Published online Dec 28, 2019. doi: 10.3748/wjg.v25.i48.6890
Peer-review started: September 29, 2019
First decision: November 27, 2019
Revised: December 3, 2019
Accepted: December 14, 2019
Article in press: December 14, 2019
Published online: December 28, 2019
Esophageal cancer is one of the most poorly diagnosed and fatal cancers in the world. Although a series of studies on esophageal cancer have been reported, the molecular pathogenesis of the disease remains elusive.
To investigate comprehensively the molecular process of esophageal cancer.
Differential expression analysis was performed to identify differentially expressed genes (DEGs) in different stages of esophageal cancer from The Cancer Genome Atlas data. Exacting gene interaction modules were generated, and hub genes in the module interaction network were found. Further, through survival analysis, methylation analysis, pivot analysis, and enrichment analysis, some important molecules and related functions/pathways were identified to elucidate potential mechanisms in esophageal cancer.
A total of 7457 DEGs and 14 gene interaction modules were identified. These module genes were significantly involved in the positive regulation of protein transport, gastric acid secretion, insulin-like growth factor receptor binding, and other biological processes as well as p53 signaling pathway, epidermal growth factor signaling pathway, and epidermal growth factor receptor signaling pathway. Transcription factors (including hypoxia inducible factor 1A) and non-coding RNAs (including colorectal differentially expressed and hsa-miR-330-3p) that significantly regulate dysfunction modules were identified. Survival analysis showed that G protein subunit gamma transducin 2 (GNGT2) was closely related to survival of esophageal cancer. DEGs with strong methylation regulation ability were identified, including SST and SH3GL2. Furthermore, the expression of GNGT2 was evaluated by quantitative real time polymerase chain reaction, and the results showed that GNGT2 expression was significantly upregulated in esophageal cancer patient samples and cell lines. Moreover, cell counting kit-8 assay revealed that GNGT2 could promote the proliferation of esophageal cancer cell lines.
This study not only revealed the potential regulatory factors involved in the development of esophageal cancer but also deepens our understanding of its underlying mechanism.
Core tip: Based on the esophageal cancer-associated RNA-seq in The Cancer Genome Atlas, we studied differentially expressed genes of esophageal cancer at various stages, constructed a protein-protein interaction network, obtained 14 dysfunctional modules, and screened Hub genes. We performed enrichment analysis to predict non-coding RNA and transcription factors as well as methylation analysis of the genes in the module. A series of regulatory factors was predicted to regulate to a certain degree the potential dysfunction mechanism of esophageal cancer, which provides new insight for future studies of esophageal cancer.
- Citation: Liu GM, Ji X, Lu TC, Duan LW, Jia WY, Liu Y, Sun ML, Luo YG. Comprehensive multi-omics analysis identified core molecular processes in esophageal cancer and revealed GNGT2 as a potential prognostic marker. World J Gastroenterol 2019; 25(48): 6890-6901
- URL: https://www.wjgnet.com/1007-9327/full/v25/i48/6890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i48.6890
Esophageal cancer is one of the world's most common cancers with poor diagnosis and high mortality because of invasiveness and a fast growth rate[1]. From a therapeutic point of view, esophageal cancer can be divided into early esophageal cancer, locally advanced resectable esophageal cancer, locally advanced unresectable esophageal cancer, and metastatic esophageal cancer. Because of the anatomical features of esophageal cancer, esophageal cancer is usually detected in the late stage, which vitally affects the treatment options and prognosis of patients[2].
During the development of esophageal cancer, the rs11473 polymorphism of the miR-483-5p binding site plays a vital role in the 3'-untranslated region of the basigin gene[3]. Single nucleotide polymorphisms in telomerase reverse transcriptase may be associated with susceptibility to esophageal cancer and contribute to the development of esophageal cancer[4]. MiR-20b may play an essential role in the tumorigenesis of esophageal cancer by regulating phosphatase and tensin homolog expression, which may be a potential therapeutic target for the treatment of esophageal cancer[5]. Growing evidence has revealed molecular targets for diagnosis and prognosis using bioinformatic analysis in the field of oncology[6-16]. These findings have deepened our understanding of the pathogenesis of esophageal cancer and have guided the direction of our research. However, the molecular pathogenesis of the disease is still elusive.
To explore comprehensively the molecular processes and potential therapeutic targets of esophageal cancer progression, we conducted a systematic module analysis. Overall, our work details the role of multifactorial mediated dysfunction modules in the growth of esophageal cancer and identifies essential genes and related biological processes, finding potential molecular mechanisms and therapeutic targets [G protein subunit gamma transducin 2 (GNGT2)] for esophageal cancer.
All esophageal cancer analyses in this study involving human participants were in accordance with the ethical standards of the Second Hospital of Jilin University and with the Declaration of Helsinki. A total of 40 esophageal cancer patients and healthy control volunteers, who matched for age and sex, were involved in this study. Informed consent was obtained from all participants. The esophageal cancer cell lines EC109 and KYSE70 were kindly provided by Laboratory in The Second Hospital of Jilin University. The cells were maintained in RPMI-1640 medium containing 10% fetal bovine serum.
Total RNA was extracted from case/control group using TRIzol. The quantitative real time-polymerase chain reaction (PCR) experiment was conducted in a real-time PCR detection system using SYBR Green qPCR Master Mix. Primers were designed and synthesized by Novogene (Beijing, China). Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. Cell counting kit-8 assay was used to measure cell proliferation. Transfected cells were cultured for 0-96 h and incubated at 37 °C for 2 h. A spectrophotometer (450 nm) was used to quantitate samples.
The Cancer Genome Atlas (TCGA) is a joint project of the National Cancer Institute and the American Human Genome Research Institute. High-throughput genomic analysis technology is a useful tool for people to understand better cancer, and it improves their abilities to prevent, diagnose, and treat disease. We downloaded esophageal cancer RNA-Seq data from the TCGA database and screened non-coding RNA (ncRNA)-mRNA interaction pairs with a score ≥ 0.5 from RNA Associated Interaction Database v2.0[17], including 431937 interacting pairs involving 5431 ncRNAs. All human transcription factor target data were downloaded and used in the general database-Transcriptional Regulatory Relationships Unraveled by Sentence-based Text mining v2 database for transcriptional studies, including 2492 transcription factors and 9396 interaction pairs.
In order to explore the molecular process of esophageal cancer staging, we selected four stages of esophageal cancer and normal samples for differential expression analysis, including healthy tissue samples vs stage 1 disease samples, stage 1 disease samples vs stage 2 disease samples, stage 2 disease samples vs stage 3 disease samples, and stage 3 disease samples vs stage 4 disease samples. We used the limma package for analysis[18-20]. Using the Correct background function, we performed background correction and normalization on the data. The normalize Between Arrays function quantile normalization method can filter out the control probe and the low expression probe. The differentially expressed genes of the data set were identified based on the lmFit and eBayes functions (P < 0.01) using default parameters.
A protein-protein interaction system was constructed based on Search Tool for the Retrieval of Interacting Genes/Proteins database data (score > 500). The gene module of more than 30 nodes was screened throughout the network using the ClusterONE plug-in[18] of the Cytoscape software[19]. We use the Cytoscape plugin CytoHubba[20] to identify hub genes in the module subnet, while CytoHubba contains 12 methods for identifying hub genes. We obtained the top 10 genes and then screened the repeat genes in the 12 sets of genes for survival analysis.
The study of the functions and signal transduction pathways involved in genes contributes to our understanding of the molecular mechanisms of disease. Gene ontology function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed using the R language Cluster profiler package[21]. The Cluster profiler is a bioconductor software package that provides statistical analysis of functional clustering of gene sets.
The transcription and post-transcriptional regulation of genes are often dominated by the regulation of transcription factors (TF) and ncRNA. Therefore, we have scientifically predicted its role in the esophageal cancer dysfunction module. If the regulatory effect between the regulator and the module exceeds 2, and the number of organizational relationships between the regulator and the module is essential (hypergeometric test, P value < 0.01), it can be confirmed that it is a regulator of the critical regulatory module.
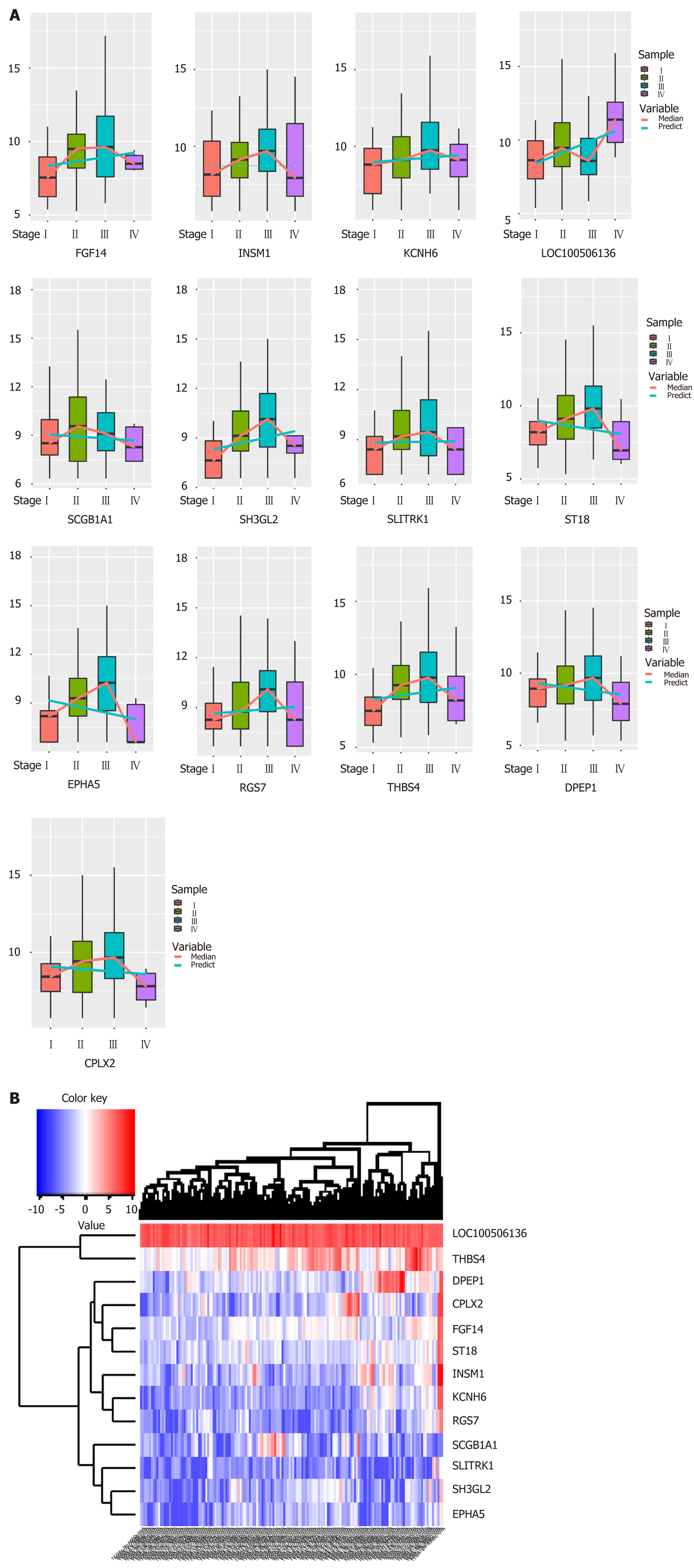
Biologists have conducted many experiments and studies on the pathogenesis of esophageal cancer and have thus identified potential pathogenic genes for the deterioration of esophageal cancer. To observe molecular changes in the progression of esophageal cancer, we performed differential expression analysis based on RNA-Seq data from four stages of esophageal cancer in the TCGA database. Based on analysis of phase 1 disease samples of normal tissue samples and esophageal cancer, analysis of phase 1 disease samples and stage 2 disease samples, analysis of stage 2 disease samples and phase 3 disease samples, and analysis of stage 3 disease samples and stage 4 disease samples, we obtained differential expression genes (DEGs) associated with each stage of esophageal cancer. A total of 7457 differentially expressed genes were received (Figure 1B). We believe that the presence of these differentially expressed genes is closely related to the development of various stages of esophageal cancer. Of the 7457 DEGs, there were 13 common genes (Table 1). The genes that were continuously down-regulated are CPLX2, DPEP1, EPHA5, SCGB1A1, ST18. The genes that were continuously up-regulated are FGF14, KCNH6, LOC100506136, RGS7, SH3GL2, THBS4 (Figure 1A).
| Common genes | DEG_S1 | DEG_S2 | DEG_S3 | DEG_S4 |
| logFC | logFC | logFC | logFC | |
| CPLX2 | -4.85047 | 2.288678 | 1.290368 | -4.17468 |
| DPEP1 | -1.33466 | 2.340936 | 1.604402 | -4.41133 |
| EPHA5 | -6.3307 | 1.829821 | 0.991225 | -3.72048 |
| FGF14 | -2.70845 | 1.254105 | 1.339303 | -2.01758 |
| INSM1 | -2.80099 | 1.759985 | 2.787167 | -3.27386 |
| KCNH6 | -3.87431 | 1.636848 | 1.717225 | -2.87864 |
| LOC100506136 | 0.757741 | 0.789295 | -0.58427 | 0.955725 |
| RGS7 | -3.27316 | 2.095696 | 1.45118 | -3.13742 |
| SCGB1A1 | -3.02471 | 3.325037 | -1.81283 | -2.46682 |
| SH3GL2 | -6.28822 | 2.761197 | 1.269058 | -2.63088 |
| SLITRK1 | -4.12073 | 2.309863 | 1.635813 | -3.57252 |
| ST18 | -2.02067 | 1.550856 | 1.289301 | -2.51987 |
| THBS4 | -2.15432 | 2.753388 | 1.06135 | -2.2027 |
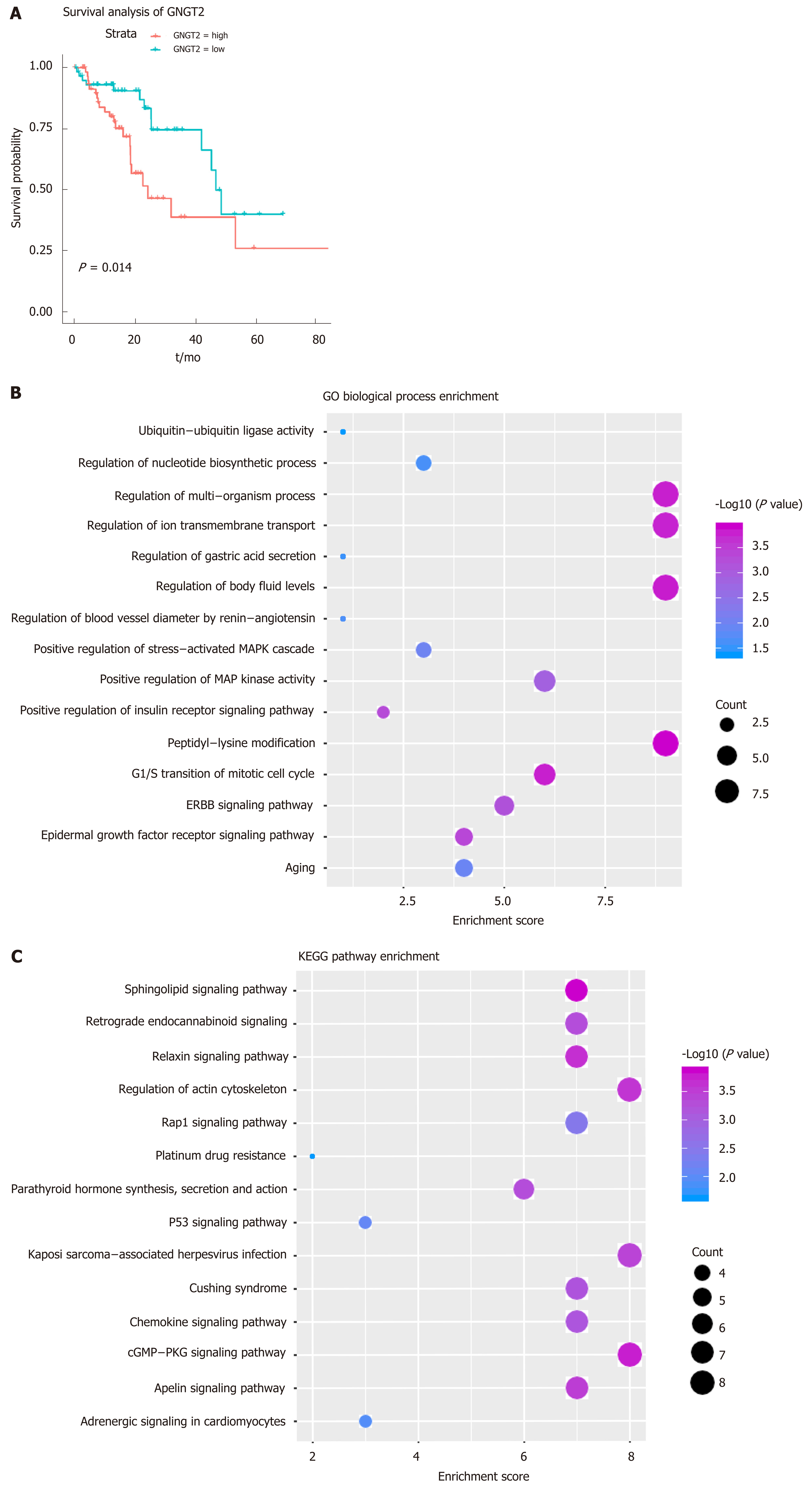
Gene module analysis helps us to study the complex collaborative relationships between multiple genes. Based on the protein interaction data of the STING database, the interaction network of differentially expressed genes was constructed, and 14 functional barrier modules were explored. Using the 12 methods in Cyto-hubba, a total of 758 hub genes were identified in the interaction sub-network of the module genes, including the gene SH3GL2, which is continuously up-regulated in Module 8. Further, 23 hub genes shared by the top10 gene set in 12 methods were screened for survival analysis. The results show that GNGT2 in module 6 is the related gene (P = 0.014) (Figure 2A). A decrease in survival rate accompanied the high expression of GNGT2 gene, and the expression level of GNGT2 gene was negatively correlated with survival rate. Function and pathway are essential mediators of the physiological response of the disease. We performed GC enrichment analysis on 14 module genes (Figure 2B) and KEGG (Figure 2C). The main biological processes include positive regulation of protein transport, gastric acid secretion, and insulin-like growth factor receptor binding. The main signal transduction pathways involved are the p53 signal transduction, the epidermal growth factor signal transduction, and the epidermal growth factor receptor signal transduction pathways. These pathways play crucial roles in the dysfunctional module for the functions and pathways involved in multiple genes.
From the perspective of systems biology and systems genetics, transcription and post-transcriptional regulation of genes have long been recognized as crucial regulators of disease development, while transcription factors and ncRNAs are universal regulators of expression and function. Although the role of TFs and ncRNA regulation of esophageal cancer progression has been evaluated by many biologists, few studies have focused on their overall global effect on dysfunctional mechanisms and the role they play in development. Therefore, in this study, based on the targeted regulation relationship between TF and ncRNA on the module gene, we performed a pivotal analysis of the conventional module to explore the crucial regulator that regulates the progression of esophageal cancer. The results showed that a total of 54 transcription factors involved 54 TF-module target pairs and 853 ncRNAs involved 944 ncRNA-module regulatory pairs. Statistical analysis revealed that TF HIF1A and ncRNA CRNDE regulate the most dysfunctional modules. These crucial transcription factors and ncRNAs may influence the development and progression of esophageal cancer by mediating dysfunctional modules. Thus, we identified these potential factors as regulators of dysfunction in esophageal cancer. Notably, hsa-miR-330-3p up-regulates the differentially expressed gene SH3GL2 throughout the esophageal cancer process, suggesting that hsa-miR-330-3p plays a crucial role in four stages of esophageal cancer.
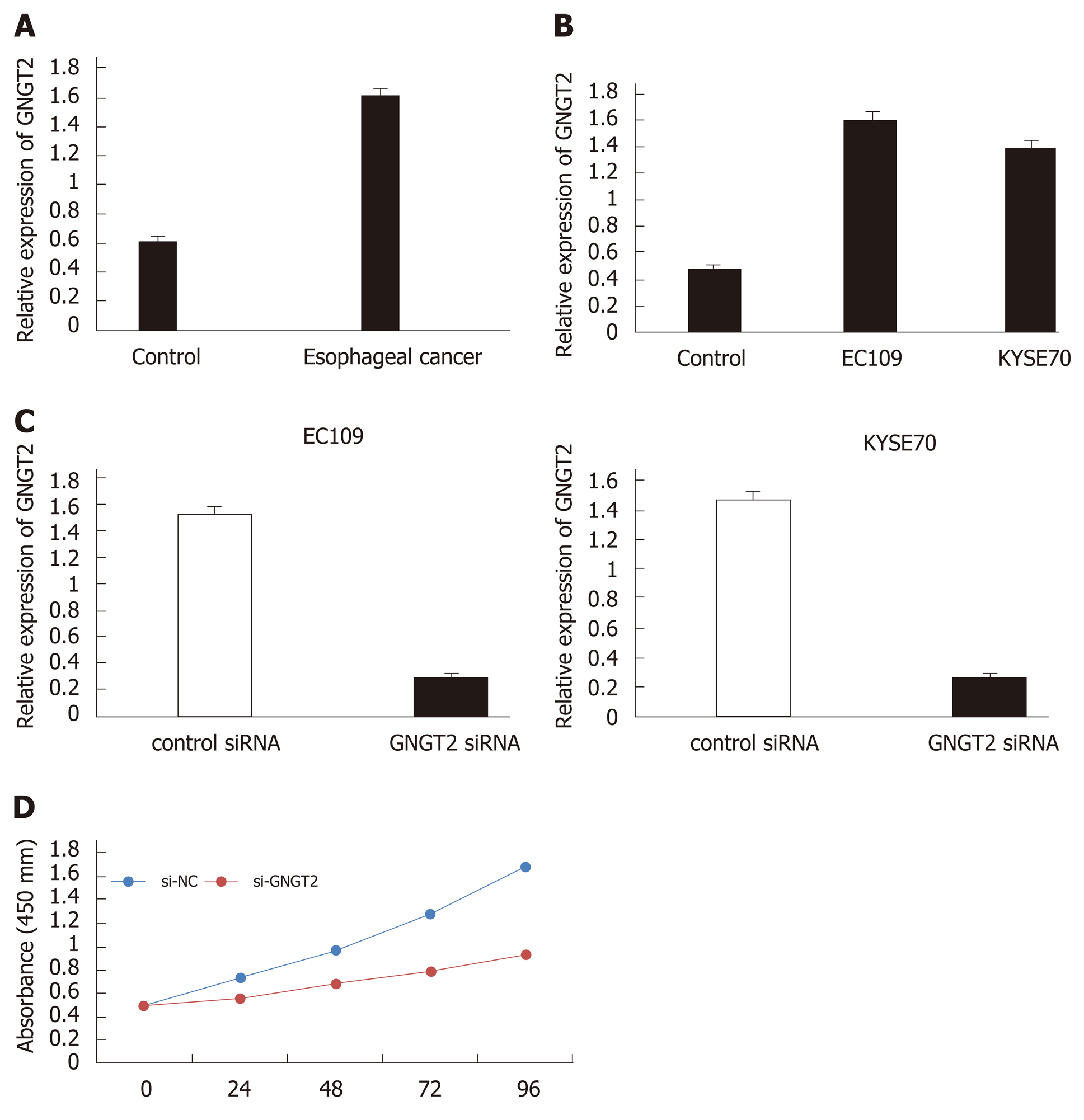
To investigate changes in GNGT2 expression during esophageal cancer development, samples from esophageal cancer patients (n = 20) and esophagus controls (n = 20) were subjected to quantitative real time-PCR analysis. As shown in Figure 3A, expression of GNGT2 gene was significantly upregulated in the esophageal epithelial cells of esophageal cancer patients. The experiment in esophageal cancer cell lines (EC109 and KYSE70) showed consistent results (Figure 3B) (P < 0.05).
To explore further the role of GNGT2 in the proliferation of EC109 and KYSE70 cells, cells were transfected with GNGT2 siRNA. As shown in Figure 3C, GNGT2 mRNA expression level was significantly decreased in EC109 and KYSE70 cells. Moreover, the proliferation of GNGT2 transfected group was significantly lower than that of the control group (Figure 3D). Taken together, the above results demonstrated that GNGT2 could promote the proliferation of esophageal cancer cell lines.
Esophageal cancer is one of the most deadly cancers, mainly because it is extremely aggressive and has a poor survival rate. Its 5-year survival rate is about 15%-25%[1]. The underlying cause of this disappointing low survival rate is that most patients have reached the late stage of disease at the time of detection. For patients with metastatic and unresectable disease, their chances of survival are limited[22]. In the present study, we collected RNA-Seq data from TCGA esophageal cancer and selected four stages of esophageal cancer disease samples and normal samples for differential analysis, and obtained four sets of time series differentially expressed genes. After screening, we found 13 common genes in four groups of DEGs. Komatsu et al[23] studied clinical biomarkers of pulmonary neuroendocrine tumors (LNET) and found that CPLX2 was strongly positive in 16.3% of the examination groups. Importantly, positive CPLX2 expression is associated with lymphatic invasion, pathological staging, and adverse disease-specific survival in LNET patients. It was concluded that CPLX2 is a novel clinical biomarker for LNET[23]. In the study of breast cancer diagnostic markers, Fu et al[24] found that changes in gene expression, such as DPEP1, may lead to cancer progression. DPEP1 has been identified as a prognostic gene for colorectal cancer (CRC). We found that DPEP1 is overexpressed in CRC. After knocking out the DPEP1 gene, cells (SW480 and HCT116) exhibited increased apoptosis and attenuated cell proliferation and cell invasion[25]. In the study of CRC, Eisenach[26] found that the expression of DPEP1 was increased in CRC tissues compared with normal mucosa. Zhang et al[27] also noted the DPEP1 gene in the study of pancreatic ductal adenocarcinoma and found that its gene expression was negatively correlated with histological grade and that lower expression of DPEP1 in tumors was associated with poor survival. Chen et al[28] analyzed the gastric cancer-associated Gene Expression Omnibus data and found that thrombospondin 4 (THBS4) was up-regulated in patients with recurrent gastric cancer and was positively correlated with the pathological stage and poor prognosis of gastric cancer. THBS4 stimulates the proliferation of gastric cancer cells. The breast-related gene explored by Huang et al[29] contains the gene THBS4, which is up-regulated in breast cancer. In the study of hepatocellular carcinoma, Su et al[30] found that knockdown of THBS4 inhibited migration and invasion of hepatocellular carcinoma cells as well as hemangiocarcinoma-induced angiogenesis. THBS4 as a target is very promising for the treatment of advanced liver cancer. Both of the above genes were present in the differential genes of the four stages of esophageal cancer in this study and were continuously down-regulated. Moreover, THBS4 was identified as a clinical biomarker gene and a therapeutic target gene in various cancers. Therefore, we can reasonably speculate that this gene plays an important role in the occurrence and development of esophageal cancer, providing a reasonable direction for further study of esophageal cancer.
The results of the methylation test showed that the SST gene was up-regulated extensively, which may be a key gene involved in methylation modification to regulate the progression of esophageal cancer. Jin et al[31] found that hypermethylation of the SST promoter is common and is associated with early tumor progression in Barrett's esophagus. The SH3GL2 gene is up-regulated. The gene is not only the common DEGs of the four-stage time series but also the Hub gene in module 6. It also may play an important role in the regulation of esophageal cancer by methylation modification. Ghosh et al[32] studied the effect of SH3GL2 methylation on the pathogenesis of head and neck squamous cell carcinoma, and abnormal SH3GL2 is an independent pathway for early developmental abnormalities of the head and neck.
Esophageal cancer is one of the most lethal malignant tumors in the world. In the past decades, although the treatment methods for esophageal cancer have improved, the overall efficacy is still poor.
In-depth analysis of molecular mechanisms related to esophageal cancer.
Exploring the molecular process of esophageal cancer comprehensively and deeply.
This study used differential expression analysis, enrichment analysis, methylation analysis, survival analysis, and statistical analyses.
A total of 7457 differentially expressed genes and 14 gene interaction modules were identified. These module genes were significantly involved in the positive regulation of protein transport, gastric acid secretion, insulin-like growth factor receptor binding and other biological processes as well as p53 signaling pathway, epidermal growth factor signaling pathway and epidermal growth factor receptor signaling pathway. In addition, transcription factors (including HIF1A) and ncRNAs (including CRNDE and hsa-mir-330-3p) that significantly regulate dysfunction modules were identified. Further, survival analysis showed that GNGT2 was closely related to survival of esophageal cancer. Differentially expressed genes with strong methylation regulation ability were identified, including SST and SH3GL2.
Overall, our work describes in detail the role of multifactor-mediated dysfunction module in the whole process of esophageal cancer, identifying key genes for staging and related biological processes, which may help to identify potential molecular mechanisms and therapeutic targets for the deterioration of esophageal cancer.
This work not only helps us to reveal the potential regulatory factors involved in the development of disease but also deepen our understanding of its deterioration mechanism.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gazouli M, Rodrigo L, Sterpetti AV S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933-7943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 554] [Cited by in F6Publishing: 612] [Article Influence: 68.0] [Reference Citation Analysis (8)] |
| 2. | Yang DH, Jung HY. [Treatment of esophageal cancer]. Korean J Gastroenterol. 2008;52:338-350. [PubMed] [Cited in This Article: ] |
| 3. | Li HY, Liu YC, Bai YH, Sun M, Wang L, Zhang XB, Cai B. SNP at miR-483-5p-binding site in the 3'-untranslated region of the BSG gene is associated with susceptibility to esophageal cancer in a Chinese population. Genet Mol Res. 2016;15(2). [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Wu Y, Yan M, Li J, Li J, Chen Z, Chen P, Li B, Chen F, Jin T, Chen C. Genetic polymorphisms in TERT are associated with increased risk of esophageal cancer. Oncotarget. 2017;8:10523-10530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Wang B, Yang J, Xiao B. MicroRNA-20b (miR-20b) Promotes the Proliferation, Migration, Invasion, and Tumorigenicity in Esophageal Cancer Cells via the Regulation of Phosphatase and Tensin Homologue Expression. PLoS One. 2016;11:e0164105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Jiao Y, Fu Z, Li Y, Meng L, Liu Y. High EIF2B5 mRNA expression and its prognostic significance in liver cancer: a study based on the TCGA and GEO database. Cancer Manag Res. 2018;10:6003-6014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Li Y, Jiao Y, Fu Z, Luo Z, Su J, Li Y. High miR-454-3p expression predicts poor prognosis in hepatocellular carcinoma. Cancer Manag Res. 2019;11:2795-2802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Jiao Y, Li Y, Fu Z, Hou L, Chen Q, Cai Y, Jiang P, He M, Yang Z. OGDHL Expression as a Prognostic Biomarker for Liver Cancer Patients. Dis Markers. 2019;2019:9037131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Li Y, Jiao Y, Li Y, Liu Y. Expression of La Ribonucleoprotein Domain Family Member 4B (LARP4B) in Liver Cancer and Their Clinical and Prognostic Significance. Dis Markers. 2019;2019:1569049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Li Y, Jiao Y, Luo Z, Li Y, Liu Y. High peroxidasin-like expression is a potential and independent prognostic biomarker in breast cancer. Medicine (Baltimore). 2019;98:e17703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Jiao Y, Li Y, Jiang P, Han W, Liu Y. PGM5: a novel diagnostic and prognostic biomarker for liver cancer. PeerJ. 2019;7:e7070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Jiao Y, Fu Z, Li Y, Zhang W, Liu Y. Aberrant FAM64A mRNA expression is an independent predictor of poor survival in pancreatic cancer. PLoS One. 2019;14:e0211291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. |
Nie Y, Jiao Y, Li Y, Li WJBRI. Investigation of the Clinical Significance and Prognostic Value of the lncRNA ACVR2B-As1 in Liver Cancer.
2019; |
| 14. | Jiao Y, Li Y, Liu S, Chen Q, Liu Y. ITGA3 serves as a diagnostic and prognostic biomarker for pancreatic cancer. Onco Targets Ther. 2019;12:4141-4152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | Zhou LL, Jiao Y, Chen HM, Kang LH, Yang Q, Li J, Guan M, Zhu G, Liu FQ, Wang S, Bai X, Song YQ. Differentially expressed long noncoding RNAs and regulatory mechanism of LINC02407 in human gastric adenocarcinoma. World J Gastroenterol. 2019;25:5973-5990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Jiao Y, Li Y, Lu Z, Liu Y. High Trophinin-Associated Protein Expression Is an Independent Predictor of Poor Survival in Liver Cancer. Dig Dis Sci. 2019;64:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Yi Y, Zhao Y, Li C, Zhang L, Huang H, Li Y, Liu L, Hou P, Cui T, Tan P, Hu Y, Zhang T, Huang Y, Li X, Yu J, Wang D. RAID v2.0: an updated resource of RNA-associated interactions across organisms. Nucleic Acids Res. 2017;45:D115-D118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Li M, Li D, Tang Y, Wu F, Wang J. CytoCluster: A Cytoscape Plugin for Cluster Analysis and Visualization of Biological Networks. Int J Mol Sci. 2017;18(9). [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24663] [Cited by in F6Publishing: 28164] [Article Influence: 1408.2] [Reference Citation Analysis (0)] |
| 20. | Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8 Suppl 4:S11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1658] [Cited by in F6Publishing: 2769] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 21. | Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11591] [Cited by in F6Publishing: 16541] [Article Influence: 1378.4] [Reference Citation Analysis (0)] |
| 22. | Tew WP, Kelsen DP, Ilson DH. Targeted therapies for esophageal cancer. Oncologist. 2005;10:590-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Komatsu H, Kakehashi A, Nishiyama N, Izumi N, Mizuguchi S, Yamano S, Inoue H, Hanada S, Chung K, Wei M, Suehiro S, Wanibuchi H. Complexin-2 (CPLX2) as a potential prognostic biomarker in human lung high grade neuroendocrine tumors. Cancer Biomark. 2013;13:171-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Fu S, Cheng J, Wei C, Yang L, Xiao X, Zhang D, Stewart MD, Fu J. Development of diagnostic SCAR markers for genomic DNA amplifications in breast carcinoma by DNA cloning of high-GC RAMP-PCR fragments. Oncotarget. 2017;8:43866-43877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Hao JJ, Zhi X, Wang Y, Zhang Z, Hao Z, Ye R, Tang Z, Qian F, Wang Q, Zhu J. Comprehensive Proteomic Characterization of the Human Colorectal Carcinoma Reveals Signature Proteins and Perturbed Pathways. Sci Rep. 2017;7:42436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Eisenach PA, Soeth E, Roder C, Kloppel G, Tepel J, Kalthoff H, Sipos B. Dipeptidase 1 (DPEP1) is a marker for the transition from low-grade to high-grade intraepithelial neoplasia and an adverse prognostic factor in colorectal cancer. Br J Cancer. 2013;109:694-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Zhang G, Schetter A, He P, Funamizu N, Gaedcke J, Ghadimi BM, Ried T, Hassan R, Yfantis HG, Lee DH, Lacy C, Maitra A, Hanna N, Alexander HR, Hussain SP. DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity and predicts clinical outcome in pancreatic ductal adenocarcinoma. PLoS One. 2012;7:e31507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Chen X, Huang Y, Wang Y, Wu Q, Hong S, Huang Z. THBS4 predicts poor outcomes and promotes proliferation and metastasis in gastric cancer. J Physiol Biochem. 2019;75:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Huang W, Zhao C, Zhong H, Zhang S, Xia Y, Cai Z. Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ Pollut. 2019;246:697-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Su F, Zhao J, Qin S, Wang R, Li Y, Wang Q, Tan Y, Jin H, Zhu F, Ou Y, Cheng Z, Su W, Zhao F, Yang Y, Zhou Z, Zheng J, Li Y, Li Z, Wu Q. Over-expression of Thrombospondin 4 correlates with loss of miR-142 and contributes to migration and vascular invasion of advanced hepatocellular carcinoma. Oncotarget. 2017;8:23277-23288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Jin Z, Mori Y, Hamilton JP, Olaru A, Sato F, Yang J, Ito T, Kan T, Agarwal R, Meltzer SJ. Hypermethylation of the somatostatin promoter is a common, early event in human esophageal carcinogenesis. Cancer. 2008;112:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Ghosh A, Ghosh S, Maiti GP, Sabbir MG, Alam N, Sikdar N, Roy B, Roychoudhury S, Panda CK. SH3GL2 and CDKN2A/2B loci are independently altered in early dysplastic lesions of head and neck: correlation with HPV infection and tobacco habit. J Pathol. 2009;217:408-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |