Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1424
Peer-review started: October 27, 2016
First decision: December 1, 2016
Revised: December 16, 2016
Accepted: January 4, 2017
Article in press: January 4, 2017
Published online: February 28, 2017
Processing time: 123 Days and 4.6 Hours
To evaluate the clinical and prognostic significance of preoperative and postoperative cytokeratin 19 (CK19) and carcinoembryonic antigen (CEA) mRNA levels in peripheral blood of patients with gastric cardia cancer (GCC).
We detected the preoperative and postoperative mRNA levels of CK19 and CEA in peripheral blood of 129 GCC patients by using reverse transcription-polymerase chain reaction and evaluated their clinical and prognostic significance by univariate Kaplan-Meier survival analysis and multivariate Cox proportional hazard analysis. A new prognostic model which stratified patients into three different risk groups was established based on the independent prognostic factors.
Elevated preoperative and postoperative CK19 and CEA mRNA levels in peripheral blood of GCC patients were associated with lymph node metastasis. Univariate analysis showed that tumor size, histological grade, depth of tumor invasion, lymph node metastasis, preoperative CK19 mRNA, and preoperative and postoperative CEA mRNA levels were correlated with the prognosis of GCC patients. The multivariate analysis showed that lymph node status (P = 0.018), preoperative CK19 (P = 0.035) and CEA (P = 0.011) mRNA levels were independent prognostic factors for overall survival (OS). The 5-year OS rates for the low-, intermediate-, and high-risk groups were 48.3%, 22.6%, and 4.6%, respectively (P < 0.001).
Elevated preoperative CK19 and CEA mRNA levels may be regarded as promising biomarkers for predicting lymph node metastasis and poor prognosis in patients with GCC. This new prognostic model may help us identify the subpopulations of GCC patients with the highest risk.
Core tip: This is a retrospective study that evaluated the clinical and prognostic significance of the preoperative (pre-) and postoperative (post-) mRNA levels of cytokeratin 19 (CK19) and carcinoembryonic antigen (CEA) in peripheral blood of patients with gastric cardia cancer. Increased pre- and post-CK19 and CEA mRNA levels were associated with positive lymph node metastasis. Elevated pre- but not post-CK19 and CEA mRNA levels were independent prognostic factors for overall survival (OS). A new prognostic model was established based on independent prognostic factors (lymph node status, pre-CK19 and pre-CEA mRNA levels), and there was a significant difference in OS among the three different risk groups.
- Citation: Qiao YF, Chen CG, Yue J, Ma MQ, Ma Z, Yu ZT. Prognostic significance of preoperative and postoperative CK19 and CEA mRNA levels in peripheral blood of patients with gastric cardia cancer. World J Gastroenterol 2017; 23(8): 1424-1433
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1424
Gastric cancer (GC) is the third leading cause of cancer-related death worldwide[1]. Recently, the incidence of gastric cardia cancer (GCC) has increased around the world[2,3]. The prognosis of patients with GCC was worse than that of patients with non-cardiac GC[4]. Although the important advances in surgical techniques and adjuvant chemotherapies have improved patient outcomes, the 5-year survival rate remains low (10%-30%)[5]. Metastasis and recurrence after surgery are the main causes of treatment failure and poor prognosis[6]. Therefore, it is of great significance to establish molecular biomarkers for GCC to detect early metastasis and improve survival.
Carcinoembryonic antigen (CEA), one of the most commonly used serum tumor markers in GC, plays an important role in tumor metastasis and may be partially associated with prognosis in GC[7]. Cytokeratin 19 (CK19), which originates from epithelial cells, is a reliable tissue-specific marker for epithelial tumor micrometastasis and is highly expressed in the gastrointestinal tract but not in normal lymph node tissue or blood. The detection of CEA and CK19 expression has been used to examine tumor micrometastasis and predict the prognosis in GC and esophageal squamous cell carcinoma[8,9]. Nevertheless, there is no valid marker to predict the lymph node metastasis and prognosis in patients with GCC. Recently, quantitative real-time polymerase chain reaction (qRT-PCR) technology, a sensitive, specific and rapid method, has been widely used to detect the presence of circulating cancer cells in peripheral blood, the expression of tumor markers, and micrometastases, as well as predict prognoses[10]. However, there are few reports on the detection and clinical significance of CEA and CK19 mRNAs in the peripheral blood of GCC patients. With the decrease in tumor burden after surgery, the levels of tumor marker expression will change. Some studies have supported the use of preoperative (pre-) CEA levels as prognostic markers in GC[11,12], whereas other studies have reported the prognostic value of postoperative (post-) CEA levels[13]. There are limited data regarding the prognostic significance of pre- and post-CK19 levels in GCC.
In the present study, we detected the pre- and post-CEA and CK19 mRNA levels in peripheral blood of patients with GCC by using qRT-PCR and estimated the clinical and prognostic value of these biomarkers in patients with GCC.
