Published online Aug 14, 2017. doi: 10.3748/wjg.v23.i30.5530
Peer-review started: March 17, 2017
First decision: May 16, 2017
Revised: May 26, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: August 14, 2017
To investigate whether autophagic cell death is involved in hyperthermic sensitization to ionizing radiation in human hepatocellular carcinoma cells, and to explore the underlying mechanism.
Human hepatocellular carcinoma cells were treated with hyperthermia and ionizing radiation. MTT and clonogenic assays were performed to determine cell survival. Cell autophagy was detected using acridine orange staining and flow cytometric analysis, and the expression of autophagy-associated proteins, LC3 and p62, was determined by Western blot analysis. Intracellular reactive oxygen species (ROS) were quantified using the fluorescent probe DCFH-DA.
Treatment with hyperthermia and ionizing radiation significantly decreased cell viability and surviving fraction as compared with hyperthermia or ionizing radiation alone. Cell autophagy was significantly increased after ionizing radiation combined with hyperthermia treatment, as evidenced by increased formation of acidic vesicular organelles, increased expression of LC3II and decreased expression of p62. Intracellular ROS were also increased after combined treatment with hyperthermia and ionizing radiation. Pretreatment with N-acetylcysteine, an ROS scavenger, markedly inhibited the cytotoxicity and cell autophagy induced by hyperthermia and ionizing radiation.
Autophagic cell death is involved in hyperthermic sensitization of cancer cells to ionizing radiation, and its induction may be due to the increased intracellular ROS.
Core tip: Increased cell autophagy and intracellular reactive oxygen species (ROS), accompanied by decreased cell viability and surviving fraction, were observed in HepG2 cells treated with hyperthermia and ionizing radiation. Pretreatment with N-acetylcysteine, an ROS scavenger, markedly inhibited the above cytotoxicity and cell autophagy. The results suggest that autophagic cell death is involved in the hyperthermic sensitization to ionizing radiation, and its induction may be due to the increased intracellular ROS.
- Citation: Yuan GJ, Deng JJ, Cao DD, Shi L, Chen X, Lei JJ, Xu XM. Autophagic cell death induced by reactive oxygen species is involved in hyperthermic sensitization to ionizing radiation in human hepatocellular carcinoma cells. World J Gastroenterol 2017; 23(30): 5530-5537
- URL: https://www.wjgnet.com/1007-9327/full/v23/i30/5530.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i30.5530
Hepatocellular carcinoma (HCC) is the third most common cancer and the second leading cause of cancer-associated mortality in China[1]. A majority of patients with HCC present unresectable or advanced disease at the time of diagnosis; the choice of treatment is limited and the prognosis is poor[2]. With the development of radiotherapy techniques, including intensity-modulated radiotherapy (IMRT) and stereotactic body radiation therapy (SBRT), the use of radiotherapy has increasingly been adopted for the treatment of HCC[3]. Hyperthermia, elevation of temperature inside tumor up to 40-42 °C, is an effective treatment modality for cancer[4]. Hyperthermia is a potent radiation sensitizer[5], and its combination with radiotherapy is a promising method for cancer treatment. Several studies have demonstrated the efficacy of combined treatment with radiotherapy and hyperthermia against HCC, head and neck cancer, breast cancer, and melanoma[6-9]. Hyperthermia can increase tumor perfusion and oxygenation, and inhibit repair of DNA damage in tumor cells, all of which may enhance tumor radiosensitivity[10,11]. However, the underlying mechanisms of the radio-sensitizing effect of hyperthermia are not fully elucidated.
