Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5525
Peer-review started: November 12, 2018
First decision: December 7, 2018
Revised: December 8, 2018
Accepted: December 13, 2018
Article in press: December 13, 2018
Published online: December 28, 2018
Processing time: 49 Days and 2.1 Hours
Given the shortage of suitable liver grafts for liver transplantation, proper use of hepatitis B core antibody-positive livers might be a possible way to enlarge the donor pool and to save patients with end-stage liver diseases. However, the safety of hepatitis B virus core antibody positive (HBcAb+) donors has been controversial. Initial studies were mainly conducted overseas with relatively small numbers of HBcAb+ liver recipients, and there are few relevant reports in the population of mainland China. We hypothesized that the safety of HBcAb+ liver grafts is not suboptimal.
To evaluate the safety of using hepatitis B virus (HBV) core antibody-positive donors for liver transplantation in Chinese patients.
We conducted a retrospective study enrolling 1071 patients who underwent liver transplantation consecutively from 2005 to 2016 at West China Hospital Liver Transplantation Center. Given the imbalance in several baseline variables, propensity score matching was used, and the outcomes of all recipients were reviewed in this study.
In the whole population, 230 patients received HBcAb+ and 841 patients received HBcAb negative (HBcAb-) liver grafts. The 1-, 3- and 5-year survival rates in patients and grafts between the two groups were similar (patient survival: 85.8% vs 87.2%, 77.4% vs 81.1%, 72.4% vs 76.7%, log-rank test, P = 0.16; graft survival: 83.2% vs 83.6%, 73.8% vs 75.9%, 70.8% vs 74.4%, log-rank test, P = 0.19). After propensity score matching, 210 pairs of patients were generated. The corresponding 1-, 3- and 5-year patient and graft survival rates showed no significant differences. Further studies illustrated that the post-transplant major complication rates and liver function recovery after surgery were also similar. In addition, multivariate regression analysis in the original cohort and propensity score-matched Cox analysis demonstrated that receiving HBcAb+ liver grafts was not a significant risk factor for long-term survival. These findings were consistent in both HBV surface antigen-positive (HBsAg+) and HBsAg negative (HBsAg-) patients.
Newly diagnosed HBV infection had a relatively higher incidence in HBsAg- patients with HBcAb+ liver grafts (13.23%), in which HBV naive recipients suffered most (31.82%), although this difference did not affect patient and graft survival (P = 0.50 and P = 0.49, respectively). Recipients with a high HBV surface antibody (anti-HBs) titer (more than 100 IU/L) before transplantation and antiviral prophylaxis with nucleos(t)ide antiviral agents post-operation, such as nucleos(t)ide antiviral agents, had lower de novo HBV infection risks.
HBcAb+ liver grafts do not affect the long-term outcome of the recipients. Combined with proper postoperative antiviral prophylaxis, utilization of HBcAb+ grafts is rational and feasible.
Core tip: Considering the shortage of suitable liver grafts for liver transplantation, using hepatitis B virus core antibody positive (HBcAb+) livers might be a possible way to enlarge the donor pool. However, the safety is controversial and not widely evaluated in Chinese patients. Our retrospective study enrolling 1071 patients found that HBcAb+ grafts did not affect the long-term outcome. Although post-transplant hepatitis B virus (HBV) infection had a relatively higher incidence in HBV surface antigen-negative patients with such grafts, it did not affect patient and graft survival. We also found that sufficient anti-HBs titers in recipients might be a protective factor against de novo HBV infection. Combined with proper postoperative antiviral prophylaxis, utilization of HBcAb+ grafts is feasible.
- Citation: Lei M, Yan LN, Yang JY, Wen TF, Li B, Wang WT, Wu H, Xu MQ, Chen ZY, Wei YG. Safety of hepatitis B virus core antibody-positive grafts in liver transplantation: A single-center experience in China. World J Gastroenterol 2018; 24(48): 5525-5536
- URL: https://www.wjgnet.com/1007-9327/full/v24/i48/5525.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i48.5525
At present, liver transplantation is the only curative method for end-stage liver diseases. However, the limited organs available cannot meet the liver graft demand in Chinese patients. The shortage has promoted the enlargement of the donor pool, and accepting non-optimal livers offers a possibility to solve this troubling problem[1,2].
China is an area of high-intermediate hepatitis B virus (HBV) endemicity, with a prevalence of HBV infection close to 8%[3,4], and studies report that the number of HBV core antibody positive (HBcAb+) liver grafts is up to 50% of the door pool[5-8]. However, liver grafts from HBcAb+ donors carry the potential risk of HBV transmission, which limits the further use of such grafts, especially for patients naïve for HBV infection[9]. Indeed, several studies have found HBV covalently closed circular DNA (cccDNA) in some HBsAg- & HBcAb+ liver grafts[5,7,10] and further verified the association between serum anti-HBc levels and intra-hepatic HBV cccDNA[11]. Thus, it is suggested that post-transplantation immunosuppression could trigger cccDNA and enhance its replication[12]. Additionally, early reports have confirmed the risk of post-transplantation HBV infection in HBcAb+ liver graft recipients to be between 33% to 100%[7,10,13-17].
However, the lack of a larger study population[18-22], the wide variation in postoperative antiviral prophylaxis[7,20,21,23-25] and the clinical characteristics of the patients resulted in the availability of vague information concerning the risk of post-transplantation HBV infection in HBcAb+ liver graft recipients. Moreover, the outcome of recipients receiving HBcAb+ liver grafts has been discussed in previous studies with contradictory results. The survival rate of recipients with HBcAb+ donors was lower than that of recipients with anti-HBc negative donors in two United States studies[19,26] in 1997 and was confirmed in another investigation 5 years later[18]. However, in a similar Spanish study, the difference in survival was not statistically significant (68% vs 76%, P > 0.05)[27]. And a UNOS-based study conducted in 2009[17] found that HBsAg- recipients receiving HBcAb+ liver grafts had a significantly worse unadjusted graft survival, but the difference disappeared in multivariable analysis. Furthermore, studies concerning the risk of post-transplantation HBV infection and the long-term graft and patient survival in HBcAb+ liver graft recipients were mainly conducted overseas, and there are few relevant reports on the population of mainland China. Herein, we conducted a retrospective study enrolling 1071 patients who consecutively underwent liver transplantation from 2005 to 2016 in the Chinese population and aimed to evaluate the safety of utilization of HBcAb+ liver grafts in a relatively large number of patients.
This retrospective study concerns 1112 Chinese patients who consecutively underwent liver transplantation from 2005 to 2016 performed at the West China Hospital Liver Transplantation Center. Re-transplantation and multi-organ transplantation were excluded. We also did not include liver transplantations using HBV surface antigen-positive (HBsAg+) and HCV-positive donors. All liver grafts were voluntarily donated after cardiac death or by living donors, and all donations were approved by the West China Hospital Ethics Committee in accordance with the ethical principles of the 1975 Declaration of Helsinki. Written informed consent was given by all participants, and those receiving HBcAb+ liver grafts were fully informed of the possible risks before transplantation. Basic information of liver transplantations, the outcome and follow-up results were derived from The China Liver Transplant Registry.
Antiviral prophylaxis was given to all HBV-positive recipients, consisting of hepatitis B immunoglobulin (HBIG) and/or lamivudine or entecavir. A dosage of 2000 IU HBIG was intramuscularly injected during the anhepatic phase, followed by 800 IU intramuscularly daily for the first week, and then weekly for 3 wk, and monthly thereafter[28-30]. These patients maintained HBV prophylaxis indefinitely.
In HBV-negative recipients, considering that there were no practice guidelines on prophylaxis of de novo hepatitis B in China until 2016[31], the post-transplant antiviral prophylaxis varied from different periods. Generally, HBIG was administered to all patients the same way as in HBV-positive recipients, but the lasting time varied from 1-6 mo. Eighteen patients additionally received nucleos(t)ide analogue reverse transcriptase inhibitors (nRTI) including lamivudine (used in 15 patients), tenofovir (used in 1 patient) and entecavir (used in 2 patients).