A total of 129 patients who were diagnosed with GCC and underwent curative surgery between January 2009 and December 2011 at Tianjin Medical University Cancer Institute and Hospital were recruited for the study. The inclusion criteria of the study were as follows: (1) complete clinicopathological and follow-up data; (2) no neoadjuvant chemotherapy, radiotherapy or chemoradiotherapy; (3) radical gastrectomy for the primary tumor and D2 lymph node dissection following the Japanese Research Society for Gastric Cancer guidelines[14]; (4) no gross or microscopic residual or recurrent gastric tumor; (5) no distant metastases prior to surgery; and (6) no other synchronous malignancies. The histological diagnosis and tumor-node-metastasis (TNM) staging were based on 7th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system[15]. The study and acquisition of blood specimens were approved by the Research Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. In addition, all of the patients provided written informed consent.
Three to five millilambert peripheral blood samples were obtained through a catheter inserted into a peripheral vessel and collected into EDTA tubes from each GCC patient before and after surgical resection. Sample processing was performed within 2 h after blood collection. Karyocytes were isolated from the blood samples using a lymphocyte separation medium according to the manufacturer’s instructions (Solarbio, Beijing, China). Briefly, blood samples were subjected to Ficoll-sodium diatrizoate density gradient centrifugation. Then, after discarding the plasma layer, the samples were mixed with a wash buffer and centrifuged at 200 g for 10 min. The pelleted cells were resuspended in red blood cell lysis buffer (Solarbio) and centrifuged another two times. The remaining cells, which are karyocytes in PB, were washed with PBS, and the total cellular RNA isolation and cDNA synthesis were performed as previously described[9]. Total RNA was extracted from the cell lysate using TRIzol reagent (Invitrogen, Gaithersburg, MD, United States) according to the manufacturer’s instructions. The concentration and purity of RNA were determined by using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, United States). A260/A280 ratios in the range of 1.8 to 2.0 were considered satisfactory for purity standards in this study. First-strand cDNA was synthesized using the SuperScript First-Strand cDNA Synthesis kit (Invitrogen) according to the manufacturer’s instructions and then stored at -20 °C for subsequent quantitative polymerase chain reaction experiments.
The mRNA expression levels of CK19 and CEA were detected by real-time PCR using the ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, United States). Glyceraldehyde-3-phosphatedehydrogenase (GADPH) was amplified as an internal control to correct for differences in the amount of total RNA per sample by normalizing the mRNA levels of CK19 and CEA to the corresponding GAPDH levels. The relative levels of the normalized gene expression were calculated with the equation 2-ΔCT, in which ΔCT = CT gene- CT control. All of the reactions were run in triplicate. The primers targeting CK19, CEA and GAPDH were as follows: 5’-ATGAAAGCTGCCTTGGAAGA-3’ (CK19, forward) and 5’-TGATTCTGC- CGCTCACTATCAG-3’ (CK19, reverse); 5’-AACTGGTGT- CCCGGATATCA-3’ (CEA, forward) and 5’-ATATTCTTTGCTCCTTGCCA-3’ (CEA, reverse); 5′-AGAAGGCTGGGGCTCATTTG-3′ (GAPDH, forward) and 5′-AGGGGCCATCCACAGTCTTC-3′ (GAPDH, reverse). The following thermocycling conditions were used under the standard mode according to the manufacturer’s recommendations: 30 s at 95 °C followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s.
After curative resection, all patients were followed regularly every 3 mo during the first two years, every 6 mo during the third to fifth years, and every 1 year thereafter until death or the last follow-up. Postoperative follow-up observations involved physical examinations, laboratory blood tests, tumor markers, chest radiography, abdominal computed tomography scan, and endoscopy examinations. The final follow-up date was December 2015. Overall survival (OS) was calculated from the date of surgery to death or the final follow-up date.
All statistical analyses were performed using SPSS 17.0 statistical software (SPSS Inc, Chicago, IL, United States). The receiver operating characteristic (ROC) curves were plotted to determine the optimal cutoff points for pre- and post-CK19 and CEA mRNA levels in predicting 5-year survival. Interdependence between the CK19 mRNA levels, CEA mRNA levels, and clinical data were calculated using the χ2 test and are displayed in cross-tables. Survival analysis was performed using the Kaplan-Meier method and compared by the log-rank test. The variables significantly affecting OS were investigated by multivariate analysis according to the Cox proportional hazard model. P values < 0.05 were considered statistically significant.
A total of 129 patients with GCC were included in our study. There were 27 (20.9%) females and 102 (79.1%) males, and the median age was 61 years (range, 38-84 years). All patients were histologically confirmed with adenocarcinoma after surgery. According to the histopathological grading, well and moderately differentiated tumors were observed in 99 (76.7%) patients, and 30 (23.3%) patients presented poorly differentiated or undifferentiated tumors. Based on the criteria of the 7th edition of the UICC/AJCC TNM staging system, 34 (26.4%) and 95 (73.6%) patients had T1-T2 and T3-4 disease, respectively. Postoperative histological examinations confirmed that lymph node metastasis was present in 51 (39.5%) cases. With regard to the TNM stage, 11 (8.5%) cases were subsequently diagnosed with stage I, 75 (58.1%) with stage II, and 43 (33.3%) with stage III.