Autophagy is an evolutionarily conserved process in which cellular organelles and long-lived proteins are sequestered into double-membrane vesicles, the autophagosomes, and subsequently delivered to the lysosomes to be degraded or recycled[12]. It can be induced by a variety of stimuli, such as nutrient deprivation, hypoxia, reactive oxygen species (ROS), protein aggregates, and damaged organelles[13]. Anticancer therapies, such as chemotherapy, radiotherapy and hyperthermia, are also shown to induce autophagy within tumor cells[14-16]. Studies have shown that increased basal autophagy is required for cells to survive after physical or chemical damage[17]. However, excessive autophagy can induce type II programmed cell death (autophagic cell death), a form of nonapoptotic cell death[18]. In the present study, we showed that autophagic cell death is involved in hyperthermic sensitization to ionizing radiation in human HCC cells, and its induction may be due to the increased intracellular ROS.
Human HCC cell line HepG2 was obtained from Wuhan University Cell Center. Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL streptomycin and 100 U/mL penicillin in a humidified 5% CO2 atmosphere at 37 °C.
Water-bath warming was performed and the temperature was controlled at 43 °C. Cell culture bottles were packaged and put into the water at 43 °C for 0.5 h. Then cells were irradiated with 4 Gy of 6 MV X-ray beam (field, 10 cm × 10 cm; source-to-surface distance, 100 cm; dose rate, 0.8 Gy/min), using a Varian Clinac 23iX (Varian Medical Systems, Inc.) at room temperature. The period between the two treatments was less than 1 h. After irradiation, the cells were immediately returned to the cell incubator and incubated at 37 °C for 72 h.
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] colorimetric assay was used to determine the survival rate of cells. After the above treatment, the cells were washed with phosphate buffered saline (PBS), and incubated with 1 × MTT at 37 °C for 4 h. Then the absorbance at 570 nm was measured, and the survival rate of the cells was calculated according to the following equation: survival rate = (experimental absorbance value/control absorbance value) × 100%.
After treatment with hyperthermia or ionizing radiation, the cells were trypsinized into single-cell suspension, plated in 60-mm dishes and incubated for 14 d to allow for colony growth. Then, the cells were fixed and stained with crystal violet, and colonies having at least 50 cells were counted using a microscope.
Formation of acidic vesicular organelles, a morphological characteristic of autophagy, was detected using acridine orange staining. Cells were incubated with acridine orange (Invitrogen) at 1 μg/mL for 15 min. Red (650 nm, stained cytoplasmic vesicles) vs green (510-530 nm, stained nuclei) fluorescence (FL3/FL1) from cells illuminated with blue (488 nm) excitation light was measured with a FACScan flow cytometer (Beckman Coulter, Brea, CA, United States). The data are presented as the fold changes with an arbitrary setting of autophagy in cells without treatment of drug, hyperthermia or radiation.
Protein lysates were prepared using a total protein extraction kit (ProMab, SJ-200501), and stored at -20 °C until assay. The protein concentrations were assayed using the Bradford method. Equivalent aliquots of protein were separated by 10% SDS-PAGE, and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in PBS for 2 h at 37 °C, washed with PBST (PBS with Tween 20) and incubated with rabbit polyclonal antibody against LC3 (dilution 1:500, CST) or p62 (dilution 1:500, CST) or mouse polyclonal antibody against GAPDH (glyceraldehyde 3-phosphate dehydrogenase, dilution 1:800, SANTA) at 4 °C overnight. After washing with PBST four times, the membranes were incubated with a secondary antibody (HRP-conjugated goat anti-rabbit IgG, SANTA, dilution 1:40000, for LC3 and p62; goat anti-mouse IgG, ZYMED, dilution 1:80000, for GAPDH) for 1 h at room temperature. The immunoreactive proteins were detected using an enhanced chemiluminescent detection system.
Intracellular ROS were measured using a ROS assay kit. After the above designated treatment, the cells were harvested and incubated with 10 μmol/L of DCFH-DA (a fluorescent probe, which may be oxidized by ROS in viable cells to 2’,7’-dichlorofluorescein, DCF) for 30 min at 37 °C. After washing three times with PBS, DCF fluorescence was quantified with a multi-detection microplate reader (485 nm excitation and 535 nm emission).
N-acetylcysteine is an ROS scavenger. Cells were pretreated with N-acetylcysteine (10 mmol/L) for 1 h and then treated with hyperthermia or ionizing radiation as above.
Data were pooled from at least three independent experiments, and presented as mean ± SD unless otherwise indicated. Differences between groups were analyzed using one-way analysis of variance (ANOVA). All the statistical analyses were performed with SPSS13.0. P values less than 0.05 were considered statistically significant.
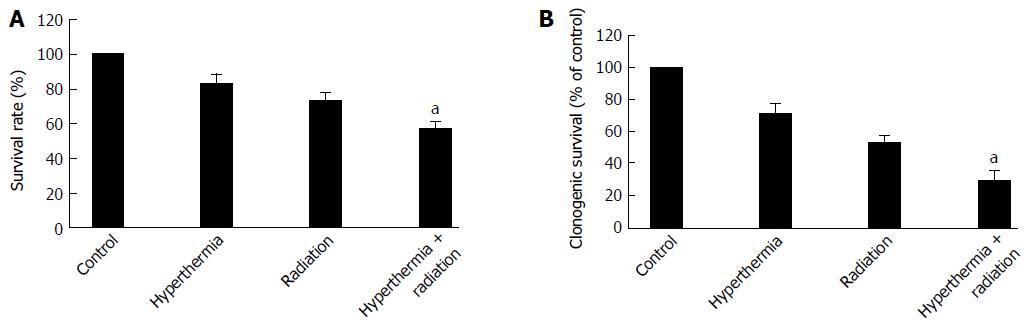
The cytotoxicity induced by ionizing radiation with or without hyperthermia was assessed by MTT and clonogenic survival assays. As shown in Figure 1A, cell viability was decreased when the cells were treated with ionizing radiation or hyperthermia. The cell viability was significantly decreased after combined treatment with ionizing radiation and hyperthermia when compared with each treatment alone. Furthermore, the clonogenic survival of the cells was also significantly decreased after ionizing radiation with hyperthermia as compared with radiation alone (Figure 1B).
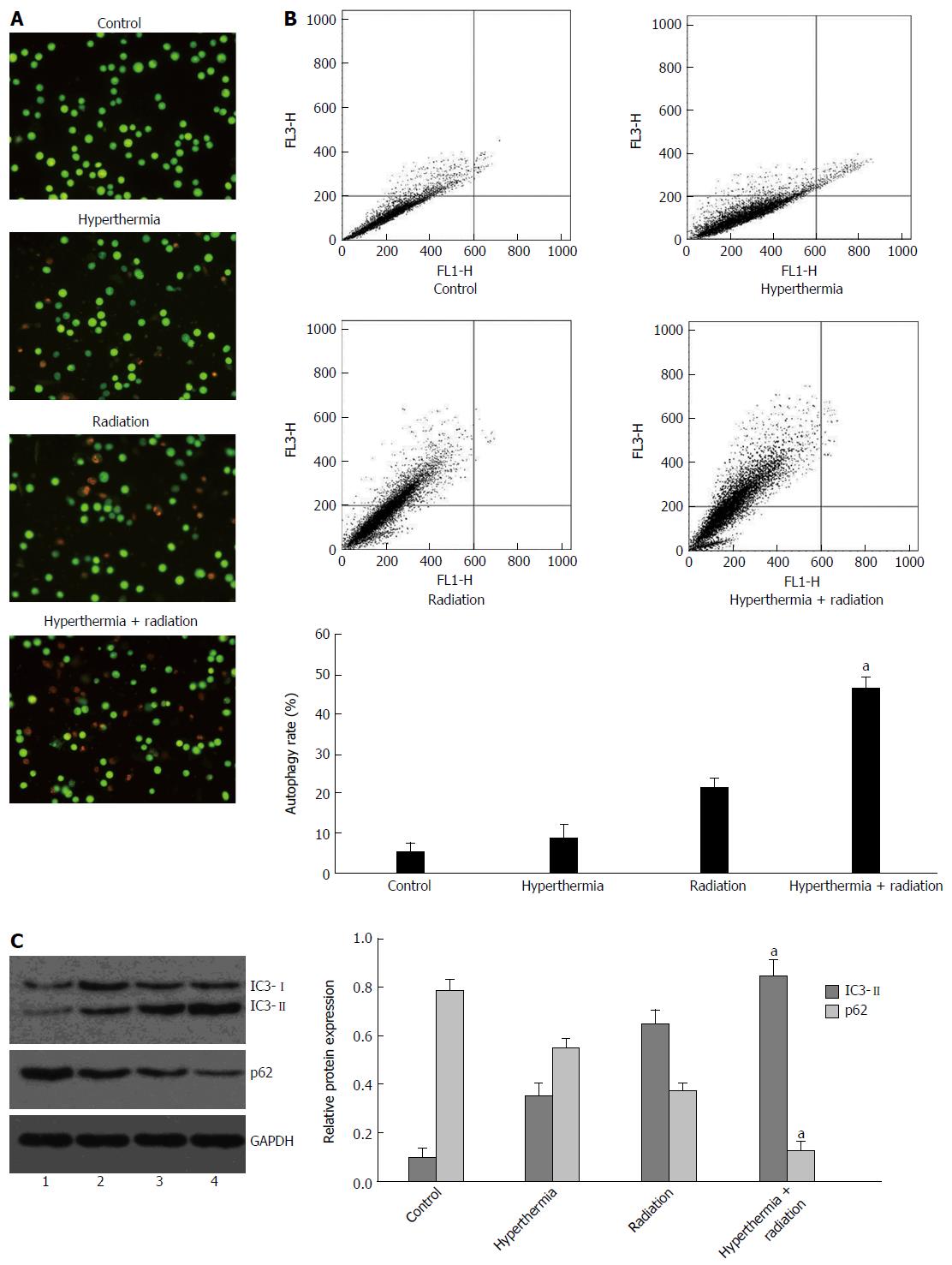
Cell autophagy is characterized by the formation of numerous acidic vesicular organelles, which can be detected using acridine orange staining[19]. The acridine orange staining was quantified using flow cytometry. No obvious increase in cell autophagy was observed in HepG2 cells following 2 Gy ionizing radiation, or until 48 h after 4 Gy ionizing radiation. Therefore, in the present study, 4 Gy ionizing radiation was given to cells, and the cells were tested 72 h after ionizing radiation. As shown in Figure 2A and B, cell autophagy was significantly increased after combined treatment with ionizing radiation and hyperthermia compared with each treatment alone.
The expression of autophagy-related proteins was also detected by Western blot, among which the increase of LC3II protein and reduction of p62 protein are the hallmarks of the induction of autophagy[19]. A significant increase in LC3II expression and a reduction of p62 expression were observed in cells undergoing combined treatment as compared with those receiving ionizing radiation or hyperthermia treatment alone (Figure 2C).
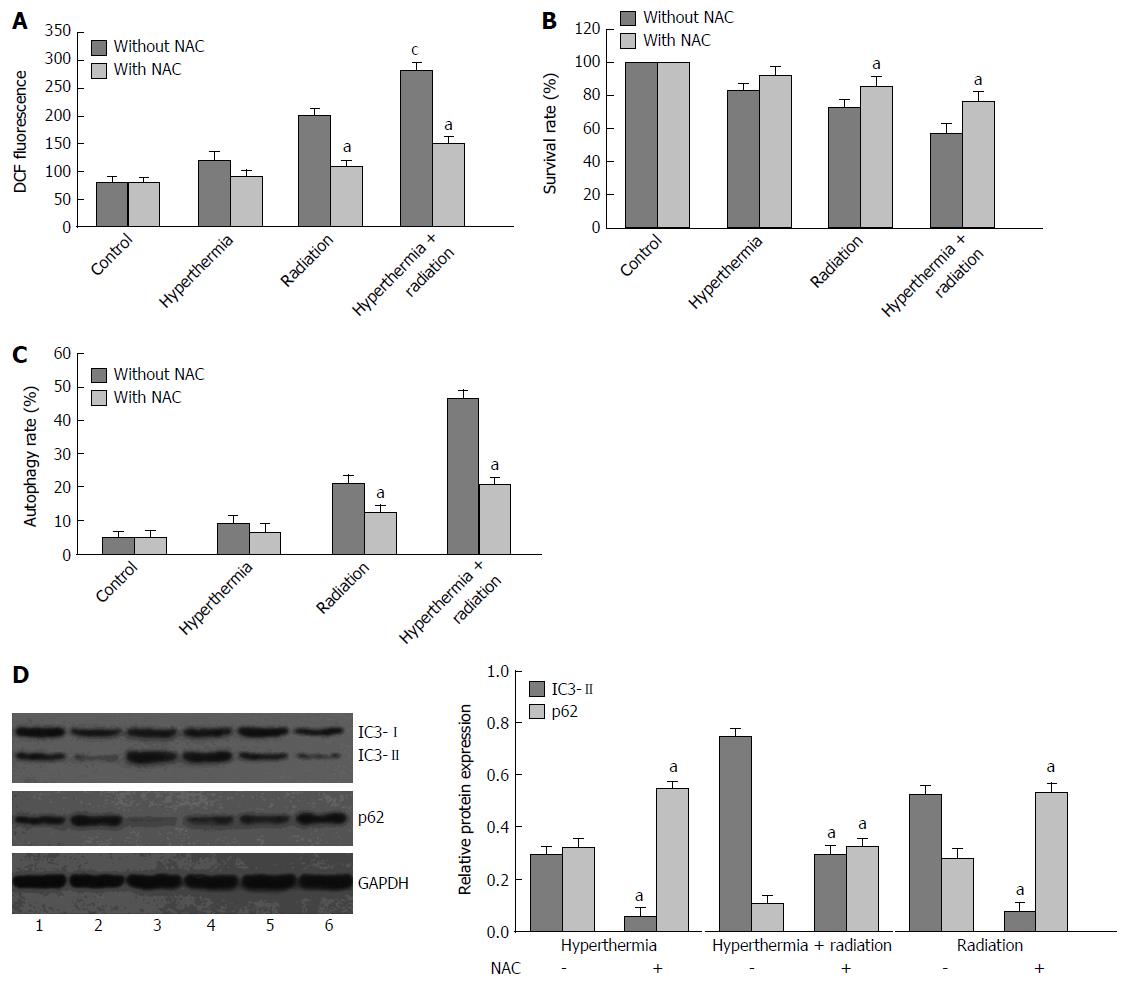
The intracellular ROS in ionizing radiation or hyperthermia-treated HepG2 cells were measured using a fluorescent probe, DCFH-DA. Treatment with ionizing radiation or hyperthermia induced an increase in intracellular ROS content, which was further increased by the combined treatment; however, the increase was completely inhibited by N-acetylcysteine, an ROS scavenger (Figure 3A).
To evaluate whether ionizing radiation or hyperthermia-induced autophagic cell death is related to intracellular ROS in HepG2 cells, pretreatment with N-acetylcysteine was performed. N-acetylcysteine pretreatment significantly improved cell viability in ionizing radiation or hyperthermia-treated HepG2 cells using MTT assay (Figure 3B). Furthermore, the autophagy rate was decreased (Figure 3C), and the expression of autophagy-related proteins, LC3II and p62, was reversed by N-acetylcysteine pretreatment in the ionizing radiation or hyperthermia-treated HepG2 cells (Figure 3D), suggesting that cell autophagy was inhibited.
In the past, radiotherapy was less considered in the treatment of HCC due to the belief that HCC is not a “radiosensitive” tumor and that radiotherapy is too “toxic” for the liver. However, recent studies have demonstrated that HCC is a radiosensitive tumor and its radiosensitivity is equivalent to poorly differentiated squamous cell carcinomas[20]. With the development of radiotherapy techniques, including IMRT and SBRT, a high radiation dose can be delivered to the tumor while the dose to the normal tissues can be simultaneously reduced. Therefore, at present radiotherapy is increasingly used for the treatment of HCC[3]. In the present study, we observed that the cell viability and clonogenic survival of HCC cells were decreased following ionizing radiation, suggesting that HCC cells are sensitive to radiation. Hyperthermia is a potent radiation sensitizer[5], and combined treatment with radiotherapy and hyperthermia has been shown to be efficacious against HCC[6]. Our study also showed that ionizing radiation combined with hyperthermia led to a significant decrease in the cell viability and clonogenic survival of HCC cells as compared with each treatment alone.
Autophagy is a major intracellular degradation mechanism for long-lived proteins and cytoplasmic organelles, the products of which are recycled to maintain cellular homeostasis. Increased basal autophagy is a cell survival mechanism in response to several stresses, such as nutrient deprivation, hypoxia, damaged mitochondria, protein aggregation and pathogens[17,21]. However, excessive autophagy may result in cell death, which is designated as type II programmed cell death (autophagic cell death)[18]. Autophagy is frequently activated in tumor cells following anticancer therapies, such as chemotherapy, radiotherapy and hyperthermia[14-16]. Although several studies showed that induction of autophagy after irradiation acts as a protective and prosurvival mechanism and contributes to radioresistance in breast tumor cells and glioma cells[22,23], most experimental data have suggested that radiation-induced autophagy in cancer cell lines is related to cell death mechanisms and that autophagy-inducing agents may act as radiosensitizers[24]. In the present study, radiation-induced autophagy was significantly increased after combined treatment with hyperthermia in HCC cells, as evidenced by increased formation of acidic vesicular organelles, increased expression of LC3II and decreased expression of p62. The increased autophagy was accompanied with decreased cell viability and clonogenic survival in HCC cells, suggesting that autophagic cell death is involved in the enhancement of cellular radiosensitivity by hyperthermia.
ROS are small and highly reactive molecules that can oxidize proteins, DNA and lipids. They are generated as by-products of cellular metabolism primarily in mitochondria, and can also be produced in response to a variety of stimuli such as growth factors, inflammatory cytokines, ionizing radiation, chemotherapy agents and toxins[25]. Hyperthermia has been also shown to induce production of ROS. A sharp increase in ROS generation has been observed after hyperthermia in a cellular model, using electron paramagnetic resonance spin trapping[26]. In the present study, we showed that intracellular ROS content was increased after treatment with ionizing radiation or hyperthermia, and was further increased by their combined treatment. Once ROS are produced, they act as signaling molecules that trigger diverse physiological and pathological responses, including induction of autophagy. Accumulating evidence has shown that oxidative stress is the converging point of many different inducers of autophagy, such as nutrient deprivation, viral infection and genotoxic stress[27]. In the present study, we found that pretreatment with N-acetylcysteine, an ROS scavenger, abolished the induction of autophagy by ionizing radiation or hyperthermia, and improved cell viability of HepG2 cells after the above treatment. These facts suggest that the induction of autophagic cell death by ionizing radiation and hyperthermia treatment in HCC cells is due to the increased intracellular ROS.
In conclusion, autophagic cell death is involved in hyperthermic sensitization of cancer cells to ionizing radiation, and its induction may be due to the increased intracellular ROS.
With the development of radiotherapy techniques, radiotherapy has increasingly been used for the treatment of hepatocellular carcinoma (HCC). Hyperthermia is a useful adjuvant to radiation therapy in the treatment of many cancers, and it is a potent radiation sensitizer. However, the underlying mechanisms of the radio-sensitizing effect of hyperthermia are not fully elucidated. Autophagy is a major intracellular degradation mechanism for long-lived proteins and cytoplasmic organelles, and excessive autophagy may result in cell death, which is designated as type II programmed cell death (autophagic cell death). The role autophagic cell death in hyperthermic sensitization to ionizing radiation has not been explored.
Many studies have shown that radiation-induced autophagy is related to cell death mechanisms in cancer cell lines and that autophagy-inducing agents may act as radiosensitizers. Oxidative stress has been proven to be the converging point of many different inducers of autophagy, such as nutrient deprivation, viral infection and genotoxic stress.
The novel findings of this study are that autophagic cell death is involved in hyperthermic sensitization to ionizing radiation, and its induction may be due to the increased intracellular reactive oxygen species.
The study provides a novel theoretical basis for hyperthermia in the treatment of malignant tumors, especially combined with radiotherapy.
Autophagy is an evolutionarily conserved process in which cellular organelles and long-lived proteins are sequestered into double-membrane vesicles, the autophagosomes, and subsequently delivered to the lysosomes to be degraded or recycled. Autophagic cell death is a kind of nonapoptotic programmed cell death (also known as type II programmed cell death), characterized by using autophagosome to degrade cell content in dying cells.
HCC is still the object of research and this paper provides some information on the mechanism of hyperthermic sensitization of cancer cells to ionizing radiation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Desiderio J, Sun BC S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Chen W, Zheng R, Zeng H, Zhang S. The incidence and mortality of major cancers in China, 2012. Chin J Cancer. 2016;35:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [PubMed] [Cited in This Article: ] |
| 3. | Park SH, Kim JC, Kang MK. Technical advances in external radiotherapy for hepatocellular carcinoma. World J Gastroenterol. 2016;22:7311-7321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 4. | Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Semin Oncol. 2014;41:714-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 2007;19:418-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Dong Y, Wu G. Analysis of short and long term therapeutic effects of radiofrequency hyperthermia combined with conformal radiotherapy in hepatocellular carcinoma. J BUON. 2016;21:407-411. [PubMed] [Cited in This Article: ] |
| 7. | Datta NR, Rogers S, Ordóñez SG, Puric E, Bodis S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Datta NR, Puric E, Klingbiel D, Gomez S, Bodis S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94:1073-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Werthmöller N, Frey B, Rückert M, Lotter M, Fietkau R, Gaipl US. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int J Hyperthermia. 2016;32:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, Evans SS, Hylander BL, Repasky EA. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011;71:3872-3880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Oei AL, Vriend LE, Crezee J, Franken NA, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. 2015;10:165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Lin L, Baehrecke EH. Autophagy, cell death, and cancer. Mol Cell Oncol. 2015;2:e985913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | McEwan DG, Dikic I. The Three Musketeers of Autophagy: phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011;21:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Notte A, Leclere L, Michiels C. Autophagy as a mediator of chemotherapy-induced cell death in cancer. Biochem Pharmacol. 2011;82:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Gewirtz DA, Hilliker ML, Wilson EN. Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother Oncol. 2009;92:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Zhao Y, Gong S, Shunmei E, Zou J. Induction of macroautophagy by heat. Mol Biol Rep. 2009;36:2323-2327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Anding AL, Baehrecke EH. Autophagy in Cell Life and Cell Death. Curr Top Dev Biol. 2015;114:67-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Kang C, Avery L. To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4:82-84. [PubMed] [Cited in This Article: ] |
| 19. | Zhang Z, Singh R, Aschner M. Methods for the Detection of Autophagy in Mammalian Cells. Curr Protoc Toxicol. 2016;69:20.12.1-20.12.26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010;25:664-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Song C, Song C, Tong F. Autophagy induction is a survival response against oxidative stress in bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2014;16:1361-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Chaachouay H, Ohneseit P, Toulany M, Kehlbach R, Multhoff G, Rodemann HP. Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother Oncol. 2011;99:287-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, Mikkelsen T, Brodie C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125:717-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 24. | Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Autophagy. 2009;5:442-450. [PubMed] [Cited in This Article: ] |
| 25. | Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:646354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 486] [Cited by in F6Publishing: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 26. | Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014;30:513-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 413] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 27. | Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1051] [Cited by in F6Publishing: 1306] [Article Influence: 130.6] [Reference Citation Analysis (0)] |