The immunosuppressive therapy followed standard protocols, consisting of corticosteroids, tacrolimus and mycophenolate (1.0 g/d to 1.5 g/d) according to our previous report[32]. Methylprednisolone was started at a dose of 200 mg daily and was generally withdrawn within 3 mo. Tacrolimus was started at a dose of 0.05-0.10 mg/kg per day and was adjusted on the basis of liver function and trough concentration[32].
All recipients were followed until June 2018 or until death or withdrawal. Patients were monitored regularly after liver transplantation, including clinical, biochemical and virological testing. Ultrasound examination was conducted every day for 2 wk after the operation and every month after discharge, with computed tomography performed every half a year[33].
HBV DNA > 100 copies/mL was detectable. Recurrence of HBV infection was defined as a detectable serum HBsAg and/or viral load (HBV DNA) in patients who were HBsAg positive at the time of transplantation, and de novo HBV infection was defined as a detectable serum HBsAg and/or viral load (HBV DNA) in patients without positive serum HBsAg or viral load before transplantation.
Primary non-function was defined according to criteria described by Ploeg et al[34] as a graft with poor initial function requiring re-transplantation or leading to death within 7 d after the primary procedure without any identifiable cause of graft failure[34]. Acute rejection was defined either by liver biopsy or recovery of liver function via high-dose methylprednisolone therapy[32]. If chronic rejection was suspected, liver biopsy was performed for confirmation[32].
Since some continuous covariates presented significant differences from the normal distribution, they are shown as medians and first and third quartiles. Categorical variables are described as absolute numbers and relative frequencies. For continuous variables, either the Mann-Whitney U-test or Student’s-t test were performed, and the differences between rates were tested using the Chi square test or Fisher’s exact test, if appropriate. The Kaplan-Meier method with log-rank test was used for cumulative survival analysis. Independent prognostic factors were identified using multivariate analysis based on the Cox proportional hazards model. The assumption for proportional hazard was evaluated using scaled Schoenfeld residuals.
Given the differences in the baseline characteristics between the two groups (Table 1), propensity score matching was used to balance out selection bias and potential confounding between the two groups[35]. Propensity scores were estimated using a non-parsimonious multivariable logistic regression model, which included all covariates that might have affected patient assignment to different groups. A nearest-neighbor 1:1 matching scheme with a caliper width of 0.1 was used for propensity score matching[36]. Statistical inference was performed in the matched cohort with the use of a Cox proportional-hazards regression model stratified on the matched pair to preserve the benefit of matching[36]. Analyses were further performed on the basis of HBsAg status of the recipients (HBsAg+ and HBsAg- recipients). Propensity score matching was also conducted in HBsAg+ and HBsAg- cohorts. The statistical methods of this study were reviewed by Jia-Wei Luo from West China School of Public Health, Sichuan University.
| Factor | Before propensity matching | After propensity matching | ||||
| HBcAb+ (n = 230) | HBcAb- (n = 841) | P value | HBcAb+ (n = 210) | HBcAb- (n = 210) | P value | |
| Baseline characteristic | ||||||
| Gender (male) | 77.4% (178) | 80.10% (674) | 0.36 | 80.5% (169) | 79.5% (167) | 0.90 |
| Age (yr) | 41.03 ± 15.58 | 42.04 ± 15.19 | 0.63 | 40.67 ± 16.57 | 40.55 ± 15.47 | 0.94 |
| BMI | 22.1 (19.7-24.2) | 22.0 (19.8-24.4) | 0.54 | 22.4 (19.7-24.4) | 22.2 (19.7-24.2) | 0.8 |
| MELD | 13.0 (9.0-21.0) | 13.0 (9.0-20.0) | 0.62 | 14.5 (10.0-20.8) | 13.0 (9.0-20.0) | 0.25 |
| CTP | 9.0 (7.0-11.0) | 8.0 (7.0-10.0) | < 0.01 | 9.0 (7.0-11.0) | 9.0 (7.0-11.0) | 0.14 |
| Creatinine (µmol/L) | 67.0 (53.1-81.9) | 71.9 (59.3-85.3) | < 0.01 | 68.4 (55.9-82.6) | 67.5 (53.6-82.4) | 0.36 |
| Total bilirubin (µmol/L) | 43.2 (18.8-198.3) | 37.8 (18.9-139.9) | 0.31 | 51.2 (22.8-201.9) | 40.2 (18.6-195.0) | 0.20 |
| Albumin (g/L) | 35.4 (30.9-40.5) | 34.6 (30.1-40.1) | 0.11 | 33.8 (30.1-40.1) | 35.4 (31.1-41.3) | 0.06 |
| HBV-positive | 64.78% (149) | 77.28% (650) | < 0.01 | 62.9% (132) | 69.0% (145) | 0.18 |
| HCV-positive | 1.3% (3) | 7.4% (62) | < 0.01 | 0.95% (2) | 3.33% (7) | 0.18 |
| Etiology | ||||||
| Liver cancer | 48.26% (111) | 46.13% (388) | 0.57 | 43.3% (91) | 49.5% (104) | 0.20 |
| Acute liver failure | 11.30% (26) | 4.99% (42) | < 0.01 | 8.57% (18) | 11.4% (24) | 0.32 |
| Cirrhosis | ||||||
| Biliary | 9.13% (21) | 7.84% (66) | 0.53 | 8.57% (18) | 9.05% (19) | 0.86 |
| HBV related | 49.13% (113) | 55.41% (466) | 0.09 | 56.19% (118) | 54.76% (15) | 0.76 |
| HCV related | 0.86% (2) | 1.90% (16) | 0.28 | 0.95% (2) | 2.38% (5) | 0.45 |
| Alcoholic | 2.17% (5) | 3.21% (27) | 0.41 | 1.90% (4) | 0.95% (2) | 0.69 |
| Autoimmune | 1.74% (4) | 1.07% (9) | 0.41 | 0.95% (2) | 0.95% (2) | 1.00 |
| Donor | ||||||
| Gender (male) | 74.35% (171) | 79.30% (666) | 0.12 | 61.4% (129) | 61.9% (130) | 0.92 |
| Age | 41.28 ± 13.01 | 33.66 ± 10.16 | < 0.01 | 39.08 ± 10.59 | 40.82 ± 12.36 | 0.12 |
| BMI | 22.7 (20.8-24.6) | 22.2 (20.8-23.9) | 0.06 | 22.8 (21.4-24.8) | 22.6 (20.8-24.5) | 0.37 |
| Serum Na+ (mmol/L) | 143.0 (140.1-153.6) | 139.9 (136.2-142.7) | < 0.01 | 141.6 (138.5-143.9) | 141.9 (140.0-153.5) | 0.23 |
| Creatinine (µmol/L) | 73.1 (54.5-98.5) | 74.6 (62.8-84.6) | < 0.01 | 75.3 (63.0-88.5) | 74.4 (54.0-95.6) | 0.39 |
| Total bilirubin (µmol/L) | 13.8 (9.4-18.9) | 15.1 (10.2-23.3) | 0.05 | 14.9 (10.1-21.7) | 13.8 (9.4-18.7) | 0.25 |
| Albumin (g/L) | 40.3 (32.6-45.3) | 41.9 (36.2-46.6) | 0.06 | 42.1 (36.2-46.7) | 41.8 (34.9-46.0) | 0.12 |
| ALT (IU/L) | 23.0 (16.0-45.0) | 28.0 (16.0-47.0) | 0.32 | 27.0 (17.8-46.2) | 26.0 (16.0-43.0) | 0.10 |
| AST (IU/L) | 27.0 (20.8-42.0) | 26.0 (18.0-39.0) | 0.15 | 27.0 (19.0-39.8) | 27.5 (20.0-47.0) | 0.12 |
| Cold ischemic time (h) | 5.0 (3.2-7.8) | 6.0 (5.0-10.0) | 0.05 | 5.5 (4.0-8.0) | 5.5 (3.0-7.0) | 0.11 |
| Warm ischemic time (min) | 6.0 (3.0-9.0) | 4.5 (2.0-6.5) | 0.05 | 6.0 (3.0-10.0) | 5.0 (3.0-7.0) | 0.08 |
All reported P-values are two-sided, and P-values less than 0.05 were considered statistically significant. All data were analyzed using SPSS22.0 (IBM Corporation, Armonk, NY, United States) and R statistical software, version 2.3.15 (R Foundation, Inc.; http://cran.r-project.org/).