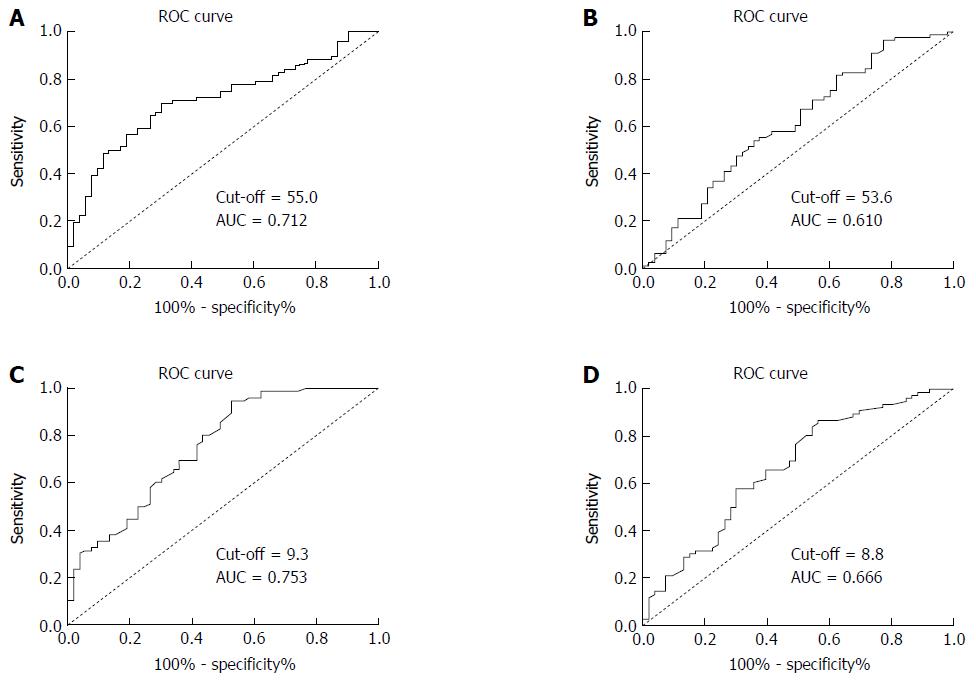
According to the ROC curve shown in Figure 1, the cutoff values of the pre- and post-CK19 mRNA levels were set at 55.0 and 53.6, respectively. Based on these cutoff values in predicting 5-year survival, the sensitivity and specificity were 69.7% and 69.8%, respectively, for pre-CK19 mRNA, and 57.9% and 58.5%, respectively, for post-CK19 mRNA. The corresponding areas under the curve (AUCs) were 0.712 and 0.610, respectively. Thus, we divided the patients into “low pre-CK19” (< 55.0, n = 63) and “high pre-CK19” (> 55.0, n = 66) groups as well as “low post-CK19” (< 53.6, n = 67) and “high post-CK19” (> 53.6, n = 62) groups.
The correlations between the pre- and post-CK19 mRNA levels and the clinicopathological characteristics are shown in Table 1. The pre-CK19 mRNA levels were significantly correlated with tumor size (P = 0.008), lymph node status (P = 0.033), and TNM stage (P = 0.012), but showed no significant correlations with other measured clinicopathological characteristics (P > 0.05 for all). Furthermore, the post-CK19 mRNA levels were only correlated with lymph node status (P = 0.048) and not with any other clinicopathological characteristics measured (P > 0.05 for all).
| Clinicopathological feature | Cases | CK19 mRNA level (pre-) | χ2 test | CK19 mRNA level (post-) | χ2 test | ||||
| Low | High | χ2 | P value | Low | High | χ2 | P value | ||
| Gender | 1.484 | 0.223 | 1.663 | 0.197 | |||||
| Male | 102 | 47 (46.1) | 55 (53.9) | 50 (49.0) | 52 (51.0) | ||||
| Female | 27 | 16 (59.3) | 11 (40.7) | 17 (63.0) | 10 (37.0) | ||||
| Age (yr) | 0.411 | 0.521 | 0.052 | 0.820 | |||||
| ≤ 60 | 59 | 27 (45.8) | 32 (54.2) | 30 (50.8) | 29 (49.2) | ||||
| > 60 | 70 | 36 (51.4) | 34 (48.6) | 37 (52.9) | 33 (47.1) | ||||
| Tumor size (cm) | 6.928 | 0.008 | 3.250 | 0.071 | |||||
| ≤ 4 | 75 | 44 (58.7) | 31 (41.3) | 44 (58.7) | 31 (41.3) | ||||
| > 4 | 54 | 19 (35.2) | 35 (64.8) | 23 (42.6) | 31 (57.4) | ||||
| Histological grade | 0.474 | 0.491 | 0.030 | 0.861 | |||||
| Well/moderately differentiated | 99 | 50 (50.5) | 49 (49.5) | 51 (51.5) | 48 (48.5) | ||||
| Poorly differentiated/undifferentiated | 30 | 13 (43.3) | 17 (6.7) | 16 (53.3) | 14 (46.7) | ||||
| Depth of tumor invasion | 3.088 | 0.079 | 3.015 | 0.082 | |||||
| T1-T2 | 34 | 21 (61.8) | 13 (38.2) | 22 (64.7) | 12 (35.3) | ||||
| T3-T4 | 95 | 42 (44.2) | 53 (55.8) | 45 (47.4) | 50 (52.6) | ||||
| Lymph node status | 4.528 | 0.033 | 3.913 | 0.048 | |||||
| Negative | 78 | 44 (56.4) | 34 (43.6) | 46 (59.0) | 32 (41.0) | ||||
| Positive | 51 | 19 (37.3) | 32 (62.7) | 21 (41.2) | 30 (58.8) | ||||
| TNM stage | 8.861 | 0.012 | 2.848 | 0.241 | |||||
| I | 11 | 9 (81.8) | 2 (18.2) | 7 (63.6) | 4 (36.4) | ||||
| II | 75 | 39 (52.0) | 36 (48.0) | 42 (56.0) | 33 (44.0) | ||||
| III | 43 | 15 (34.9) | 28 (65.1) | 18 (41.9) | 25 (58.1) | ||||
According to the plotted ROC curves (Figure 1C and D), the optimal cutoff values of the pre- and post-CEA mRNA levels were set at 9.3 and 8.8, respectively. Based on these cutoff values in predicting 5-year survival, the sensitivity and specificity were 72.4% and 59.7%, respectively, for pre-CEA mRNA, and 67.1% and 54.7%, respectively, for post-CEA mRNA. The AUCs were 0.753 and 0.666, respectively. Thus, we divided the patients into “low pre-CEA” (< 9.3, n = 52) and “high pre-CEA” (> 9.3, n = 77) groups as well as “low post-CEA” (< 8.8, n = 69) and “high post-CEA” (> 8.8, n = 70) groups.
The associations between the pre- and post CEA mRNA levels and the clinicopathological characteristics are shown in Table 2. Pre-CEA mRNA levels were closely correlated with tumor size (P = 0.031), lymph node status (P = 0.002), and TNM stage (P = 0.048). However, no statistical significance was observed in the correlation between pre- CEA mRNA levels and the other measured clinicopathological features (P > 0.05 for all). Furthermore, there was a significant correlation between post-CEA mRNA levels and tumor size (P = 0.016), lymph node status (P = 0.008), and TNM stage (P = 0.025), but no statistical significance was observed in the correlation between post-CEA mRNA levels and the other measured clinicopathological features (P > 0.05 for all).
| Clinicopathological feature | Cases | CEA mRNA level (pre-) | χ2 test | CEA mRNA level (post-) | χ2 test | ||||
| Low | High | χ2 | P value | Low | High | χ2 | P value | ||
| Gender | 1.891 | 0.169 | 1.327 | 0.249 | |||||
| Male | 102 | 38 (37.3) | 64 (62.7) | 44 (43.1) | 58 (56.9) | ||||
| Female | 27 | 14 (51.9) | 13 (48.1) | 15 (55.6) | 12 (44.4) | ||||
| Age (yr) | 0.413 | 0.521 | 1.121 | 0.290 | |||||
| ≤ 60 | 59 | 22 (37.3) | 37 (62.7) | 24 (40.7) | 35 (59.3) | ||||
| > 60 | 70 | 30 (42.9) | 40 (57.1) | 35 (50.0) | 35 (50.0) | ||||
| Tumor size (cm) | 0.078 | 0.780 | 5.757 | 0.016 | |||||
| ≤ 4 | 75 | 31 (41.3) | 44 (58.7) | 41 (54.7) | 34 (45.3) | ||||
| > 4 | 54 | 21 (38.9) | 33 (61.1) | 18 (33.3) | 36 (66.7) | ||||
| Histological grade | 3.024 | 0.082 | 2.423 | 0.12 | |||||
| Well/moderately differentiated | 99 | 44 (44.4) | 55 (55.6) | 49 (49.5) | 50 (50.5) | ||||
| Poorly differentiated/undifferentiated | 30 | 8 (26.7) | 22 (73.3) | 10 (33.3) | 20 (66.7) | ||||
| Depth of tumor invasion | 4.653 | 0.031 | 0.966 | 0.326 | |||||
| T1-T2 | 34 | 19 (55.9) | 15 (44.1) | 18 (52.9) | 16 (47.1) | ||||
| T3-T4 | 95 | 33 (34.7) | 62 (65.3) | 41 (43.2) | 54 (56.8) | ||||
| Lymph node status | 9.871 | 0.002 | 7.012 | 0.008 | |||||
| Negative | 78 | 40 (51.3) | 38 (48.7) | 43 (55.1) | 35 (44.9) | ||||
| Positive | 51 | 12 (23.5) | 39 (76.5) | 16 (31.4) | 35 (68.6) | ||||
| TNM stage | 6.063 | 0.048 | 7.379 | 0.025 | |||||
| I | 11 | 6 (54.5) | 5 (45.5) | 9 (81.8) | 2 (18.2) | ||||
| II | 75 | 35 (46.7) | 40 (53.3) | 34 (45.3) | 41 (54.7) | ||||
| III | 43 | 11 (25.6) | 32 (74.4) | 16 (37.2) | 27 (62.8) | ||||
The median follow-up period for the entire cohort was 42 mo (range, 6-84 mo). The 5-year OS rate for all the patients was 31.7% with a median survival time of 39 mo.
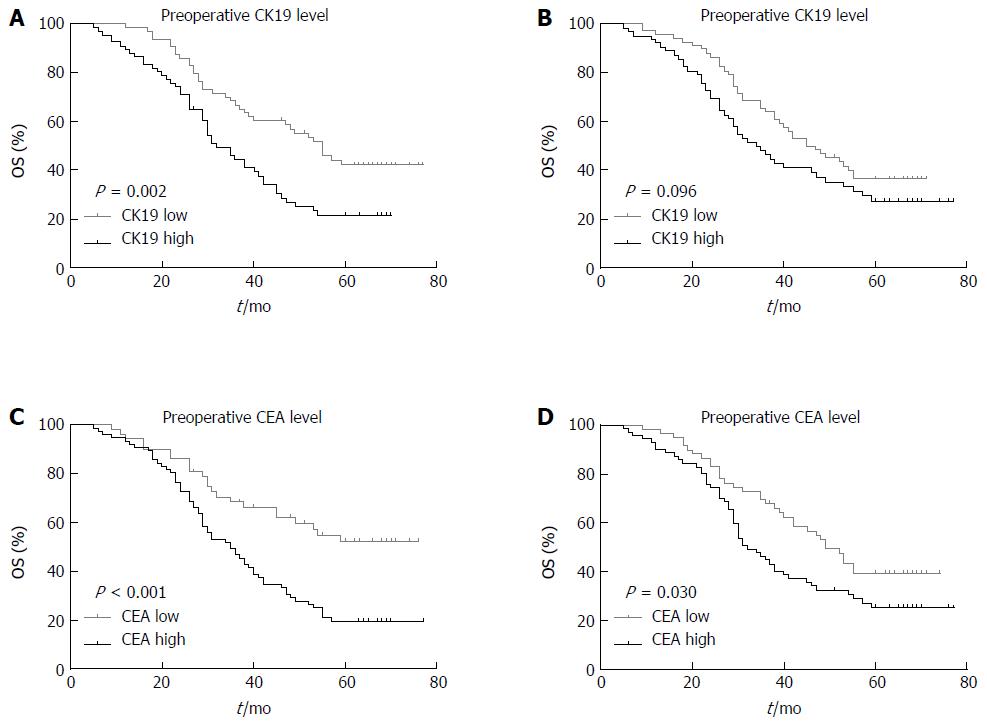
The 5-year OS curves of patients based on the pre- and post-CK19 mRNA and CEA mRNA levels are shown in Figure 2. The 5-year OS rates for the low pre-CK19 and high pre-CK19 groups were 42.1% and 21.5%, respectively (χ2 = 9.183, P = 0.002; Figure 2A). The 5-year OS rates for low post-CK19 and high post-CK19 groups were 36.5% and 27.4%, respectively (χ2 = 2.773, P = 0.096; Figure 2B). The 5-year OS rates for low pre-CEA and high pre-CEA groups were 52.2% and 19.5%, respectively (χ2 = 12.890, P = 0.000, Figure 2C). The 5-year OS rates for low post-CEA and high post-CEA groups were 39.5% and 25.4%, respectively (χ2 = 4.721, P = 0.030; Figure 2D).
Univariate Kaplan-Meier analysis showed that tumor size (P = 0.015), histological grade (P = 0.039), depth of tumor invasion (P = 0.010), lymph node status (P < 0.001), pre-CK19 mRNA level (P = 0.002), pre-CEA mRNA level (P < 0.001), and post-CEA mRNA level (P = 0.030) significantly affected the prognosis of patients with GCC (Table 3).
| Clinicopathological feature | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender | 1.377 (0.797-2.377) | 0.245 | ||
| Age (yr) | 0.812 (0.529-1.247) | 0.336 | ||
| Tumor size(cm) | 1.688 (1.097-2.596) | 0.015 | 1.305 (0.816-2.089) | 0.267 |
| Histological grade | 1.277 (1.008-1.619) | 0.039 | 1.104 (0.860-1.418) | 0.436 |
| Depth of tumor invasion | 1.985 (1.162-3.393) | 0.010 | 1.191 (0.660-2.151) | 0.562 |
| Lymph node status | 2.894 (1.864-4.492) | 0.000 | 1.848 (1.109-3.079) | 0.018 |
| Pre-CK19 mRNA | 1.932 (1.247-2.992) | 0.002 | 1.625 (1.035-2.553) | 0.035 |
| Post-CK19 mRNA | 1.431 (0.932-2.197) | 0.096 | ||
| Pre-CEA mRNA | 2.337 (1.443-3.784) | 0.000 | 1.918 (1.162-3.166) | 0.011 |
| Post-CEA mRNA | 1.609 (1.039-2.492) | 0.030 | 1.213 (0.753-1.955) | 0.427 |
The seven factors with prognostic potential for OS were subsequently subjected to multivariate analysis using the Cox proportional hazards model. As shown in the Table 3, the multivariate survival analysis indicated that lymph node status (P = 0.018), pre-CK19 mRNA levels (P = 0.035), and pre-CEA mRNA levels (P = 0.011) were independent prognostic factors for patients with GCC.
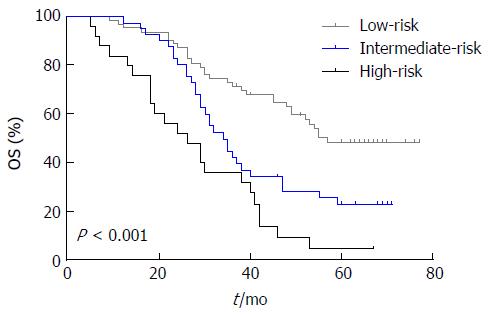
To better identify GCC patients at high risk for lymph node metastasis and poor OS, we proposed a new prognostic model by combining the three identified independent prognostic factors and stratified patients into three groups as follows: the low-risk group, comprising patients with 0 or 1 risk factor; the intermediate-risk group, comprising patients with 2 factors; and the high-risk group, comprising patients with all 3 factors. Finally, there were 57, 34, and 26 patients in the low-, intermediate-, and high-risk groups, respectively. The 5-year OS rates for the low-, intermediate-, and high-risk groups were 48.3%, 22.6%, and 4.6%, respectively, and a statistically significant difference was observed (χ2 = 28.319, P < 0.001; Figure 3).
Invasion and distant metastasis are the leading factors influencing the clinical outcome of patients with GC[16]. Many GC patients with resectable tumors died of postoperative distant metastasis and had a poor prognosis. GCC has been reported to be a distinct clinical entity based on its pathogenesis and risk factors and has a higher incidence of lymph node metastasis and a poorer prognosis than non-cardiac GC[17,18]. Therefore, identifying one method or marker to determine the potential of cancer cell spreading and disease progression in patients with GCC will certainly help to tailor postoperative adjuvant therapies and improve clinical outcomes. However, there are no markers available that can predict lymph node metastasis and evaluate the prognosis of patients with GCC.
Numerous factors are associated with patient prognosis, including the expression of growth factors and their receptors, cell cycle regulators, cell adhesion molecules and matrix-degrading enzymes, all of which play important roles in tumor cell proliferation, invasion, and metastasis[19]. For GC, several tumor-specific markers, including CEA and CK19 in either serum or tumor tissue (as evaluated by either enzyme immunoassay kit or immunohistochemistry, respectively), have been used to detect metastasis and predict prognosis[8,11]. Recently, qRT-PCR has been shown to have a sensitivity 10- to 100-fold higher than the routine immunological methods and to be a reliable method for detecting circulating tumor cells, measuring the mRNA expression of tumor markers, and predicting patient prognosis[10]. In addition, compared with the acquisition of bone marrow, lymph nodes or tumor tissues, peripheral blood collection is a minimally invasive procedure and can be collected throughout the entire disease process[6]. All these data have garnered increasing attention in this field of research to analyze the clinical and prognostic value of CEA and CK19 mRNAs in the peripheral blood of GCC patients by using qRT-PCR.
In the present study, we detected the CK19 and CEA mRNA levels in peripheral blood of GCC patients by using qRT-PCR and analyzed the correlation between CK19 and CEA mRNA levels with clinicopathological variables. Our results showed significant correlations between the pre-CK19 mRNA levels and tumor size, lymph node status, and TNM stage as well as between the pre-CEA mRNA levels and T status, lymph node status, and TNM stage. Furthermore, a significant association could be found between post-CEA mRNA levels and tumor size, lymph node status, and TNM stage. However, the post-CK19 mRNA levels were only correlated with lymph node status. These results indicated that pre- and post-CEA mRNA and CK19 mRNA levels had different clinical values in GCC, and it seems that pre-CEA and CK19 mRNA levels may have a stronger prognostic role than post-CEA and CK19 mRNA levels. During disease progression, tumor cells lose their intercellular adhesion molecules, and unpredictable lymphatic spread can occur. Lymph node metastasis is one of the most important prognostic factors for GCC. However, it is difficult to detect lymph node micrometastasis by routine pathological examination[8]. In the present study, our results showed that both pre- and post-CEA mRNA levels as well as pre- and post-CK19 mRNA levels were associated with positive lymph node metastasis. Our results was similar to the reports by Yanagita et al[20] and Kochi et al[11], who demonstrated that CK19 and CEA mRNA levels in peripheral blood were correlated with lymph node metastasis in GC. CK19, a cytoskeletal protein, is exclusively expressed in epithelial tissues and any tumor tissues derived from the epithelium. If the CK19 transcript is detected in lymph nodes of patients with epithelial tumors, the presence of disseminated cancer cells could be considered[21]. Therefore, our results indicated that both CEA mRNA and CK19 mRNA levels may serve as potential markers to detect lymph node metastasis in GCC.
Previous studies have suggested the potential role of CEA in monitoring disease recurrence and treatment response as well as in predicting the prognosis of many malignancies[22-24]. Cytokeratins are components of epithelial cells and the intermediate filaments of epithelial cancer cells; these proteins are involved in the dynamic remodeling of tumor cells during invasion and metastasis. CK19, a tissue-specific marker for epithelial tumor micrometastasis, has been reported to be a prognostic marker in esophageal cancer[9], hepatocellular carcinoma[25], and lung cancer[21]. Based on the previously published data of CEA and CK19 in other malignancies, we further explored the relationships between pre- and post-CEA and CK19 mRNA levels with OS in patients with GCC. To analyze the prognostic value of pre- and post-CEA and CK19 mRNA levels in GCC, Kaplan-Meier survival analysis and multivariate analysis were performed in the following analysis. The Kaplan-Meier survival analysis showed that both pre- and post-CEA mRNA levels as well as elevated pre-CK19 mRNA levels (but not post-CK19 mRNA levels) were correlated with a poor prognosis in GCC. In addition, the current study showed that the rate of elevated pre-CEA mRNA expression was 59.6%, which was higher than the rate of increased pre-CK19 mRNA expression (51.2%). These results indicated that CEA mRNA levels in peripheral blood of GCC patients may have a stronger prognostic prediction power than CK19 mRNA and that CEA mRNA levels seem to be a potential marker with a high sensitivity for prognostic prediction. Our observation was similar to that from a study by Ikeguchi et al[26], who proposed that CEA was more reliable than cytokeratins in detecting disseminated tumor cells. Multivariate analysis further showed that lymph node status, pre-CK19 mRNA levels and pre-CEA mRNA levels negatively affected the prognosis of GCC patients. These data indicate that pre-CK19 and CEA mRNA levels in peripheral blood of GCC patients who underwent a radical gastrectomy are more accurate indicators of prognosis than post-CK19 and CEA mRNA levels. One reason may be because the levels of CK19 and CEA mRNAs are proportional to the tumor load and TNM stage, and the CK19 and CEA mRNA levels will inevitably decrease after tumor removal. Thus, the pre-CK19 and CEA mRNA levels may have a stronger power to reflect the entire status of disease and predict prognosis compared with post-CK19 and CEA mRNA levels.
In clinical practice, we noticed that GCC patients with three independent risk factors (positive lymph node metastasis, elevated pre-CK19 and CEA mRNA levels) tended to have a poorer prognosis. Therefore, we proposed a prognostic model based on these three risk factors and classified GCC patients into low-, intermediate- and high-risk groups. We further compared the survival curves of the three groups and found that there was a significant difference in OS among the three different risk groups. This prognostic model can be easily constructed and may potentially help clinicians make a more accurate judgment for prognosis and individual therapeutic treatment to improve survival and quality of life based on the risk stratification. GCC patients with a high-risk score may benefit from closer monitoring or more aggressive postoperative adjuvant therapy.
In conclusion, to the best of our knowledge, this is the first report showing that both elevated CK19 and CEA mRNA levels are correlated with positive lymph node metastasis in GCC. Elevated pre-CK19 and CEA mRNA levels were independently associated with poor prognosis in GCC patients undergoing curative surgery. Additionally, the new proposed prognostic model may help clinicians provide better individual therapeutic approaches and improve the outcome of patients with GCC based on the TNM stage.
We would like to thank Yu-Mei Feng from the Department of Biochemistry and Molecular Biology, Tianjin Medical University Cancer Institute and Hospital, for their support and assistance in this study.
Gastric cardia cancer (GCC) is a distinct clinical entity, with a higher incidence of lymph node metastasis and poor prognosis. Meanwhile, there is no valid marker to predict the lymph node metastasis and prognosis in GCC. Carcinoembryonic antigen (CEA) and cytokeratin 19 (CK19) are related to lymph node metastasis and prognosis in gastrointestinal tumors. The level of tumor marker expression will change after surgery. Real-time polymerase chain reaction (qRT-PCR) technology, a sensitive, specific and rapid method, has been widely used to detect the expression of tumor markers. However, the clinical and prognostic significance of CEA and CK19 mRNAs in GCC patients has been few reported.
Recent studies have reported that pre- or post-CEA and CK19 mRNA levels are correlated with lymph node metastasis and prognosis in gastric cancer. However, few studies have reported and compared the clinical and prognostic significance of pre- and post-CEA and CK19 mRNA levels in GCC.
qRT-PCR, a sensitive, specific and rapid method, was used to detect the expression of CEA and CK19 mRNAs in GCC. In addition, this study is the first to report and compare the clinical and prognostic significance of pre- and post-CEA and CK19 mRNA levels in GCC.
These results provide evidence that either pre- and post-CEA or pre- and post-CK19 mRNA levels could help predict lymph node metastasis in patients with GCC, and the prognostic value of pre-CEA and pre-CK19 mRNA levels supersedes that of post-CEA and post-CK19 mRNA levels for clinical applications.
CK19, a cytoskeletal protein, is exclusively expressed in epithelial tissues and any tumor tissues derived from the epithelium. CEA is a glycoprotein found in epithelial cells of colon cancer and colonic mucosa.
This is a strong study that evaluated the clinical and prognostic significance of pre- and post-CK19 and CEA mRNA levels in GCC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hasan M, Garcia-Olmo D S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20469] [Article Influence: 2046.9] [Reference Citation Analysis (19)] |
| 2. | Cai L, Zheng ZL, Zhang ZF. Risk factors for the gastric cardia cancer: a case-control study in Fujian Province. World J Gastroenterol. 2003;9:214-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Kubo A, Corley DA. Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer. 2002;95:2096-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Hsu NY, Chow KC, Chen WJ, Lin CC, Chou FF, Chen CL. Expression of nm23 in the primary tumor and the metastatic regional lymph nodes of patients with gastric cardiac cancer. Clin Cancer Res. 1999;5:1752-1757. [PubMed] |
| 5. | Green D, Ponce de Leon S, Leon-Rodriguez E, Sosa-Sanchez R. Adenocarcinoma of the stomach: univariate and multivariate analysis of factors associated with survival. Am J Clin Oncol. 2002;25:84-89. [PubMed] |
| 6. | Wu CH, Lin SR, Hsieh JS, Chen FM, Lu CY, Yu FJ, Cheng TL, Huang TJ, Huang SY, Wang JY. Molecular detection of disseminated tumor cells in the peripheral blood of patients with gastric cancer: evaluation of their prognostic significance. Dis Markers. 2006;22:103-109. [PubMed] |
| 7. | Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Ru Y, Zhang L, Chen Q, Gao SG, Wang GP, Qu ZF, Shan TY, Qian N, Feng XS. Detection and clinical significance of lymph node micrometastasis in gastric cardia adenocarcinoma. J Int Med Res. 2012;40:293-299. [PubMed] |
| 9. | Qiao YF, Chen CG, Yue J, Ma Z, Yu ZT. Clinical significance of preoperative and postoperative cytokeratin 19 messenger RNA level in peripheral blood of esophageal cancer patients. Dis Esophagus. 2016;29:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Yeh KH, Chen YC, Yeh SH, Chen CP, Lin JT, Cheng AL. Detection of circulating cancer cells by nested reverse transcription-polymerase chain reaction of cytokeratin-19 (K19)--possible clinical significance in advanced gastric cancer. Anticancer Res. 1998;18:1283-1286. [PubMed] |
| 11. | Kochi M, Fujii M, Kanamori N, Kaiga T, Kawakami T, Aizaki K, Kasahara M, Mochizuki F, Kasakura Y, Yamagata M. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer. 2000;3:177-186. [PubMed] |
| 12. | Wang W, Chen XL, Zhao SY, Xu YH, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget. 2016;7:35423-35436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Lee EC, Yang JY, Lee KG, Oh SY, Suh YS, Kong SH, Yang HK, Lee HJ. The value of postoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the early detection of gastric cancer recurrence after curative resection. J Gastric Cancer. 2014;14:221-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1893] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 15. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6431] [Article Influence: 428.7] [Reference Citation Analysis (0)] |
| 16. | Tang Z, Sheng H, Zheng X, Ying L, Wu L, Liu D, Liu G. Upregulation of circulating cytokeratin 20, urokinase plasminogen activator and C-reactive protein is associated with poor prognosis in gastric cancer. Mol Clin Oncol. 2015;3:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Jang KY, Hwang SH, Kwon KS, Kim KR, Choi HN, Lee NR, Kwak JY, Park BH, Park HS, Chung MJ. SIRT1 expression is associated with poor prognosis of diffuse large B-cell lymphoma. Am J Surg Pathol. 2008;32:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Feng AN, Zhang LH, Fan XS, Huang Q, Ye Q, Wu HY, Yang J. Expression of SIRT1 in gastric cardiac cancer and its clinicopathologic significance. Int J Surg Pathol. 2011;19:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Yanagita S, Uenosono Y, Arigami T, Daisuke M, Okubo K, Kijima T, Arima H, Hirata M, Haraguchi N, Hagihara T. The clinical usefulness of the intraoperative detection of sentinel lymph node metastases by a rapid RT-PCR system in patients with gastric cancer. Cancer. 2016;122:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Ge MJ, Wu QC, Wang M, Zhang YH, Li LB. Detection of disseminated lung cancer cells in regional lymph nodes by assay of CK19 reverse transcriptase polymerase chain reaction and its clinical significance. J Cancer Res Clin Oncol. 2005;131:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Chae HD, Kim IH. Prognostic significance of CEA expression by RT-PCR in peritoneal wash from patients with gastric cancer: result of a 5-year follow-up after curative resection. Scand J Gastroenterol. 2016;51:956-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Nam DH, Lee YK, Park JC, Lee H, Shin SK, Lee SK, Lee YC, Cheong JH, Hyung WJ, Noh SH. Prognostic value of early postoperative tumor marker response in gastric cancer. Ann Surg Oncol. 2013;20:3905-3911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 503] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 25. | Yang XR, Xu Y, Shi GM, Fan J, Zhou J, Ji Y, Sun HC, Qiu SJ, Yu B, Gao Q. Cytokeratin 10 and cytokeratin 19: predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res. 2008;14:3850-3859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Ikeguchi M, Ohro S, Maeda Y, Fukuda K, Yamaguchi K, Shirai H, Kondo A, Tsujitani S, Kaibara N. Detection of cancer cells in the peripheral blood of gastric cancer patients. Int J Mol Med. 2003;11:217-221. [PubMed] |