Copyright
©The Author(s) 2019.
World J Gastroenterol. May 28, 2019; 25(20): 2416-2429
Published online May 28, 2019. doi: 10.3748/wjg.v25.i20.2416
Published online May 28, 2019. doi: 10.3748/wjg.v25.i20.2416
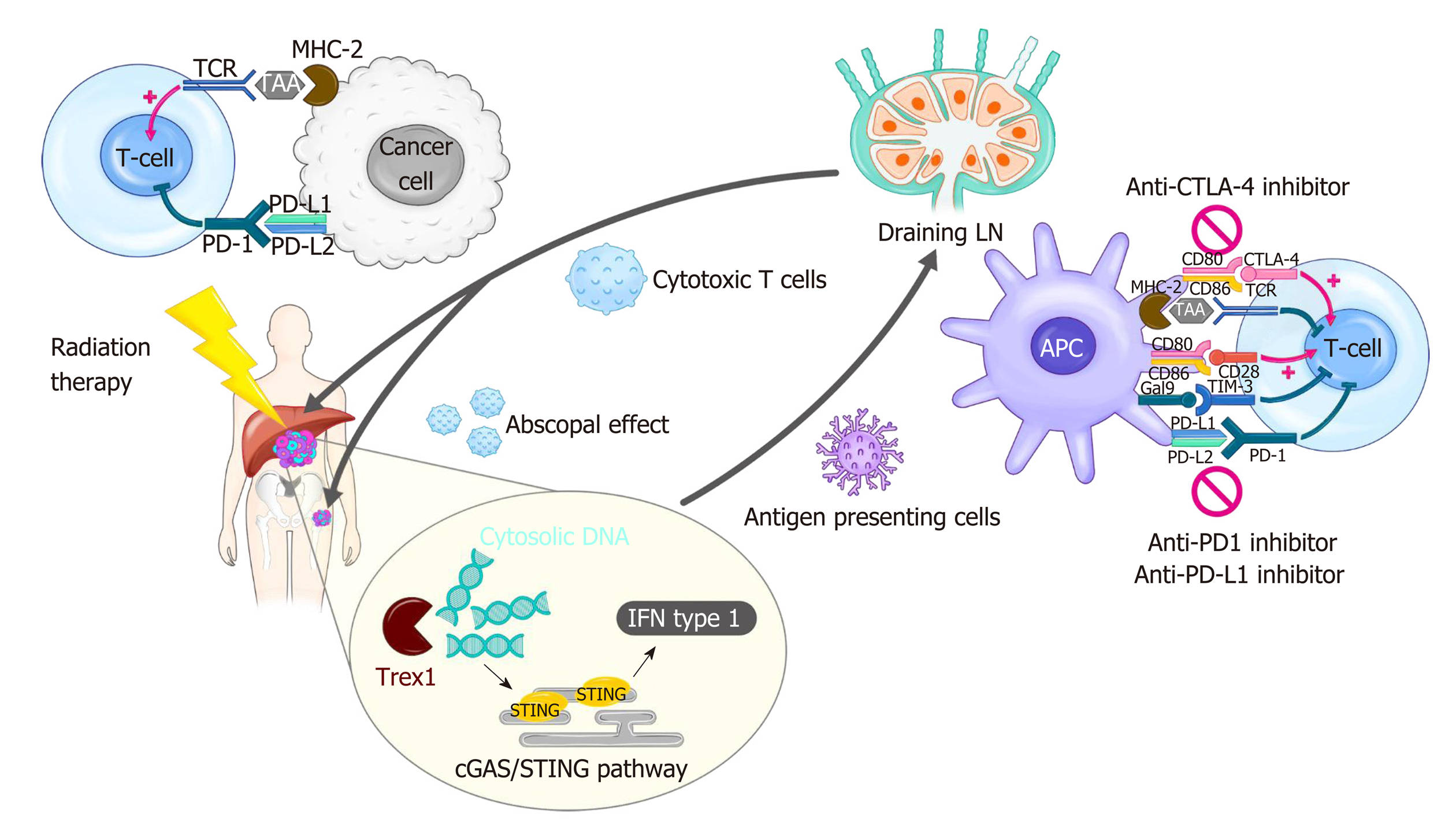
Figure 1 Modulation of tumor immunity by radiotherapy and immune checkpoint blockade.
Radiation-induced cell death results in cytosolic DNA accumulation in the tumor, which in turn activates the production of type I interferon (IFN) genes via cGAS/STING pathway. Increased IFN activates antigen presenting cells such as dendritic cells (DCs), which can prime T cells within draining lymph node. IFN also mediates recruitment of effector CD8+ T cells capable of killing cancer cells into irradiated tumor sites. Radiation triggers the release of tumor antigens and danger-associated molecular patterns, which can also activate DCs. Radiation-induced secretion of cytokines and chemokines play both pro-immunogenic and immunosuppressive roles in the tumor microenvironment. The antitumor effect of radiation therapy (RT) is frequently hindered by activation of immune checkpoint pathways. Thus, the combination of RT and immune checkpoint inhibitors such as anti-programmed death 1 inhibitor shows a synergistic effect in many types of cancer. The immune checkpoint blockade also enhances RT-induced systemic effect, called abscopal effect, which refers to the regression of an unirradiated tumor. cGAS: Cyclic guanosine monophosphate-adenosine monophosphate synthase; CTLA-4: Cytotoxic T lymphocyte-associated protein 4; IFN: Interferon; LN: Lymph node; MHC: Major histocompatibility complex; PD-1: Programmed death 1; PD-L1: Programmed death-ligand 1; STING: Stimulator of interferon genes; TAA: Tumor-associated antigen; TCR: T-cell receptor; Trex1: Three prime repair exonuclease 1.
- Citation: Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol 2019; 25(20): 2416-2429
- URL: https://www.wjgnet.com/1007-9327/full/v25/i20/2416.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i20.2416