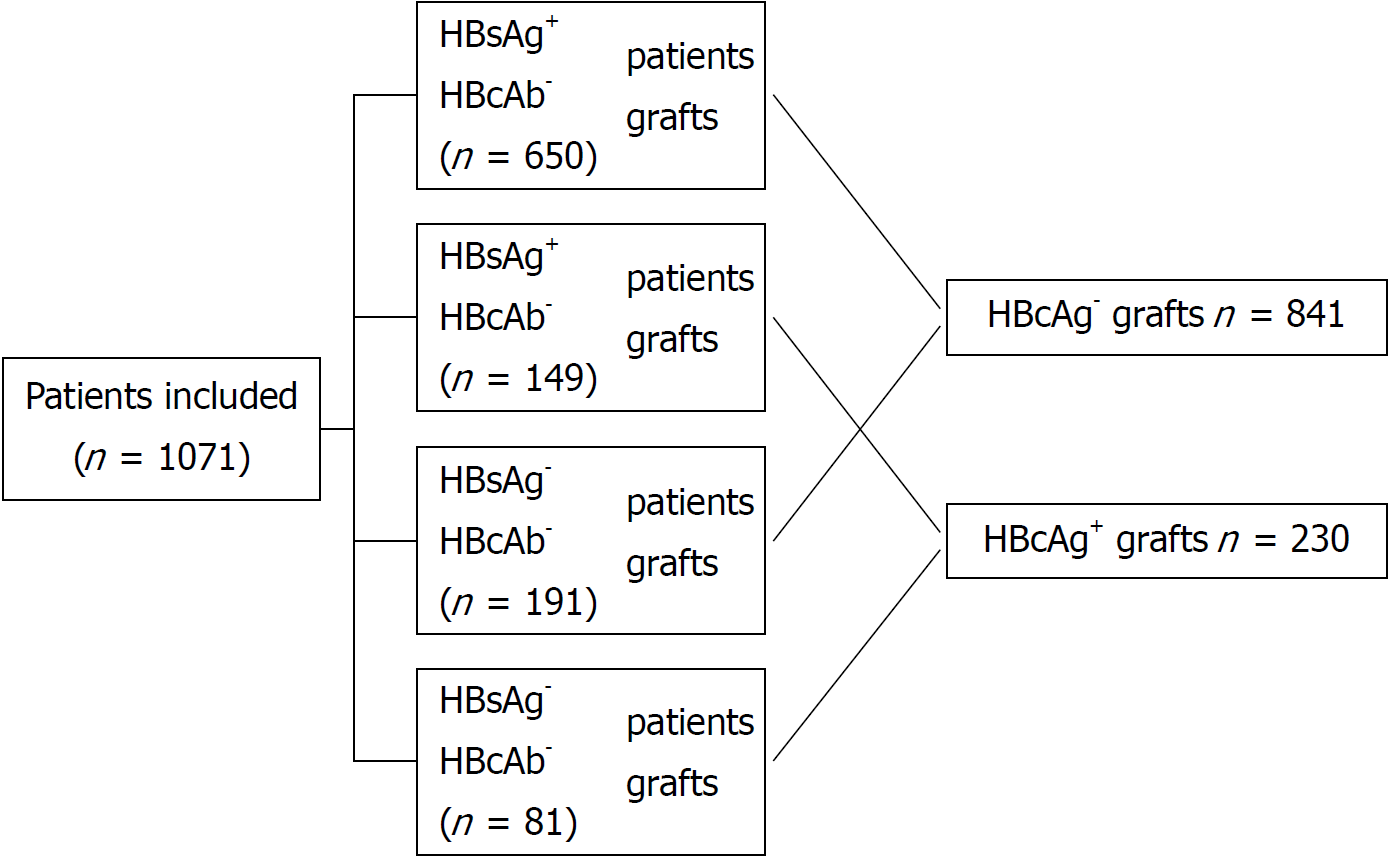
From 2005 to 2016, 1071 liver transplantation patients met our inclusion criteria, of which 230 patients received HBcAb+ liver grafts and 841 patients received HBcAb- liver grafts. The median following time was 48.72 ± 33.37 mo (range from 0.1 to 128.1 mo). In these patients, 799 were HBsAg+ (149 receiving HBcAb+ liver grafts and 650 receiving HBcAb- liver grafts), and 272 were HBsAg- (81 receiving HBcAb+ liver grafts and 191 receiving HBcAb- liver grafts) at the time of transplantation. A flow diagram of patient disposition is shown in Figure 1.
The clinical data of the recipients and donors between the two groups are summarized in Table 1. There were differences between the two groups in several baseline variables. For example, recipients of HBcAb+ grafts had a lower HBsAg+ rate (64.78% vs 77.28%, P < 0.01) and a lower HCV-positive rate (1.3% vs 7.4%, P < 0.01). HBcAg+ donors were relatively older than HBcAg- donors (41.28 ± 13.01 vs 33.66 ± 10.16, P < 0.01). With the utilization of propensity score matching, 210 pairs of patients were generated, and the characteristics of the two groups were balanced (Table 1).
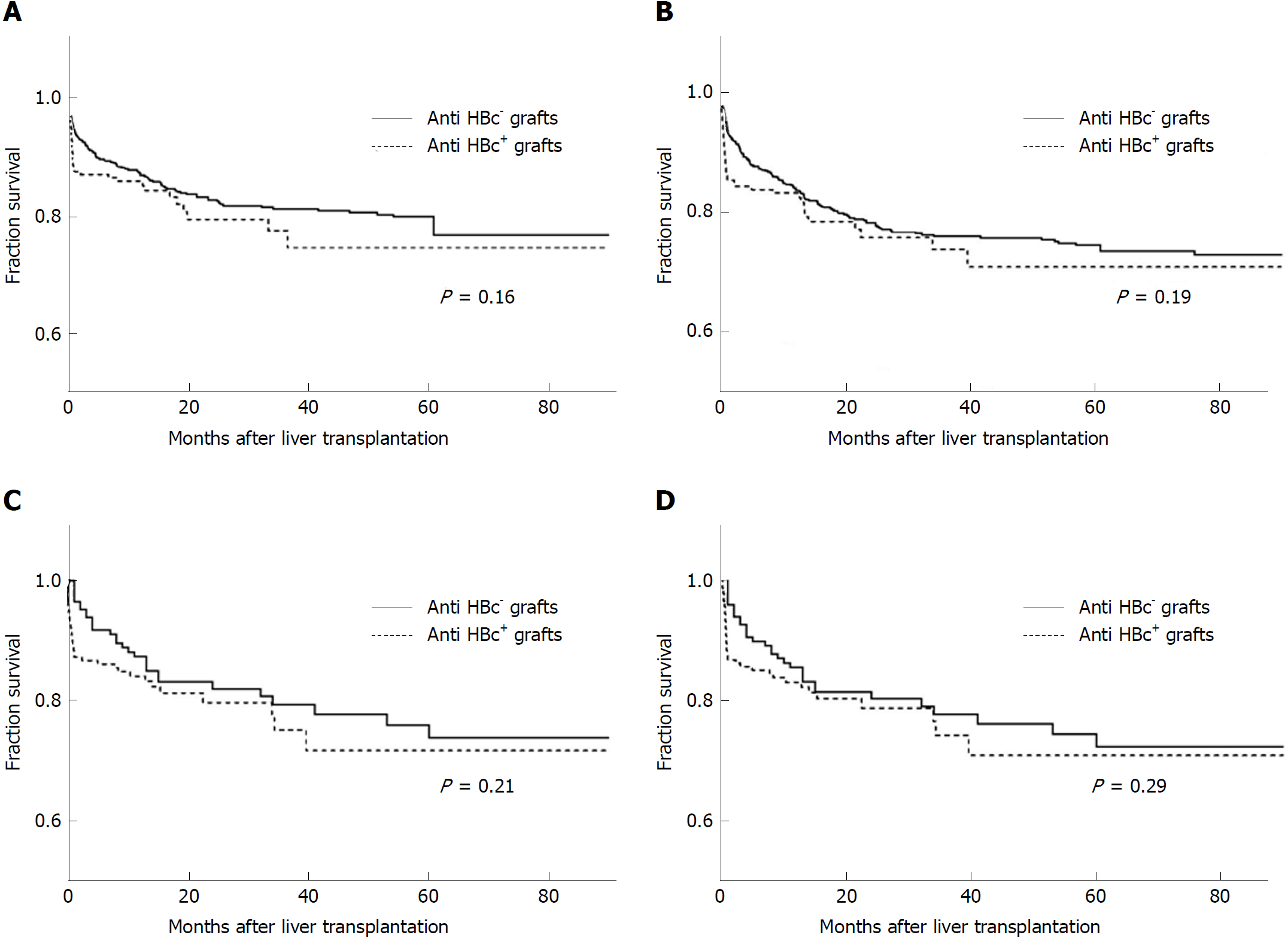
In the original cohort, which included 1071 patients, the 1-, 3- and 5-year survival rates between the HBcAb+ and HBcAb- liver graft recipients were similar (85.8%, 77.4% and 72.4% vs 87.2%, 81.1% and 76.7%, log-rank test, χ2 = 1.97, P = 0.16, Figure 2A). Meanwhile, graft survival rates between the two groups were also comparable (83.2%, 73.8% and 70.8% vs 83.6%, 75.9% and 74.4%, log-rank test, χ2 = 1.65, P = 0.19, Figure 2B). Propensity score matching was used for the differences in several baseline variables between the two groups as noted above, and the 1-, 3- and 5-year patient survival rates in the new HBcAb+ and HBcAb- groups presented no significant differences (84.1%, 75.2% and 71.7% vs 87.4%,79.4% and 73.8%, log-rank test, χ2 = 1.53, P = 0.21, Figure 2C). The corresponding 1-, 3- and 5-year graft survival rates in the HBcAb+ group were 83.1%,74.3% and 70.7% compared with 85.6%, 77.8% and 72.3% in the controls (log-rank test, χ2 = 1.10, P = 0.29, Figure 2D).
In HBsAg+ patients, HBcAb+ liver grafts did not have deleterious effects on survival when compared with HBcAb- grafts (log-rank test, χ2 = 1.59, P = 0.20, Supplement Figure 1A), similar to the HBsAg- recipients (log-rank test, χ2 = 0.81, P = 0.37, Supplement Figure 1B).
To identify the independent prognostic factors, Cox regression analysis was conducted in the whole cohort, and multivariate regression analysis showed that a higher BMI in the recipients was a strong indicator for worse patient (P = 0.001) and graft survival (P = 0.001) (Table 2). Blood transfusion amount and mechanical ventilation time were also shown as risk factors both for patient (P = 0.018 and 0.002, respectively) and graft survival (P = 0.021 and P = 0.004, respectively) (Table 2). However, parallel to the results of the survival curves, the multivariate regression analysis did not support HBcAb+ liver grafts as an independent risk factor for death or graft loss after liver transplantation ( P = 0.675 and P = 0.982, Table 2).
| Patient survival | Graft survival | |||||||
| Equation variable | P value | HR | 95%CI | P value | HR | 95%CI | ||
| Recipient | ||||||||
| BMI | 0.001 | 1.006 | 1.002 | 1.010 | 0.001 | 1.007 | 1.002 | 1.011 |
| MELD | 0.942 | 1.002 | 0.948 | 1.059 | 0.912 | 1.003 | 0.949 | 1.059 |
| CTP | 0.429 | 1.049 | 0.835 | 1.263 | 0.398 | 1.046 | 0.834 | 1.258 |
| HBsAb (negative vs positive) | 0.208 | 1.058 | 0.768 | 1.347 | 0.258 | 1.027 | 0.739 | 1.374 |
| Operation | ||||||||
| Anhepatic phase (min) | 0.271 | 1.003 | 0.997 | 1.01 | 0.169 | 1.004 | 0.998 | 1.011 |
| RBC transfusion (U) | 0.018 | 1.055 | 1.009 | 1.104 | 0.021 | 1.052 | 1.006 | 1.103 |
| After operation | ||||||||
| Mechanical ventilation (h) | 0.002 | 1.003 | 1.001 | 1.006 | 0.004 | 1.004 | 1.001 | 1.006 |
| Postoperative HBV infection (negative vs positive) | 0.502 | 0.597 | 0.132 | 2.695 | 0.490 | 0.527 | 0.118 | 2.340 |
| Donor | ||||||||
| Age | 0.215 | 1.015 | 0.991 | 1.042 | 0.155 | 1.018 | 0.992 | 1.045 |
| AST (IU/L) | 0.65 | 1.002 | 0.993 | 1.011 | 0.563 | 1.002 | 0.994 | 1.011 |
| Na (mmol/L) | 0.243 | 1.016 | 0.988 | 1.045 | 0.408 | 1.011 | 0.983 | 1.041 |
| Anti HBc-positive (negative vs positive) | 0.675 | 1.175 | 0.551 | 2.505 | 0.982 | 1.008 | 0.480 | 2.118 |
Propensity score-matched analysis also revealed that receiving HBcAb+ liver grafts was not associated with a significantly higher risk of death (Cox regression analysis, P = 0.45, 95%CI: 0.73-2.03, Table 3).
| Group | No. of patients | HR (95%CI) | P value |
| Entire cohort, multivariable-adjusted (n = 1071) | |||
| HbcAb-positive grafts | 230 | 1.18 (0.55,2.51) | 0.68 |
| HbcAb-negative grafts | 841 | Reference | |
| Entire cohort, propensity score-matched (n = 210 pairs) | |||
| HbcAb-positive grafts | 210 | 1.21 (0.73,2.03) | 0.45 |
| HbcAb-negative grafts | 210 | Reference | |
| HBsAg-positive cohort, multivariable-adjusted (n = 799) | |||
| HbcAb-positive grafts | 149 | 1.07 (0.98,1.17) | 0.12 |
| HbcAb-negative grafts | 650 | Reference | |
| HBsAg-positive cohort, propensity score-matched (n = 132 pairs) | |||
| HbcAb-positive grafts | 132 | 1.06 (0.93,1.20) | 0.37 |
| HbcAb-negative grafts | 132 | Reference | |
| HBsAg-negative cohort, multivariable-adjusted (n = 272) | |||
| HbcAb-positive grafts | 81 | 1.42 (0.71,2.81) | 0.32 |
| HbcAb-negative grafts | 191 | Reference | |
| HBsAg-negative cohort, propensity score-matched (n = 77 pairs) | |||
| HbcAb-positive grafts | 77 | 1.37 (0.60,3.14) | 0.45 |
| HbcAb-negative grafts | 77 | Reference | |
Consistent with the above results, both multivariate regression analysis in the original cohort and propensity score-matched Cox analysis did not support that receiving HBcAb positive liver grafts was associated with worse outcome for either HBsAg+ or HBsAg- patients (Table 3).
During the observation period, 21 patients died in the entire cohort within 30 d. In patients receiving HBcAb+ liver grafts, 5 patients died because of multi-organ failure with sepsis and 3 patients died of abdominal hemorrhage, while in the HBcAb- group, patients died within 30 d because of multi-organ failure with sepsis (n = 8), liver failure due to portal vein embolism (n = 2) and abdominal hemorrhage (n = 3). The death rates within 30 d of the two groups were not significantly different [Chi square test, 3.47% (8/230) vs 1.54% (13/841), P = 0.10]. None of the causes of death were HBV-related.
In the post-transplant observation, no differences between HBcAb+ and HBcAb- graft recipients were observed in rates of major complications in either the early stage (within 30 d post LT) or the later period (beyond 30 d post LT; Tables 4 and 5)
| Major complication | Before propensity matching | After propensity matching | ||||
| Anti-HBc+ (n = 230) | Anti-HBc- (n = 841) | P value | Anti-HBc+ (n = 210) | Anti-HBc- (n = 210) | P value | |
| Primary non-function | 0.43 (1/230) | 0.24 (2/841) | 0.52 | 0 | 0 | 1.00 |
| Biliary complications | 0.87 (2/230) | 1.30 (11/841) | 0.75 | 0.47 (1/210) | 0.47 (1/210) | 1.00 |
| Hepatic artery stenosis/embolism | 2.61 (6/230) | 1.54 (13/841) | 0.26 | 2.38 (5/210) | 1.43 (3/210) | 0.72 |
| Portal vein stenosis/embolism | 3.91 (9/230) | 2.49 (21/841) | 0.25 | 3.33 (7/210) | 0.95 (2/210) | 0.17 |
| Outflow tract stenosis/embolism | 0.87 (2/230) | 0.36 (3/841) | 0.29 | 0.47 (1/210) | 0.47 (1/210) | 1.00 |
| Bacterial infection | 21.30 (49/230) | 20.33 (171/841) | 0.75 | 8.10 (17/210) | 7.62 (16/210) | 0.85 |
| Intra-abdominal hemorrhage | 7.39 (17/230) | 5.35 (45/841) | 0.24 | 5.24 (11/210) | 2.38 (5/210) | 0.20 |
| Pleural effusion | 10.86 (25/230) | 15.21 (128/841) | 0.09 | 5.71 (12/210) | 3.33 (7/210) | 0.24 |
| Celiac effusion | 11.74 (27/230) | 14.86 (125/841) | 0.23 | 7.62 (16/210) | 8.10 (17/210) | 0.85 |
| Pneumonedema | 0.43 (1/230) | 0.36 (3/841) | > 0.9 | 0.47 (1/210) | 0.47 (1/210) | 1.00 |
| Major complication | Before propensity matching | After propensity matching | ||||
| Anti-HBc+ (n = 230) | Anti-HBc- (n = 841) | P value | Anti-HBc+ (n = 210) | Anti-HBc- (n = 210) | P value | |
| Acute rejection | 11.30 (26/230) | 11.05 (93/841) | 0.91 | 9.52 (20/210) | 8.10 (17/210) | 0.60 |
| Chronic rejection | 0.87 (2/230) | 0.95 (8/841) | > 0.9 | 0.95 (2/210) | 0.95 (2/210) | 1.00 |
| GVHD | 0.43 (1/230) | 0.59 (5/841) | > 0.9 | 0.47 (1/210) | 0.47 (1/210) | 1.00 |
| Biliary complications | 9.56 (22/230) | 9.15 (77/841) | 0.85 | 7.14 (15/210) | 10.95 (23/210) | 0.17 |
| Hepatic artery stenosis/embolism | 0.87 (2/230) | 0.71 (6/841) | 0.68 | 0.95 (2/210) | 2.38 (5/210) | 0.45 |
| Portal vein stenosis/embolism | 0.87 (2/230) | 1.78 (15/841) | 0.55 | 0.95 (2/210) | 0.95 (2/210) | 1.00 |
| Outflow tract stenosis/embolism | 0.43 (1/230) | 0.71 (6/841) | > 0.9 | 0.47 (1/210) | 0.47 (1/210) | 1.00 |
| Tumor recurrence | 5.65 (13/230) | 4.52 (38/841) | 0.51 | 4.76 (10/210) | 6.19 (13/210) | 0.52 |
| CMV infection | 0.87 (2/230) | 0.83 (7/841) | > 0.9 | 0.95 (2/210) | 1.90 (4/210) | 0.68 |
| Opportunistic infection | 2.17 (5/230) | 1.43 (12/841) | 0.38 | 1.43 (3/210) | 2.38 (5/210) | 0.72 |
| Diabetes mellitus | 7.82 (18/230) | 7.97 (67/841) | 0.94 | 7.14 (15/210) | 4.76 (10/210) | 0.3 |
| Hypertension | 4.78 (11/230) | 3.57 (30/841) | 0.39 | 3.33 (7/210) | 2.38 (5/210) | 0.77 |
| Hyperlipemia | 0.43 (1/230) | 0.95 (8/841) | 0.69 | 0.47 (1/210) | 1.90 (4/210) | 0.37 |
In the entire cohort, 3 patients with HBcAb+ liver graft underwent re-transplantations for primary non-function (n = 1), biliary complications (n = 1) and portal vein embolism (n = 1), while 7 recipients in the HBcAb- group underwent re-transplantations for primary non-function (n = 1), biliary complications (n = 2), portal vein embolism (n = 1) and hepatic artery embolism (n = 3). The re-transplantation rates of the two groups were 1.30% (3/230) and 0.83% (7/841), without a significant difference (Fisher exact test, P = 0.45).
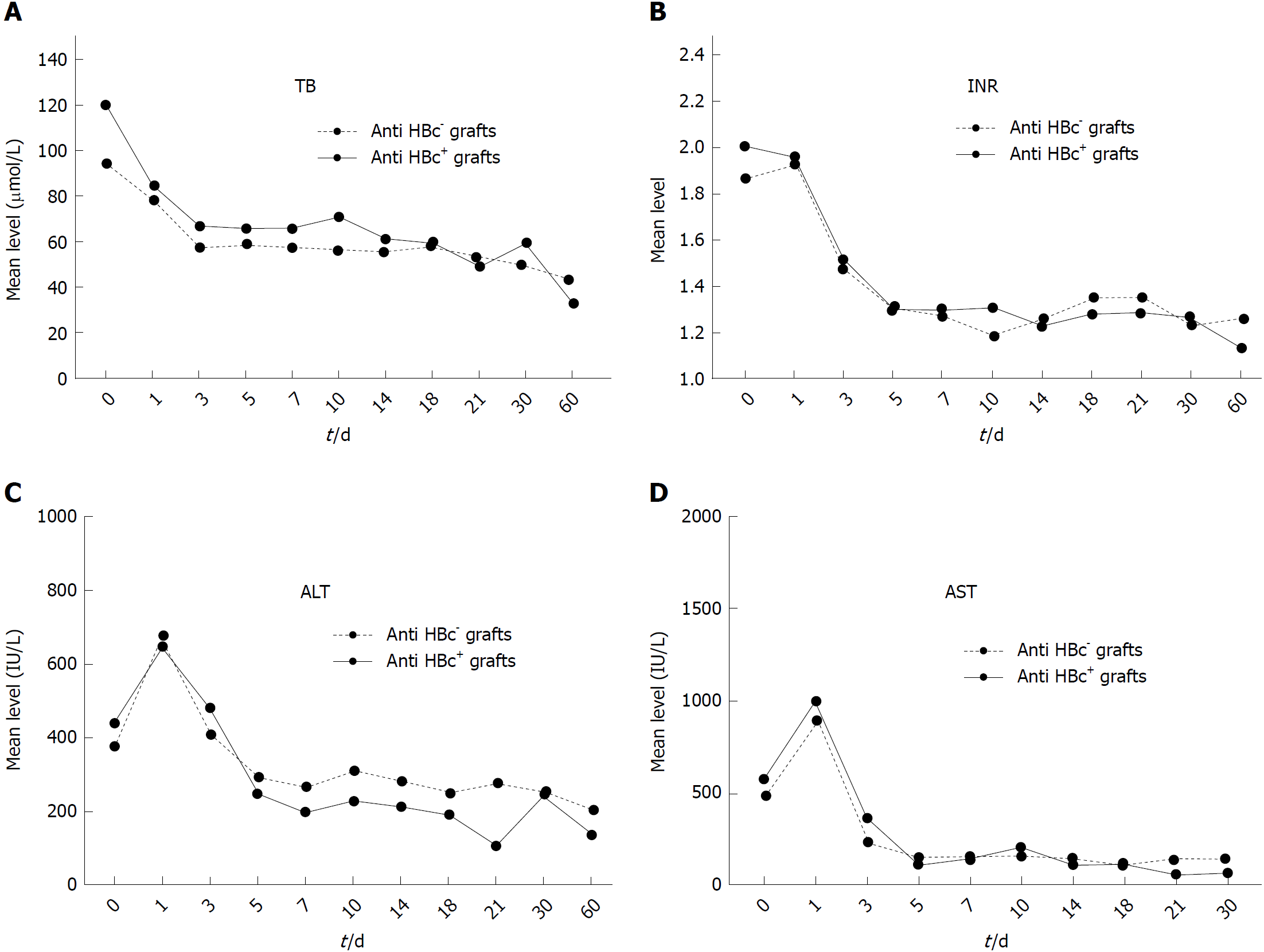
Furthermore, postoperative follow-up showed that there was no significant difference in liver function recovering after liver transplantation between the HBcAb+ and HBcAb- groups (Figure 3).
As was noted in the beginning, it is well acknowledged that HBcAb+ liver grafts retain the capability to transmit HBV, especially to HBsAg- patients[7,9,23], which is one of the major concerns in utilization of such grafts.
First, we analyzed de novo HBV infection in the HBsAg negative cohort and its risk factors. We found that 9 patients receiving HBcAb+ liver grafts developed hepatitis B after a mean time of 15.66 ± 5.52 mo, with an infection rate of 13.23% (9/68, 13 patients died within 1 mo after liver transplantation or without any post-transplant HBV serological data), whereas no patients receiving HBcAb- liver grafts were newly diagnosed with HBV after the operation (0/163, 28 patients died within 1 mo after liver transplantation or without any post-transplant HBV serological data). Thus, the data indicate a higher prevalence of de novo HBV infection in recipients of HBcAb+ liver grafts (Fisher exact test, 13.23% vs 0, P < 0.0001).
Once the diagnosis of de novo hepatitis B was made, anti-viral therapy was switched to entecavir or tenofovir. Only one patient died 3 mo after a new diagnosis of HBV due to severe rejection-caused multi-organ failure without HBV flare-up. The other patients are still alive at present. The multivariate regression analysis revealed that the occurrence of newly diagnosed HBV infection is not an independent threat to patient and graft survival (P = 0.502 and 0.490, respectively, Table 2).
Considering the status of HBV surface antibody (anti-HBs) and HBV core antibody (anti-HBc), there were three different HBV serological statuses in recipients (Figure 4). There was a clear tendency that the HBV-naïve patients had the highest de novo HBV infection rate (31.82%, 7/22), while the anti-HBs+ recipients had the lowest de novo HBV infection rate (2.56%, 1/39). The comparison showed a significant difference (Fisher’s exact test, P = 0.002). The results suggested that the presence of anti-HBs in recipients might be a protective factor against HBV infection.
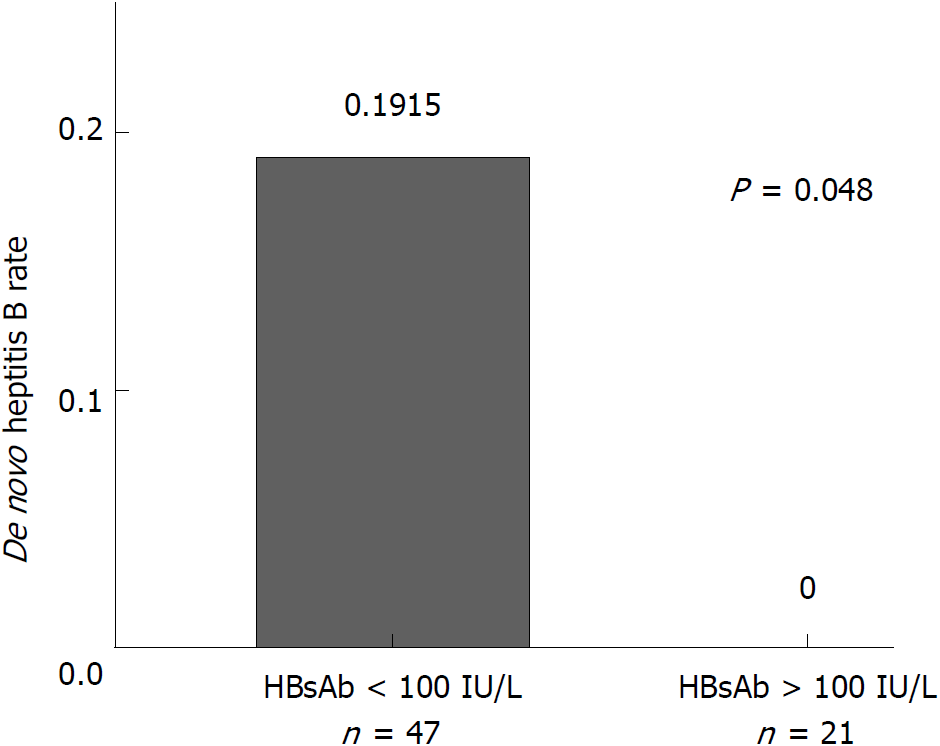
Furthermore, the anti-HBs titer at the time of transplantation has a significant impact on the outcome of the HBV infection. We found that no patients with anti-HBs titer more than 100 IU/l before surgery (n = 21) developed de novo HBV infection, while those whose anti-HBs titers were less than 100 IU/l before surgery had a de novo infection rate of 19.15% (9/47) (Fisher exact test, P = 0.048, Figure 5).
Next, we divided the 68 patients into two groups with respect to antiviral prophylaxis: an HBIG monotherapy group (n = 50) and an nRTI combination group (n = 18). The corresponding de novo HBV infection rates of each group were 16.00% (8/50) and 5.55% (1/18). There was a higher risk tendency in the HBIG monotherapy group compared with the other, but without a significant difference (Fisher exact test, P = 0.43).
It is important to note that the only patient who developed HBV positivity in the nRTI combination group had an HBIG administration of 3 mo but did not use nRTI regularly until 8 mo after surgery and was diagnosed 4 mo later.
Finally, we also analyzed the recurrence of HBV infection in the HBsAg+ cohort and found that 20 (2.5%) of the 799 HBsAg+ patients developed recurrence of HBV infection, in which 7 patients received HBcAb+ liver grafts and 13 received HBcAb- liver grafts. Thus, the recurrence rates between the two groups were 4.7% (7/149) and 2.0% (13/650); this difference was not statistically significant (Chi square test, P = 0.06).
Recent years have witnessed an increasing trend in the utilization of marginal organs, including HBV core antigen-positive liver grafts, to address the problem of organ shortage[8,37]. In early times, transplantation centers adhered to the principle that the occurrence of anti-HBc in liver grafts was a contraindication to organ use in fear of deleterious effects[9,18,19]. However, as discussed above, the postoperative safety of receiving such grafts has become debatable over the following years.
Recent studies conducted in Austrian[20]and Italian[23] populations still associated HBcAb+ livers with inferior outcomes, but without explained mechanisms. The Italian study[23] suggested that HBcAb positivity represents a surrogate marker of suboptimal graft quality. Nevertheless, none of the recorded causes of death or graft loss differed between HBcAb+ or HBcAb- donors, and no graft loss was attributable to recurrence or de novo HBV infection, in line with other studies[20,38]. Notably, the authors in both articles[20,23] all acknowledged the high prevalence of HCV (20% to 50%) in the study population and the high rate of HCV recurrence[23] leading to cirrhosis in 20-30% of patients[39] when direct antiviral agents were not widely applied in the past. The Austrian study[20] also demonstrated that anti-HCV positivity was a strong indicator for worse patient survival (HR: 2.38, 95%CI: 1.18-4.78, P = 0.015), in agreement with an earlier report[40]. However, their findings in HCV infection rates were not the same as in our Chinese cohort (HCV infection rate: less than 8%), which might partially explain the difference in the impacts of HBcAb+ liver grafts.
In our study, the number of HBcAb+ liver grafts was greater than the numbers in most previous studies[7,18-20,22,26-27,41,42], which allows a more reliable conclusion. Our study was the largest single-center cohort of long-term follow-up in a Chinese population, which provides an important view in the utilization of such grafts.
Although the baseline data of HBcAb+ donors and recipients shared similarity in most aspects with HBcAb- donors, as a real world study, differences existed between the two groups, consisting of a significantly higher Child-Pugh score and a higher prevalence of acute liver failure as transplantation reasons in HBcAb+ graft recipients. These differences could be explained as the relatively “imperfect” liver grafts, for example, HBcAb+ grafts were more likely to be allocated to patients with more severe and urgent conditions in short of proper grafts available in recent clinical practice. And it was notable that HBcAb+ donors were older than HBcAb- donors, which was in line with previous studies[7,20,23,26] and reflected a higher prevalence of HBV infection and anti-HBc occurrence in older people, especially in epidemic areas.
Considering these confounding factors in a real world study, a new cohort of patients was generated in a propensity-score matching method, as we have introduced, to achieve appropriate analysis validity, which was neglected in most of the previous studies. Additionally, given the different HBsAg status in recipients, a subgroup analysis was conducted, and survival was compared as in the entire population.
Using multiple rigorous strategies, we were able to come to a firm conclusion that overall survival with HBcAb+ liver grafts was not suboptimal, regardless of the HBsAg status of the recipients.
Monitoring after surgery in our study also showed that HBcAb+ grafts did not increase the post-transplant complications in either the early stage or the later stage. Furthermore, liver function recovered in HBcAb+ grafts at the same pace as in HBcAb- grafts.
Another barrier to HBcAb+ graft utilization is the risk of HBV infection in recipients, especially for patients naïve to HBV infection[26]. In the era of prophylaxis with HBIG and nRTI, the dramatically high prevalence of HBV infection in HBcAb+ liver recipients[7,10,13-17] has declined (in our cohort, de novo infection: 13.23%). Post-transplantation HBV infection did not affect patient and graft survival (P = 0.50 and 0.49, respectively, Table 2), the same as in previous articles[20,23]. Yet, there was no consensus on a prophylactic regimen for de novo HBV infection until 2015 in the United States[43] and 2016 in China[31]. Thus, a diverse array of protocols has been used worldwide in past time[7,20,21,23-25], from no prophylaxis to the use of nucleos(t)ide antiviral agents, HBIG or their combination, resulting in varied HBV infection rates in different institutes[7]. In our cohort, there was a higher risk tendency of de novo HBV infection in the HBIG monotherapy group compared with patients with prophylaxis consisting of HBIG and nRTI (16.00% vs 5.55%), highlighting the importance of nucleos(t)ide antiviral agents in HBV prophylaxis. In addition, recent studies suggested that HBIG+ nRTI combination therapy did not provide superior protection over nRTI-only treatment[7,14,16,24,25,44]. The latest guidelines overseas[43,45] also recommend nRTI (lamivudine, entecavir or tenofovir) as prophylactic agents, excluding HBIG.
However, it is worth noting that the higher cost and lower compliance of HBIG injection prevented its long-term utilization in patients who were HBV-negative before transplantation, varying from weeks to months in our study and in other reports[7,14,25,44], which might increase the HBV infection rates in HBIG monotherapy prophylaxis, as we found above.
Additionally, recipient characteristics in HBV serology did have a significant impact on postoperative HBV infection when accepting HBcAb+ grafts. In our results, HBV-naïve patients had the highest de novo HBV infection rate (31.82%), while the anti-HBs+ recipients had the lowest (2.56%), a tendency that was in accord with a previous systematic review[7]. Quantitative investigation on anti-HBs suggested that patients with an anti-HBs titer more than 100 IU/l before surgery had a lower risk of hepatitis B (P = 0.048, Figure 5). And in a recent study, patients were given HBV vaccinations as active immunizations with the aim of achieving anti-HBs > 1000 IU/L pre-transplant and > 100 IU/L post-transplant, which achieved satisfactory preventive results[46]. They also suggested that anti-viral prophylaxis could be safely discontinued in patients who obtain this immunity. Generally, these findings emphasize the prophylactic role of HBsAb and active immunization might be an economical alternative prophylaxis in patients who respond appropriately to vaccination.
Our study has limitations. It is based on retrospective data and patients at a single institution, which may be subject to bias and confounding, although we used multiple strategies (multivariable adjustment and propensity-score matching). In addition, because of the retrospective nature of our study, we were unable to obtain complete pre-LT HBV vaccination data. Further multicenter studies are needed to evaluate the ideal prophylaxis to prevent post-transplant de novo HBV infection.
In conclusion, our retrospective study revealed that HBcAb+ liver grafts could be used with similar outcomes to HBcAb- grafts regardless of the HBsAg status of the recipients. Although HBcAb+ liver recipients do have a higher de novo HBV infection rate in which HBV-naïve patients suffer more often, nucleos(t)ide antiviral agents have been regarded as effective antiviral prophylaxis that should be widely applied in clinical practice. Furthermore, maintaining sufficient anti-HBs titers in recipients might also be protective against de novo HBV infection.
Given the shortage of suitable liver grafts for liver transplantation, proper use of hepatitis B core antibody-positive livers might be a possible way to enlarge the donor pool and to save patients with end-stage liver diseases. However, the safety of hepatitis B virus core antibody positive (HBcAb+) donors has been controversial. Initial studies were mainly conducted overseas with relatively small numbers of HBcAb+ liver recipients, and there are few relevant reports in the population of mainland China.
We performed this study to evaluate the safety of HBcAb+ liver graft recipients in a Chinese population and to investigate the feasibility of wide utilization of such liver grafts.
The objectives of our study were: (1) to evaluate the long-term survival of HBcAb+ liver graft recipients; and (2) to investigate the post-transplant hepatitis B virus infection rates of HBcAb+ liver graft recipients and to elucidate possible risk factors.
We conducted a retrospective study, enrolling 1071 patients who consecutively underwent liver transplantation from 2005 to 2016 at West China Hospital Liver Transplantation Center. Given the imbalance in several baseline variables, propensity score matching was used, and the outcomes of all recipients were reviewed in this study.
Our results revealed that the 1-, 3- and 5-year survival rates in patients and grafts between the HBcAb+ and HBcAb- recipients showed no difference (P = 0.16 and 0.19, respectively), and receiving HBcAb+ liver grafts was not a significant risk factor for long-term survival. Further studies illustrated that post-transplant major complication rates and liver function recovery after surgery were also similar. These findings were consistent in both HBsAg+ and HBsAg- patients. Newly diagnosed HBV infection had a relatively higher incidence in HBsAg- patients with HBcAb+ liver grafts (13.23%), in which HBV-naïve recipients suffered most (31.82%), whereas it did not affect patient and graft survival (P = 0.50 and 0.49, respectively). Recipients with high anti-HBs titers (more than 100 IU/L) before transplantation and antiviral prophylaxis with nucleos(t)ide antiviral agents post-operation had lower de novo HBV infection risks.
HBcAb+ grafts did not increase the post-transplant mortality, nor did they affect post-transplant major complication rates and liver function recovery. HBV-naïve recipients suffered post-transplantation de novo HBV infection more often, and sufficient anti-HBs titers in recipients might be a protective factor. Combined with proper postoperative antiviral prophylaxis, such as nucleos(t)ide antiviral agents, utilization of HBcAb+ grafts is rational and feasible.
Further multicenter studies are needed to investigate more interval time groups with a large sample size on the outcome of HBcAb+ graft recipients. The findings of this study should spur more investigators to evaluate the ideal postoperative antiviral therapy, which may involve active immunization prophylaxis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gordon LG, Jin M, Roohvand F, Shimizu Y S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Alkofer B, Samstein B, Guarrera JV, Kin C, Jan D, Bellemare S, Kinkhabwala M, Brown R Jr, Emond JC, Renz JF. Extended-donor criteria liver allografts. Semin Liver Dis. 2006;26:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Merion RM, Goodrich NP, Feng S. How can we define expanded criteria for liver donors? J Hepatol. 2006;45:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550-6557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 710] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 4. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1983] [Article Influence: 198.3] [Reference Citation Analysis (3)] |
| 5. | Merrill RM, Hunter BD. Seroprevalence of markers for hepatitis B viral infection. Int J Infect Dis. 2011;15:e78-e121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Bohorquez HE, Cohen AJ, Girgrah N, Bruce DS, Carmody IC, Joshi S, Reichman TW, Therapondos G, Mason AL, Loss GE. Liver transplantation in hepatitis B core-negative recipients using livers from hepatitis B core-positive donors: a 13-year experience. Liver Transpl. 2013;19:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010;52:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Anwar N, Sherman KE. Transplanting organs from hepatitis B positive donors: Is it safe? Is it ethical? J Viral Hepat. 2018;25:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Burton JR Jr, Shaw-Stiffel TA. Use of hepatitis B core antibody-positive donors in recipients without evidence of hepatitis B infection: a survey of current practice in the United States. Liver Transpl. 2003;9:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kwan KW, Lim TR, Kumar R, Krishnamoorthy TL. Understanding the hepatitis B core positive liver donor. Singapore Med J. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Caviglia GP, Abate ML, Tandoi F, Ciancio A, Amoroso A, Salizzoni M, Saracco GM, Rizzetto M, Romagnoli R, Smedile A. Quantitation of HBV cccDNA in anti-HBc-positive liver donors by droplet digital PCR: A new tool to detect occult infection. J Hepatol. 2018;69:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 12. | Mason AL, Xu L, Guo L, Kuhns M, Perrillo RP. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology. 1998;27:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Prakoso E, Strasser SI, Koorey DJ, Verran D, McCaughan GW. Long-term lamivudine monotherapy prevents development of hepatitis B virus infection in hepatitis B surface-antigen negative liver transplant recipients from hepatitis B core-antibody-positive donors. Clin Transplant. 2006;20:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Skagen CL, Jou JH, Said A. Risk of de novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts - a systematic analysis. Clin Transplant. 2011;25:E243-E249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Avelino-Silva VI, D’Albuquerque LA, Bonazzi PR, Song AT, Miraglia JL, De Brito Neves A, Abdala E. Liver transplant from Anti-HBc-positive, HBsAg-negative donor into HBsAg-negative recipient: is it safe? A systematic review of the literature. Clin Transplant. 2010;24:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Chang MS, Olsen SK, Pichardo EM, Stiles JB, Rosenthal-Cogan L, Brubaker WD, Guarrera JV, Emond JC, Brown RS Jr. Prevention of de novo hepatitis B in recipients of core antibody-positive livers with lamivudine and other nucleos(t)ides: a 12-year experience. Transplantation. 2013;95:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Yu L, Koepsell T, Manhart L, Ioannou G. Survival after orthotopic liver transplantation: the impact of antibody against hepatitis B core antigen in the donor. Liver Transpl. 2009;15:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Muñoz SJ. Use of hepatitis B core antibody-positive donors for liver transplantation. Liver Transpl. 2002;8:S82-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Dodson SF, Issa S, Araya V, Gayowski T, Pinna A, Eghtesad B, Iwatsuki S, Montalvo E, Rakela J, Fung JJ. Infectivity of hepatic allografts with antibodies to hepatitis B virus. Transplantation. 1997;64:1582-1584. [PubMed] |
| 20. | Brandl A, Stolzlechner P, Eschertzhuber S, Aigner F, Weiss S, Vogel W, Krannich A, Neururer S, Pratschke J, Graziadei I. Inferior graft survival of hepatitis B core positive grafts is not influenced by post-transplant hepatitis B infection in liver recipients-5-year single-center experience. Transpl Int. 2016;29:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Marciano S, Gaite LA, Bisignano L, Descalzi VI, Yantorno S, Mendizabal M, Silva MO, Anders M, Orozco OF, Traverso R. Use of liver grafts from anti-hepatitis B core-positive donors: a multicenter study in Argentina. Transplant Proc. 2013;45:1331-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Lee S, Kim JM, Choi GS, Park JB, Kwon CH, Choe YH, Joh JW, Lee SK. De novo hepatitis b prophylaxis with hepatitis B virus vaccine and hepatitis B immunoglobulin in pediatric recipients of core antibody-positive livers. Liver Transpl. 2016;22:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Angelico M, Nardi A, Marianelli T, Caccamo L, Romagnoli R, Tisone G, Pinna AD, Avolio AW, Fagiuoli S, Burra P. Hepatitis B-core antibody positive donors in liver transplantation and their impact on graft survival: evidence from the Liver Match cohort study. J Hepatol. 2013;58:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Perrillo R. Hepatitis B virus prevention strategies for antibody to hepatitis B core antigen-positive liver donation: a survey of North American, European, and Asian-Pacific transplant programs. Liver Transpl. 2009;15:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Saab S, Waterman B, Chi AC, Tong MJ. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core antibody-positive donors: a systematic review. Liver Transpl. 2010;16:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Dickson RC, Everhart JE, Lake JR, Wei Y, Seaberg EC, Wiesner RH, Zetterman RK, Pruett TL, Ishitani MB, Hoofnagle JH. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. The National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Gastroenterology. 1997;113:1668-1674. [PubMed] |
| 27. | Prieto M, Gómez MD, Berenguer M, Córdoba J, Rayón JM, Pastor M, García-Herola A, Nicolás D, Carrasco D, Orbis JF. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl. 2001;7:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Shen S, Jiang L, Xiao GQ, Yan LN, Yang JY, Wen TF, Li B, Wang WT, Xu MQ, Wei YG. Prophylaxis against hepatitis B virus recurrence after liver transplantation: a registry study. World J Gastroenterol. 2015;21:584-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Jiang L, Yan L, Li B, Wen T, Zhao J, Jiang L, Cheng N, Wei Y, Yang J, Xu M. Prophylaxis against hepatitis B recurrence posttransplantation using lamivudine and individualized low-dose hepatitis B immunoglobulin. Am J Transplant. 2010;10:1861-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Jiang L, Yan L, Wen T, Li B, Zhao J, Yang J, Xu M, Wang W. Hepatitis B prophylaxis using lamivudine and individualized low-dose hepatitis B immunoglobulin in living donor liver transplantation. Transplant Proc. 2013;45:2326-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Chinese Society of Organ Transplantation, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. The practice guideline on prophylaxis and treatment of hepatitis B for liver transplantation in China (2016 edition). Zhonghua Gan Zang Bing Za Zhi. 2016;24:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Shao ZY, Yan LN, Wang WT, Li B, Wen TF, Yang JY, Xu MQ, Zhao JC, Wei YG. Prophylaxis of chronic kidney disease after liver transplantation--experience from west China. World J Gastroenterol. 2012;18:991-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Yu S, Yu J, Zhang W, Cheng L, Ye Y, Geng L, Yu Z, Yan S, Wu L, Wang W. Safe use of liver grafts from hepatitis B surface antigen positive donors in liver transplantation. J Hepatol. 2014;61:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation-a multivariate analysis. Transplantation. 1993;55:807-813. [PubMed] |
| 35. | Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, Robins JM. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 547] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 36. | Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 1109] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 37. | Nadig SN, Bratton CF, Karp SJ. Marginal donors in liver transplantation: expanding the donor pool. J Surg Educ. 2007;64:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Brock GN, Mostajabi F, Ferguson N, Carrubba CJ, Eng M, Buell JF, Marvin MR. Prophylaxis against de novo hepatitis B for liver transplantation utilizing hep B core (+) donors: does hepatitis B immunoglobulin provide a survival advantage? Transpl Int. 2011;24:570-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14 Suppl 2:S36-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Berenguer M, Prieto M, San Juan F, Rayón JM, Martinez F, Carrasco D, Moya A, Orbis F, Mir J, Berenguer J. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 494] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 41. | MacConmara MP, Vachharajani N, Wellen JR, Anderson CD, Lowell JA, Shenoy S, Chapman WC, Doyle MB. Utilization of hepatitis B core antibody-positive donor liver grafts. HPB (Oxford). 2012;14:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Douglas DD, Rakela J, Wright TL, Krom RA, Wiesner RH. The clinical course of transplantation-associated de novo hepatitis B infection in the liver transplant recipient. Liver Transpl Surg. 1997;3:105-111. [PubMed] |
| 43. | Huprikar S, Danziger-Isakov L, Ahn J, Naugler S, Blumberg E, Avery RK, Koval C, Lease ED, Pillai A, Doucette KE. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant. 2015;15:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 44. | Vizzini G, Gruttadauria S, Volpes R, D’Antoni A, Pietrosi G, Filì D, Petridis I, Pagano D, Tuzzolino F, Santonocito MM. Lamivudine monoprophylaxis for de novo HBV infection in HBsAg-negative recipients with HBcAb-positive liver grafts. Clin Transplant. 2011;25:E77-E81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2796] [Article Influence: 399.4] [Reference Citation Analysis (0)] |
| 46. | Wang SH, Loh PY, Lin TL, Lin LM, Li WF, Lin YH, Lin CC, Chen CL. Active immunization for prevention of De novo hepatitis B virus infection after adult living donor liver transplantation with a hepatitis B core antigen-positive graft. Liver Transpl. 2017;23:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |