the analysis (Figure 1). The characteristics of the participants are shown in Supplementary Table 1.
| Characteristic | LNN (n = 668) | LN (n = 142) | ONN (n = 135) | ON (n = 519) | P value | ||
| LN-LNN | LN-ONN | LN-ON | |||||
| Age (yr) | 55.81 ± 7.92 | 54.33 ± 9.24 | 58.83 ± 6.96 | 56.44 ± 7.26 | 0.258 | 0.000 | 0.049 |
| Male sex, n (%) | 310 (46.4%) | 60 (42.3%) | 54 (40.0%) | 202 (38.9%) | 0.283 | 0.307 | 0.199 |
| BMI (kg/m2) | 21.23 ± 1.33 | 21.74 ± 1.01 | 26.69 ± 2.27 | 27.98 ± 2.84 | 0.000 | 0.000 | 0.000 |
| Waist circumference (cm) | 73.49 ± 5.66 | 76.27 ± 6.03 | 88.22 ± 5.53 | 90.8 ± 6.53 | 0.000 | 0.915 | 0.000 |
| Waist-to-hip ratio | 0.83 ± 0.06 | 0.84 ± 0.05 | 0.85 ± 0.06 | 0.92 ± 0.1 | 0.115 | 0.839 | 0.000 |
| Waist-to-height ratio | 0.46 ± 0.03 | 0.48 ± 0.03 | 0.51 ± 0.03 | 0.58 ± 0.04 | 0.002 | 0.000 | 0.000 |
| Systolic blood pressure (mmHg) | 130.75 ± 17.05 | 137.81 ± 7.14 | 137.52 ± 17.43 | 142.85 ± 18.44 | 0.984 | 0.639 | 0.099 |
| Diastolic blood pressure (mmHg) | 80.14 ± 9.63 | 85.22 ± 9.93 | 84.24 ± 9.62 | 87.09 ± 10.31 | 0.035 | 0.045 | 0.344 |
| Hypertension, n (%) | 136 (20.4%) | 52 (36.6%) | 41 (30.4%) | 270 (52.0%) | 0.000 | 0.037 | 0.750 |
| MetS, n (%) | 85 (12.7%) | 45 (31.7%) | 42 (31.1%) | 387 (74.6%) | 0.026 | 0.987 | 0.000 |
| Hb (g/L) | 127.12 ± 14.81 | 129.98 ± 16.18 | 125.28 ± 13.17 | 130.01 ± 16.71 | 0.046 | 0.023 | 0.991 |
| PLT (109/L) | 171.54 ± 46.53 | 186.61 ± 50.94 | 172.26 ± 52.14 | 189.18 ± 57.18 | 0.018 | 0.045 | 0.742 |
| ALT (U/L) | 19.71 ± 3.69 | 28.21 ± 14.69 | 23.09 ± 11.38 | 31.57 ± 19.01 | 0.016 | 0.583 | 0.288 |
| AST (U/L) | 23.44 ± 11.88 | 24.95 ± 7.58 | 23.38 ± 6.1 | 26.15 ± 9.34 | 0.323 | 0.099 | 0.342 |
| AKP (U/L) | 65.24 ± 19.05 | 72.14 ± 15.99 | 62.26 ± 19.65 | 76.35 ± 22.97 | 0.124 | 0.074 | 0.368 |
| GGT (U/L) | 19.51 ± 3.89 | 24.31 ± 14.28 | 39.33 ± 43.73 | 46.87 ± 31.4 | 0.128 | 0.141 | 0.002 |
| LDH (U/L) | 162.23 ± 25.23 | 175.86 ± 27.05 | 172.09 ± 34.15 | 173.05 ± 26.8 | 0.019 | 0.689 | 0.684 |
| ALB (g/L) | 45.74 ± 2.67 | 46.43 ± 2.67 | 45.89 ± 6.09 | 46.76 ± 3.89 | 0.390 | 0.707 | 0.727 |
| GLU (mmol/L) | 5.56 ± 0.89 | 5.84 ± 0.92 | 5.67 ± 1.06 | 6.02 ± 1.37 | 0.841 | 0.517 | 0.209 |
| TC (mmol/L) | 4.69 ± 0.79 | 4.88 ± 1.01 | 4.72 ± 0.92 | 4.93 ± 0.9 | 0.086 | 0.578 | 0.711 |
| TG (mmol/L) | 1.19 ± 0.68 | 1.56 ± 1.28 | 1.37 ± 0.91 | 1.93 ± 1.24 | 0.026 | 0.046 | 0.325 |
| LDL-c (mmol/L) | 2.72 ± 0.66 | 2.89 ± 0.86 | 2.83 ± 0.72 | 2.94 ± 0.75 | 0.021 | 0.322 | 0.992 |
| HDL-c (mmol/L) | 1.54 ± 0.3 | 1.29 ± 0.3 | 1.46 ± 0.41 | 1.34 ± 0.26 | 0.012 | 0.037 | 0.483 |
| BUN (mmol/L) | 5.19 ± 1.22 | 5.00± 1.06 | 5.41 ± 1.34 | 5.28 ± 1.22 | 0.055 | 0.015 | 0.072 |
| SCr (μmol/L) | 62.84 ± 13.06 | 62.56 ± 13.18 | 61.13 ± 12.48 | 62.8 ± 15.2 | 0.438 | 0.442 | 0.908 |
| UA (mmol/L) | 271.83 ± 64.94 | 305.66 ± 72.2 | 284.49 ± 66.4 | 311.17 ± 76.53 | 0.217 | 0.934 | 0.659 |
| CAP (dB/m) | 197.06 ± 31.91 | 269.53 ± 2.91 | 207.21 ± 41.08 | 292.4 ± 34.4 | 0.000 | 0.000 | 0.077 |
| E (kPa) | 5.47 ± 1.98 | 4.75 ± 5.27 | 5.86 ± 3.43 | 6.33 ± 2.06 | 0.513 | 0.793 | 0.038 |
| FLI score | 11.38 ± 9.56 | 26.58 ± 13.11 | 37.75 ± 21.40 | 60.88 ± 23.35 | 0.000 | 0.036 | 0.017 |
Among those participants, 966 (35.6%) were male. The mean age of all subjects was 56.73 ± 7.52 years. A total of 1000 (36.8%) participants were classified as obese (BMI ≥ 25 kg/m2), 655 (24.1%) as overweight (23 ≤ BMI < 25 kg/m2), 1060 (39.1%) as lean (BMI < 23 kg/m2), and 810 (29.8%) as lean with a normal WC. A total of 1100 (40.5%) participants fulfilled the diagnostic criteria for NAFLD. The prevalence of NAFLD was 61.7% in the obese group, 39.1% in the overweight group, 21.4% in the lean group, and 17.5% in the lean group with a normal WC. The prevalence of lean NAFLD subjects with a normal WC was 5.2% in those 2715 participants and 12.9% in the 1100 diagnosed NAFLD subjects (Figure 2).
WC is one of the most important risk factors for the development of NAFLD. We selected the lean participants with a normal WC and the obese participants with a large or normal WC for analysis. The clinical characteristics of those participants are shown in Table 1.
Among the lean participants with a normal WC, the prevalence of NAFLD was 17.5%. The percentages of both the individual components of MetS and WHtR in those with and without NAFLD are shown in Figure 3. Compared to the non-NAFLD group, the NAFLD group had significantly higher BMI, WC, WHtR, DBP, and hypertension and MetS proportion; Hb, PLT, alanine aminotransferase, lactate dehydrogenase, TG, LDL-c, and CAP values; and FLI score. Furthermore, the level of HDL-c was significantly lower in the NAFLD group (Table 1).
Compared to the obese NAFLD group with a large WC, there were no significant differences in the level of Hb, PLT, TG, LDL-c, or HDL-c in the lean NAFLD group with a normal WC. Compared to the obese non-NAFLD group with a normal WC, subjects in the lean NAFLD group with a normal WC were younger, and had significantly lower BMI, HDL-c, and FLI score but higher WHtR, DBP, Hb, PLT, TG, blood urea nitrogen, and CAP values (Table 1).
In univariate analysis, WHtR (OR: 4.275; 95%CI: 2.242-5.167; P = 0.003), DBP (OR: 1.097; 95%CI: 1.036-1.163; P = 0.005), Hb (OR: 1.051; 95%CI: 1.019-1.084; P = 0.000), PLT (OR: 1.019; 95%CI: 1.009-1.029; P = 0.000), TG (OR: 2.243; 95%CI: 1.579-3.204; P = 0.000), HDL-c (OR: 0.013; 95%CI: 0.001-0.128; P = 0.000), and LDL-c (OR: 1.512; 95%CI: 1.060-2.156; P = 0.022) were significantly associated with NAFLD in lean participants with a normal WC. In multivariate analysis, WHtR (OR: 3.934; 95%CI: 2.543-5.854; P = 0.004), Hb (OR: 1.042; 95%CI: 1.003-1.082; P = 0.033), PLT (OR: 1.020; 95%CI: 1.006-1.033; P = 0.004), and TG (OR: 1.946; 95%CI: 1.227-3.085; P = 0.005) were significant risk factors associated with the presence of NAFLD in these participants (Table 2).
| Univariate analysis | Multivariate analysis1 | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| WHtR | 4.275 (2.242-5.167) | 0.003 | 3.934 (2.543-5.854) | 0.004 |
| Diastolic blood pressure (mmHg) | 1.097 (1.036-1.163) | 0.005 | - | - |
| Hb (g/L) | 1.051 (1.019-1.084) | 0.000 | 1.042 (1.003-1.082) | 0.033 |
| PLT (109/L) | 1.019 (1.009-1.029) | 0.000 | 1.020 (1.006-1.033) | 0.004 |
| TG (mmol/L) | 2.243 (1.579-3.204) | 0.000 | 1.946 (1.227-3.085) | 0.005 |
| HDL-c (mmol/L) | 0.013 (0.001, 0.128) | 0.000 | - | - |
| LDL-c (mmol/L) | 1.512 (1.060, 2.156) | 0.022 | - | - |
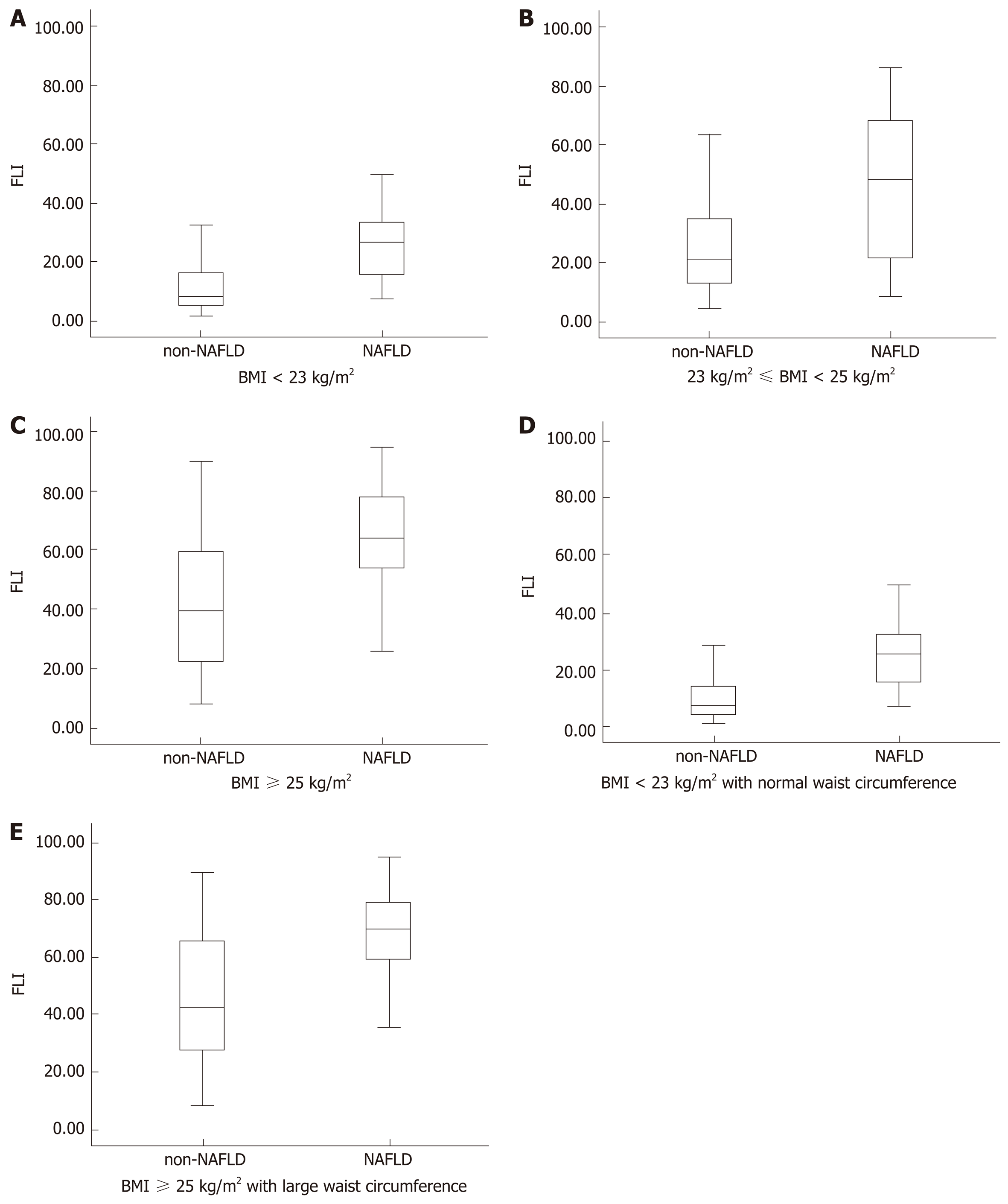
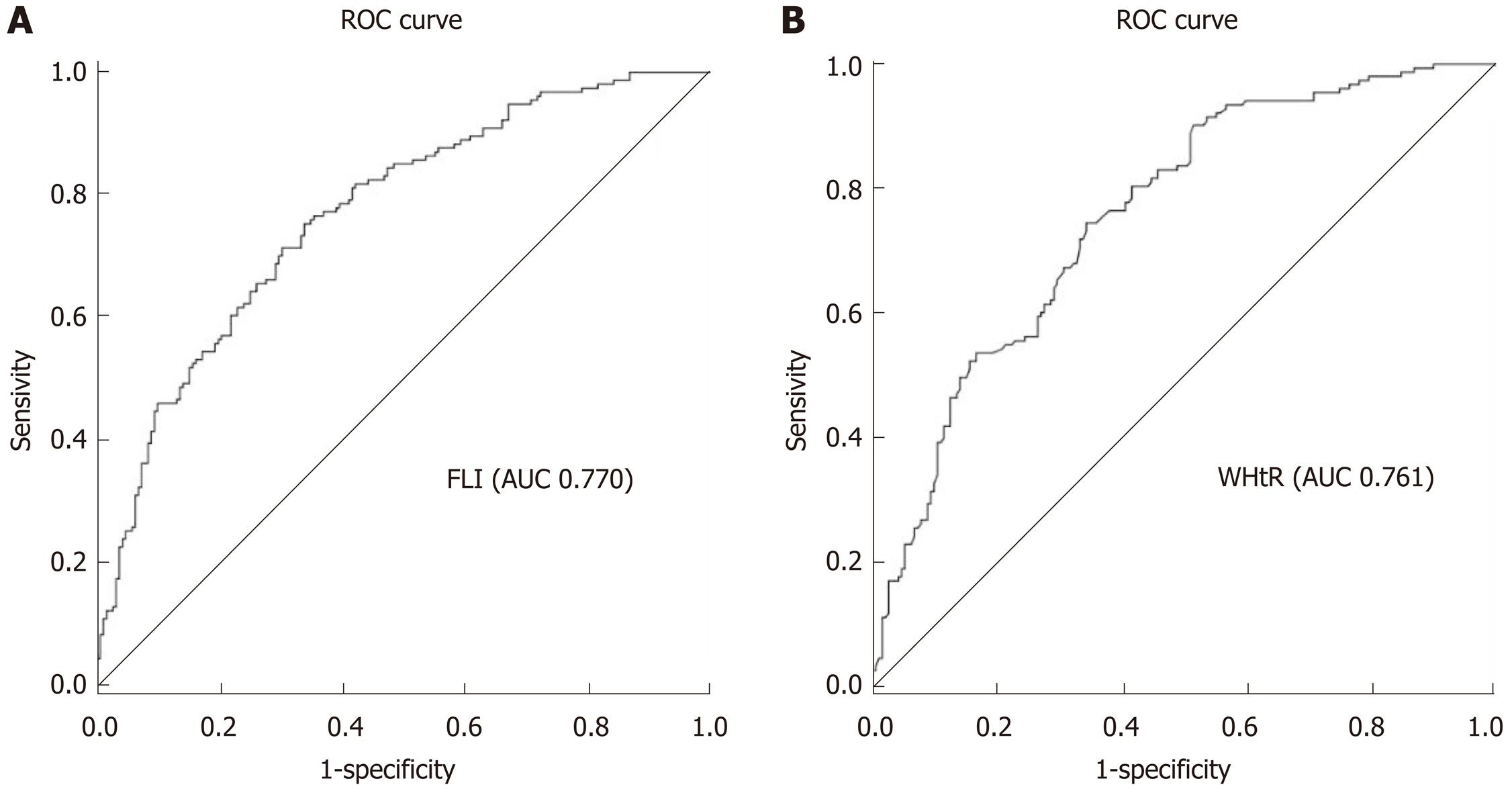
Figure 3 shows the FLI in different groups by various parameters, including BMI, WC, and the diagnosis of NAFLD. Figure 4 depicts the ROC curves of WHtR and FLI score in the lean participants with a normal WC, which were used to identify lean subjects with NAFLD. The area under the ROC curve (AUC) value was 0.761 for WHtR and 0.770 for FLI score. The FLI score had a larger AUC. The optimal cutoff points, sensitivity, and specificity of the two indices (WHtR and FLI score) in detecting these individuals with NAFLD are listed in Table 3. The appropriate cutoff value of FLI score in screening for NAFLD was 25.15, which had a 77.8% sensitivity and 75.9% specificity, and that of WHtR was 0.474, which had an 88.9% sensitivity and 62.5% specificity.
| AUROC (95%CI) | Cutoff value | Sensitivity | Specificity | Youden’s index | |
| WHtR | 0.761 (0.712-0.811) | 0.474 | 0.889 | 0.625 | 0.514 |
| FLI score | 0.770 (0.721-0.819) | 25.15 | 0.778 | 0.759 | 0.537 |
From March 2012 to March 2013, another group of unrelated adult subjects (n = 105) were recruited in the study, including NAFLD diagnosed by liver biopsy (n = 59) and healthy controls (n = 46). The characteristics of these subjects are not shown in the article.
Among those subjects, 53 (50.5%) were classified as obese (BMI ≥ 25 kg/m2), 52 (49.5%) as non-obese (BMI < 25 kg/m2), and 27 (25.7%) as lean (BMI < 23 kg/m2). A total of 59 (56.2%) fulfilled the diagnostic criteria for NAFLD. The prevalence of NAFLD was 83.0% in the obese group, 28.8% in the non-obese group, and 22.2% in the lean group. The prevalence of lean NAFLD was 5.7% in those 105 participants and 10.2% in the 59 patients with NAFLD (Table 4).
| Genotype | LNN (n = 21) | LN (n = 6) | NNN (n = 37) | NN (n = 15) | ONN (n = 9) | ON (n = 44) | P value | ||
| LN-LNN | NN-NNN | ON-ONN | |||||||
| SIRT1 rs2273773 TT/TC.CC | 10/11 | 1/5 | 22/15 | 6/9 | 2/7 | 29/15 | 0.233 | 0.363 | 0.022 |
| APOC3 rs2070666 TT/TA.AA | 8/13 | 3/3 | 16/21 | 6/9 | 7/2 | 17/27 | 1.000 | 0.374 | 0.030 |
| PNPLA3 rs738409 CC/CG.GG | 8/13 | 0/6 | 17/20 | 1/14 | 3/6 | 12/32 | 0.370 | 0.014 | 0.237 |
| PNPLA3 rs738408 CC/CT.TT | 8/13 | 0/6 | 17/20 | 1/14 | 3/6 | 12/32 | 0.370 | 0.014 | 0.237 |
| PNPLA3 rs4823173 GG/GA.AA | 8/13 | 0/6 | 16/21 | 1/14 | 3/6 | 12/32 | 0.277 | 0.010 | 0.237 |
| PNPLA3 rs2072906 AA/AG.GG | 7/14 | 0/6 | 15/22 | 1/14 | 3/6 | 12/32 | 0.332 | 0.014 | 0.208 |
| AGTR1 rs5186 AA/AC.CC | 17/4 | 5/1 | 33/4 | 13/2 | 9/0 | 39/5 | 0.900 | 0.801 | 0.024 |
| AGTR1 rs440881 CC/CA.AA | 0/21 | 0/6 | 0/37 | 0/15 | 0/9 | 0/44 | 0.639 | 0.319 | 0.054 |
The details of the significant genetic variation are presented in Table 4. Obese subjects with NAFLD had a higher incidence of genotype TT of the SIRT1 rs2273773 locus, TA/AA of the APOC3 rs2070666 locus, and genotype AA of the AGTR1 rs5186 locus than obese subjects without NAFLD. Non-obese subjects with NAFLD had a higher incidence of genotype CG/GG of the PNPLA3 rs738409 locus, genotype CT/TT of the PNPLA3 rs738408 locus, genotype GA/AA of the PNPLA3 rs4823173 locus, and genotype AG/GG of the PNPLA3 rs2072906 locus than non-obese subjects without NAFLD. There was no significant difference in the genotypes of SNPs between lean subjects with and without NAFLD (P < 0.05).
We conducted this study to characterize the prevalence, clinical and genetic features, and risk factors for NAFLD and to assess possible indicators for the prediction of NAFLD in the lean Chinese population. Our results revealed that the prevalence of NAFLD was 17.5% in the lean population with a normal WC. In the subjects with a normal WC, metabolic disorders were more common in lean participants with NAFLD than in obese controls. Hb, PLT count, and TG levels were independently associated with the prevalence of lean NAFLD with a normal WC. WHtR and FLI score were possible ideal indicators for the prediction of those subjects, although the cut-off values need to be optimized. Our study also found that there was no significant difference in the SNPs between lean subjects with and without NAFLD.
As the body weight of Asians is lower than that of other races, the prevalence of lean NAFLD has been mainly described in Asia and varies from 15%-21%[17,18]. Xu et al[19] revealed that the prevalence of NAFLD in non-obese (BMI < 25 kg/m2) subjects was 7.27%, and the incidence of NAFLD during the 5-year follow-up was 8.88%. Kim et al[20] reported that the prevalence of NAFLD in non-obese (BMI < 25 kg/m2) subjects was 22.4% at baseline and 30.3% at follow-up assessment. Thus, NAFLD has an important role even in lean individuals. In our study, the percentage of lean (BMI < 23 kg/m2) subjects with a normal WC was only 29.8% in all of these subjects. The large proportion of overweight, obesity, or abdominal obesity currently is potentially dangerous. The prevalence of NAFLD was 61.7% in the obese group, 39.1% in the overweight group, 21.4% in the lean group, and 17.5% in the lean group with a normal WC. Hence, a smaller, but significant, proportion of populations develop NAFLD despite having a relatively low BMI and even normal WC.
Dyslipidemia is a common metabolic feature in NAFLD, which is characterized by increased TG, decreased HDL-c, and increased LDL-c[21]. Dyslipidaemia, mainly hypertriglyceridemia, has been proven to be an important contributing factor for both the development and regression of NAFLD, especially in non-obese subjects. Kim et al[20] demonstrated the superiority of baseline serum TG levels in predicting the course of NAFLD compared with hyperglycaemia and hypertension in the non-obese subjects. Meanwhile, Hb and the PLT count have been suggested as simple and reliable biomarkers for NAFLD[19,22-24]. Xu et al[19] found that subjects with higher Hb levels are more likely to have NAFLD, and higher baseline Hb levels independently predicted the development of NAFLD. In the present study, among the participants with a normal WC, we found that the lean NAFLD subjects had significantly higher levels of Hb, PLT count, TG, and LDL-c and lower levels of HDL-c compared with either lean or obese participants without NAFLD. In the multivariate analysis, Hb, PLT count, and TG were all independently associated with the prevalence of NAFLD in the lean participants with a normal WC. These observations suggest that some metabolic profiles of lean NAFLD were more common than those of obese controls regardless of the WC. Therefore, clinicians should also pay more attention to the diagnosis of NAFLD by AUS in lean subjects, rather than focusing only on those with obesity. Hb, PLT count, and hypertriglyceridemia may be associated with the development of NAFLD in lean subjects with a normal WC.
BMI, WC, WHR, and WHtR are well-known and frequently used anthropometric measures in the preventative screening of central adiposity and NAFLD. The FLI is also a convenient tool for diagnosing NAFLD based on BMI, WC, TG, and GGT levels[15]. The FLI ranges from 0 to 100 and reflects the percentage of hepatic fat content. FLI < 30 rules out and FLI ≥ 60 rules in fatty liver. Koehler et al[25] found that this index showed good predictive performance in the diagnosis of NAFLD, with an AUC of 0.813. In an Italian population cohort, after 15 years of follow-up, the FLI was independently associated with liver-related mortality[26]. In our study, the FLI decreased significantly as the BMI decreased. Statistical analysis showed that WHtR and FLI both had advantages in the prediction of NAFLD over single WC, WHR, and BMI. In the analysis of the presence of NAFLD with the ROC curve, the FLI had an AUC of 0.770. Although FLI ≥ 60 has been proposed as a cutoff value to indicate NAFLD in previous articles, we propose that the FLI cutoff value of 25.15 be used to indicate NAFLD in lean Chinese adults with a normal WC. Our result is perhaps because the FLI is composed of BMI, WC, TG, and GGT levels[15], so the cutoff value should be optimized, especially in subjects with a low BMI and WC. The appropriateness of this proposed cutoff point should be tested in future studies.
Genome-wide association studies of different ethnic populations have revealed that multiple SNPs are associated with NAFLD. Several SNPs associated with NAFLD are rs738409 in PNPLA3, rs58542926 in the TM6SF2 variant, which is associated with lower hepatic and plasma lipids, and rs641738 in MBOAT7[27-30]. Thus far, the correlation between SNPs and NAFLD has not yet been studied in lean Chinese adults. Our study showed that there was no significant difference in the common SNPs between the lean subjects with and without NAFLD. Based on the above findings, we believe that metabolic factors, rather than genetic factors, may play a vital role in the occurrence and progression of NAFLD in lean Chinese adults just as in subjects with obesity. However, as the SNPs we detected were limited, the role of genetic factors in NAFLD in lean Chinese adults should be further explored in the future.
There are some limitations in this study. First, most of the NAFLD statuses were only assessed by ultrasonography and CAP values, which cannot determine the severity of NAFLD, and not by liver biopsy. Nevertheless, ultrasonography is widely used for population-based studies, with a reasonable accuracy. Further studies providing data on hepatic histological outcomes are needed to confirm our observation. Second, our initial study design did not include the diet questionnaires and the examination of insulin levels and insulin resistance, although the diet and insulin resistance may be closely associated with NAFLD in lean subjects. Third, this was a cross sectional study and may lack evidence of the predictive value of the WHtR and FLI. However, this value is not diagnostic; it is a predictor for the screening of NAFLD among lean adults with normal WC. The appropriateness of these proposed cut-off points should be tested in future studies. Despite these limitations, our results provide important insights into the prevalence, characteristics, and risk factors for NAFLD in a lean Chinese population. This is the first study to give the cut-off value of the FLI to diagnose NAFLD in the Chinese lean population with a normal WC.
In conclusion, our results have showed that NAFLD is not uncommon in Chinese lean adults even with a normal WC. Clinicians should pay more attention to the diagnosis of NAFLD by ultrasonography in these subjects, rather than focusing only on those with obesity. A lean subject with high WHtR, Hb, or hypertriglyceridemia may be the best to be referred for an ultrasonography examination. A lower cutoff value of the FLI has been obtained for Chinese lean adults with a normal WC. Further larger-sample research is needed to confirm our observations and also focus on the relationships between other risk factors and the development and progression of NAFLD in this population.
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in the world. Considerable attention has been paid to be the prevalence of and risk factors for NAFLD in obese subjects. However, the percentage of non-obese or lean NAFLD patients are increasing and these patients may have a worse outcome compared to their obese counterpart.
As the non-obese or lean individuals may not visit clinics for NAFLD diagnosis or ignore the diagnosis of NAFLD, it might lead to more problems than obese individuals. So, it is critically important to find the precise characteristics of these populations, especially the lean subgroup, which can help clinicians provide appropriate advice and treatment to these populations.
In this study, we aimed to investigate the prevalence, clinical characteristics, and risk factors for NAFLD in lean individuals, and finally identify the possible indicators in screening for NAFLD in these populations.
The participant characteristics and anthropometric indices, including height, weight, and waist circumference (WC), were obtained. Their fatty liver index (FLI), abdominal ultrasonography results, and controlled attenuation parameter were all assessed. In another small group consisting of biopsy-proven NAFLD subjects and healthy controls, genotyping for single-nucleotide polymorphisms associated with NAFLD was performed.
Among lean participants with a normal WC, the percent of the people who fulfilled the diagnostic criteria for NAFLD was 17.5%. The significant associated factors with the presence of NAFLD in these participants included waist-height-ratio, hemoglobin, platelets, and triglycerides. The appropriate cut-off value of the FLI score in screening for NAFLD in the lean subjects with normal WC was lower than the value we often used.
The results from this study suggest that NAFLD is not uncommon in Chinese lean adults even with a normal WC. A lean subject with high waist-height ratio, hemoglobin, platelet count, or hypertriglyceridemia may be the best to be referred for an abdominal ultrasound examination. A lower cutoff value of the FLI has been obtained for lean Chinese with a normal WC.
This study clearly highlights the need for paying more attention to the diagnosis of NAFLD by abdominal ultrasound even in lean subjects. The cut-off values of non-invasive diagnostic methods for NAFLD may vary based on different populations. In future, application of precise anthropometric strategies and identification of the relationships between other risk factors and the development and progression of NAFLD in this population would be required.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abenavoli L S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 424] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 943] [Cited by in F6Publishing: 1128] [Article Influence: 225.6] [Reference Citation Analysis (0)] |
| 3. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 671] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 5. | Abenavoli L, Milic N, Di Renzo L, Preveden T, Medić-Stojanoska M, De Lorenzo A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:7006-7016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 114] [Cited by in F6Publishing: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 690] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 7. | Choi JH, Rhee EJ, Bae JC, Park SE, Park CY, Cho YK, Oh KW, Park SW, Lee WY. Increased risk of type 2 diabetes in subjects with both elevated liver enzymes and ultrasonographically diagnosed nonalcoholic fatty liver disease: a 4-year longitudinal study. Arch Med Res. 2013;44:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Zeng J, Sun C, Sun WL, Chen GY, Pan Q, Yan SY, Xu ZJ, Chen YW, Fan JG. Association between non-invasively diagnosed hepatic steatosis and chronic kidney disease in Chinese adults on their health check-up. J Dig Dis. 2017;18:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 596] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 10. | Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, Chan HL, Chim AM, Woo J, Chu WC, Wong VW. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306-14; quiz 1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 11. | Cho HC. Prevalence and Factors Associated with Nonalcoholic Fatty Liver Disease in a Nonobese Korean Population. Gut Liver. 2016;10:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol Commun. 2018;2:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 13. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8720] [Cited by in F6Publishing: 9585] [Article Influence: 639.0] [Reference Citation Analysis (0)] |
| 14. | Meziri M, Pereira WC, Abdelwahab A, Degott C, Laugier P. In vitro chronic hepatic disease characterization with a multiparametric ultrasonic approach. Ultrasonics. 2005;43:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1607] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 16. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3544] [Cited by in F6Publishing: 4068] [Article Influence: 678.0] [Reference Citation Analysis (7)] |
| 17. | Liu CJ. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol. 2012;27:1555-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Fukuda T, Hamaguchi M, Kojima T, Hashimoto Y, Ohbora A, Kato T, Nakamura N, Fukui M. The impact of non-alcoholic fatty liver disease on incident type 2 diabetes mellitus in non-overweight individuals. Liver Int. 2016;36:275-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 19. | Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. Am J Gastroenterol. 2013;108:1299-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Kim NH, Kim JH, Kim YJ, Yoo HJ, Kim HY, Seo JA, Kim NH, Choi KM, Baik SH, Choi DS, Kim SG. Clinical and metabolic factors associated with development and regression of nonalcoholic fatty liver disease in nonobese subjects. Liver Int. 2014;34:604-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Angelico F, Baratta F, Pastori D, Del Ben M. Statins and non-alcoholic fatty liver disease. Liver Int. 2019;39:1787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Yu C, Xu C, Xu L, Yu J, Miao M, Li Y. Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J Hepatol. 2012;56:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Imajo K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:1300-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Petta S, Sebastiani G, Bugianesi E, Viganò M, Wong VW, Berzigotti A, Fracanzani AL, Anstee QM, Marra F, Barbara M, Calvaruso V, Cammà C, Di Marco V, Craxì A, de Ledinghen V. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 25. | Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11:1201-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 26. | Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, Bosi E, Ruotolo G, Piemonti L, Perseghin G. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54:145-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, Kluwe J, Boettler T, Lammert F, Geier A; NAFLD Clinical Study Group. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Umano GR, Caprio S, Di Sessa A, Chalasani N, Dykas DJ, Pierpont B, Bale AE, Santoro N. The rs626283 Variant in the MBOAT7 Gene is Associated with Insulin Resistance and Fatty Liver in Caucasian Obese Youth. Am J Gastroenterol. 2018;113:376-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Kitamoto T, Kitamoto A, Ogawa Y, Honda Y, Imajo K, Saito S, Yoneda M, Nakamura T, Nakajima A, Hotta K. Targeted-bisulfite sequence analysis of the methylation of CpG islands in genes encoding PNPLA3, SAMM50, and PARVB of patients with non-alcoholic fatty liver disease. J Hepatol. 2015;63:494-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Abenavoli L, Pellicano R, Boccuto L. Role of genetics and metabolism in non-alcoholic fatty liver disease. Panminerva Med. 2018;60:41-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |