Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4759
Peer-review started: January 18, 2017
First decision: February 9, 2017
Revised: March 8, 2017
Accepted: June 9, 2017
Article in press: June 12, 2017
Published online: July 14, 2017
To evaluate sustained viral response (SVR) of 8-wk ledipasvir/sofosbuvir therapy among non-cirrhotic, genotype-1 hepatitis C virus (HCV) patients with RNA < 6 million IU/mL.
We performed a retrospective cohort study to examine SVR rates, predictors of treatment failure and safety analysis of 8-wk ledipasvir/sofosbuvir (LDV/SOF) therapy among non-cirrhotic, genotype 1 HCV patients with viral load < 6 million IU/mL. Primary outcome was an achievement of SVR at 12 wk after treatment. Secondary outcomes were identifying predictors of treatment failure and adverse events during treatment.
Total 736 patients: 55% males, 51% Caucasians and 65% were genotype 1a. Non-cirrhotic state of 53% was determined by clinical judgment (imaging, AST, platelet count) and 47% had documented liver fibrosis testing (biopsy, vibration-controlled transient elastography, serum biomarkers). Overall SVR12 was 96%. No difference in SVR12 was seen between patients whose non-cirrhotic state was determined by clinical judgment and patients who had fibrosis testing. Age groups, gender, ethnicity and genotype 1 subtype did not predict SVR. Non-cirrhotic state determined by clinical judgment based on simple, non-invasive tests were not associated with lower SVR [OR = 1.02, 95%CI: 0.48-2.17, P = 0.962]. The AUROC for hepatitis C RNA viral load was 0.734 (P < 0.001, 95%CI: 0.66-0.82). HCV RNA 2.2 million IU/mL was identified as the cutoff value with sensitivity 73% and specificity 64%. HCV RNA < 2.2 million IU/mL was associated with significantly higher SVR 98% with OR = 0.22 (95%CI: 0.1-0.49, P < 0.001) compared to SVR 92% in HCV RNA ≥ 2.2 million IU/mL. No death or morbidities were reported.
Our outcomes validate safety and effectiveness of 8-wk LDV/SOF therapy in non-cirrhotic, untreated HCV genotype 1 patients with HCV RNA < 6 million IU/mL.
Core tip: We highlight that sustained viral response (SVR) outcomes in patients with their non-cirrhotic status determined by clinical judgment using simple, cheap, non-invasive tests such as platelet count, sonographic finding of spleen size and hepatic morphology, are comparable with those who had specialized tests such as liver biopsy, vibration-controlled transient elastography or specialized serum biomarker test. We also validate the fact that hepatitis C virus (HCV) RNA plays a role in predicting SVR (AUROC = 0.743, 95%CI: 0.66-0.82) with a cutoff value of 2.2 million IU/mL. Significantly higher 98% SVR was observed among HCV RNA < 2.2 million IU/mL, compared to 92% SVR with HCV RNA ≥ 2.2 million IU/mL.
- Citation: Latt NL, Yanny BT, Gharibian D, Gevorkyan R, Sahota AK. Eight-week ledipasvir/sofosbuvir in non-cirrhotic, treatment-naïve hepatitis C genotype-1 patients with hepatitis C virus-RNA < 6 million: Single center, real world effectiveness and safety. World J Gastroenterol 2017; 23(26): 4759-4766
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4759.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4759
Hepatitis C virus (HCV) infection is a major cause of cirrhosis, hepatocellular carcinoma and liver-related mortality[1]. Recent studies estimate the prevalence of HCV to be between 1.2%-1.5% in the United States, which is approximately 4.5-5 million. This population is composed of 1 million incarcerated/homeless individuals, hospitalized patients, and people living on Indian reservations, and also includes 3.6 million from the 2003-2010 National Health and Nutrition Examination Survey[2,3]. The prevalence of HCV is declining, but HCV-related cirrhosis is still expected to peak in the year 2020[4]. Therefore, effective, safe and well-tolerated treatment regimens with shorter duration are urgently needed.
Hepatitis C treatment has evolved from a 78-wk interferon monotherapy to 48-wk pegylated interferon plus ribavirin therapy and now 12-wk therapy with newer all-oral direct-acting antiviral (DAA) agents. DAA regimens have revolutionized the treatment of hepatitis C with their excellent sustained virologic response (SVR), tolerable side effect profiles and shorter duration of therapy. Despite the high cost of newer DAAs, a cost-effective analysis has demonstrated that ledipasvir/sofosbuvir (LDV/SOF)-based regimens will reduce long-term HCV-related complications and are cost-effective in the majority of chronic HCV patients [5].
ION-3 trial demonstrated that an 8-wk LDV/SOF therapy in non-cirrhotic, treatment naïve genotype 1 HCV patients with HCV RNA < 6 million IU/mL is non-inferior to 12-wk LDV/SOF therapy (SVR: 94% vs 95%)[6]. The shorter duration of treatment can remarkably increase patient compliance and substantially reduce treatment cost. Real-world studies have reported that SVR rates are comparable to those observed in ION-3 trial[7-10]. However, conflicting data has been reported by a large real-world cohort from Veteran’s Affairs in which researchers found that non-cirrhotic patients with HCV-RNA < 6 million IU/mL were less likely to achieve SVR with 8-wk LDV/SOF treatment compared to 12-wk treatment [9].
Eight-week LDV/SOF therapy for non-cirrhotic, genotype 1 HCV patients is not included in HCV guidelines by American Association for the Study of Liver Diseases and Infectious Diseases Society of America due to lack of real-world validation for comparable SVR with 12-wk therapy[11]. European Association for the Study of the Liver and United States Food and Drug Administration recommend considering 8-wk therapy with caution in treatment-naïve genotype-1 HCV patients without cirrhosis who have pre-treatment viral load < 6 million IU/mL[12,13]. We contemplated that shorter duration of treatment could provide the lower cost, the higher patient compliance and adherence as long as the shorter duration therapy can provide the comparable outcomes. We performed a retrospective cohort study to examine the SVR rates, the predictors of treatment failure and the safety analysis of 8-wk LDV/SOF therapy among non-cirrhotic, previously untreated genotype-1 HCV patients with viral load < 6 million IU/mL.
Kaiser Permanente Southern California (KPSC) is a large, integrated healthcare system with over 4 million members. Integrated healthcare is delivered to members at 14 medical centers throughout the region. All interactions with the healthcare system, such as clinic/emergency department/urgent care visits, hospital admissions and outpatient laboratory tests are captured in an integrated electronic medical record (EMR) system and the data is available for research purposes. Emergency care delivered at outside facilities is captured in a claims system that is also available. The KPSC Regional guidelines for 8-wk LDV/SOF therapy were developed and all providers were notified for eligibility criteria: genotype-1, non-cirrhotic, HCV-RNA < 6 million IU/mL and no prior treatment failure. Cirrhotic status of some patients was confirmed by liver biopsy or other non-invasive testing such as vibration-controlled transient elastography (VCTE) or FIBROSPECT II test in some KPSC centers. In some patients, non-cirrhotic status of some patients was determined by clinical judgement of treating healthcare providers using sonographic morphology of the liver, the spleen size and the platelet count in other KPSC centers. All patients with platelet count less than 150 × 109/L underwent a form of hepatic fibrosis testing such as liver biopsy, VCTE or FIROSPEcT II. Every patient had hepatic sonography and baseline laboratory testing prior to hepatitis C treatment. We developed a protocol for KPSC nurse practitioners, physician assistants and pharmacists, who specialized in hepatitis C treatment, to document intended treatment duration and rationales, pre-treatment testing and close monitoring of patients during treatment such as laboratory testing every 2 wk, calling/messaging to identify any barriers/adverse events and providing coping mechanisms/strategies if any event occurred.
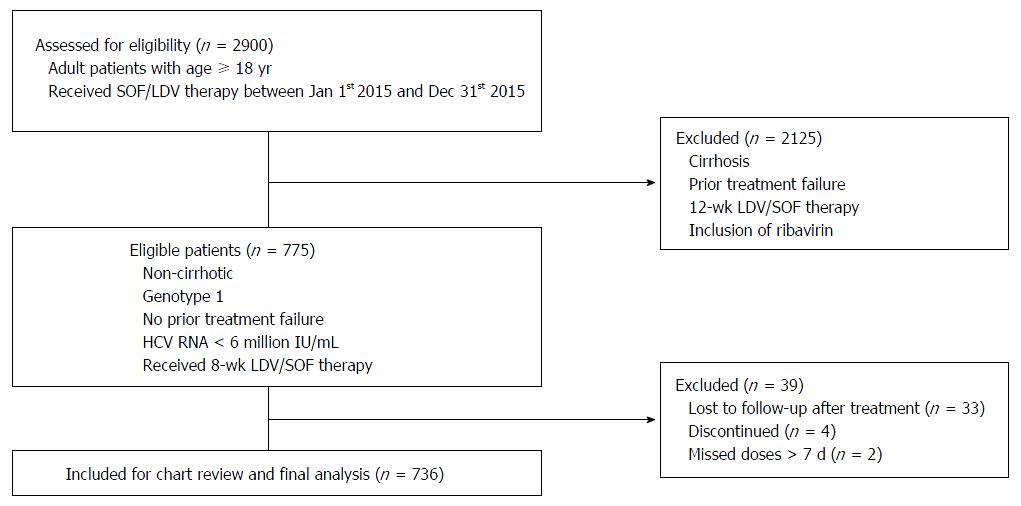
Inclusion criteria: patients with age ≥ 18 years, HCV viral load < 6 million IU/mL, no cirrhosis or prior treatment failure and who had received 8-wk LDV/SOF therapy for chronic HCV genotype-1 infection. Exclusion criteria: patients without SVR12 (SVR at 12 wk after end of treatment) data, patients who did not complete the intended therapy and patients who missed doses for more than seven consecutive days. Individuals who fulfilled above criteria were included in the final study analysis.
We conducted a retrospective cohort study from December 2015 to December 2016, of all patients who had completed 8-wk LDV/SOF therapy. Patient’s clinical and demographic information was captured from KPSC-EMR system. We developed a standardized protocol with explicit criteria for data abstraction including pre- and post-treatment laboratory results, co-morbid medical conditions, liver biopsy (Metavir fibrosis staging), VCTE (kilopascal), FIBROSPECT II test (serum biomarkers), adverse events, clinic/urgent care/emergency department visits and hospitalizations during treatment. Two data abstractors, who are familiar with the EMR system, collected the data according to the protocol criteria to maximize the inter-rater reliability of data abstraction.
Patient reported side effects such as fatigue, headache, insomnia, arthralgia/myalgia, nausea, cough, rash, dizziness, diarrhea, pruritus, irritability and edema, were recorded. Serious adverse events were defined as any event requiring care at the emergency department or hospital admission.
Primary outcome of our study was achievement of SVR at 12 wk after treatment. Secondary outcomes were identifying predictors of treatment failure and adverse events during treatment. SVR was defined as non-detectable level of HCV-RNA test.
For the primary endpoint evaluating SVR12 and for the evaluation of adherence, the final analysis was restricted to per-protocol fashion of those patients who completed therapy and returned for follow-up HCV-RNA testing 3 mo after the end of treatment. The rationale to exclude patients who were lost to follow-up was to counter artificial lowering of the calculated SVR rates. Descriptive statistics were used to compare the baseline differences between those individuals who did or did not achieve SVR12. We used cross-tabulation with Pearson χ2 test to determine the significant difference between categorical variables and 2-tailed Independent-samples t-test to determine the significant difference between 1 categorical variable and 1 quantitative variable. We used multivariate logistic regression to estimate OR and 95%CI to identify predictors of treatment failure while adjusting for confounding variables. All data were entered into and analyzed using IBM SPSS Statistics 20 (IBM, Armonk, NY, United States).
We identified total of 775 non-cirrhotic, genotype 1 HCV patients with HCV-RNA < 6 million IU/mL who received 8-wk LDV/SOF treatment. Seven hundred and thirty-six patients were included in the final analysis after exclusion of patients who reported missing doses, discontinued treatment due to adverse events and patients who did not follow-up for SVR12. Figure 1 demonstrates the flow chart of patient selection process.
The demographic and clinical characteristics of patients are outlined in Table 1. The mean age was 58 years, 55% were males, 51% were Caucasian and 65% had genotype 1a infection. Fifty-three percent of patients considered to be non-cirrhotic were determined by healthcare providers based on clinical judgement (platelet count, spleen size, hepatic morphology on ultrasound) and 47% patients had documented liver fibrosis testing (43% liver biopsy, 3% VCTE and 0.4% FIBROSPECT II). Mean HCV-RNA log10 was 6.2.
| Characteristics | n = 736 |
| Age, mean ± SD (yr) | 58 ± 10 |
| Range | (23-85) |
| Male sex | 403 (55) |
| Ethnicity | |
| Caucasian | 374 (51) |
| African American | 178 (24) |
| Hispanic | 158 (21) |
| Asian/Pacific islanders | 26 (4) |
| HCV genotype-subtype | |
| 1a | 475 (64) |
| 1b | 242 (33) |
| 1 without confirmed subtype | 19 (3) |
| Liver biopsy | 317 (43) |
| Vibration-controlled transient elastography | 25 (3) |
| FIBROSpect II | 3 (0.4) |
| Overall fibrosis score | |
| Stage 0 | 45 (13) |
| Stage 1 | 164 (48) |
| Stage 2 | 104 (30) |
| Stage 3 | 29 (8) |
| Stage 3-4 or 4 | 2 (1) |
| Non-cirrhotic state determined by clinical judgement | 391 (53) |
| HCV RNA - log10 IU/mL, mean ± SD | 6.2 ± 0.2 |
| HCV RNA ≥ 2.2 million IU/mL | 219 (30) |
| Pre-treatment laboratory values | |
| GFR, mean ± SD (Range) | 79 ± 11 (40-89) |
| Platelet count (103/mm3), mean ± SD (Range) | 218 ± 55 (45-495) |
| INR, mean ± SD (Range) | 0.99 ± 0.7 (0.8-1.3) |
| Albumin, mean ± SD (Range) | 3.9 ± 0.4 (2.1-5.1) |
| Missing | 101 (14.0) |
| Co-morbid conditions | |
| Psychiatric diagnoses | 145 (20.0) |
| Chronic kidney disease | 35 (5.0) |
| Psoriasis | 16 (2.0) |
| HIV co-infection | 5 (0.7) |
| Cryoglobulinemia | 4 (0.5) |
| HCV-related glomerulonephritis | 2 (0.3) |
| HBV co-infection | 2 (0.3) |
| Hepatocellular carcinoma | 1 (0.1) |
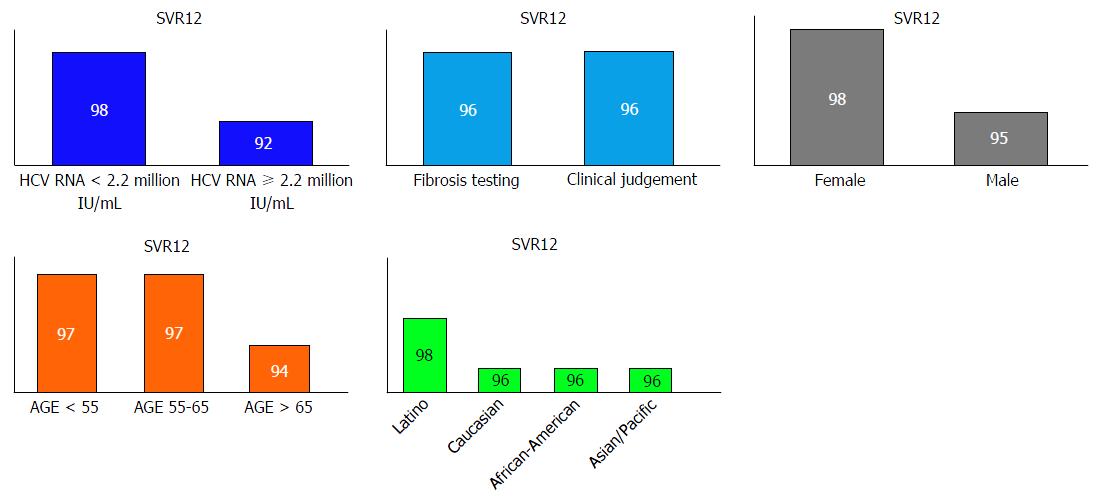
Table 2 demonstrates the study outcomes (SVR). Overall SVR12 was 96%. None of the patients who achieved SVR12 had viral relapse at 24-wk post treatment. Fifty-nine percent patients had SVR24 data at the time of analysis. We found no difference in SVR12 between patients whose non-cirrhotic state was determined by clinical judgment and patients who had fibrosis testing. No significant difference in SVR12 was seen among gender, genotype 1 subtype, ethnicity, type of fibrosis tests and fibrosis stages. Special populations; those co-infected with HIV and HBV achieved 100% SVR12. When reviewed by age groups, patients with age > 65 years had lower SVR compared to age groups 55-65 and < 55 years but no statistical significance was observed (Figure 2).
| Characteristics | SVR12 (%) (n = 736) | P value |
| Overall | 96 (708/736) | |
| Non-cirrhotic state determined by clinical judgement | 96 (376/391) | 0.962 |
| Non-cirrhotic state determined by biopsy, VCTE, FIBROSPECT II | 96 (332/345) | |
| HCV RNA ≥ 2.2 million IU/mL | 92 (201/219) | < 0.001 |
| HCV RNA < 2.2 million IU/mL | 98 (507/270) | |
| HIV co-infection | 100 (6/6) | |
| HBV co-infection | 100 (2/2) | |
| Gender | 0.071 | |
| Male | 95 (383/403) | |
| Female | 98 (325/333) | |
| HCV genotype-subtype | 0.414 | |
| 1a | 96 (454/475) | |
| 1b | 98 (236/324) | |
| Undetermined | 95 (18/19) | |
| Ethnicity | ||
| Caucasian | 96 (357/374) | |
| African American | 96 (171/178) | |
| Hispanic | 98 (155/158) | 0.544 |
| Asian/Pacific Islander | 96 (25/26) | |
| Age groups | 0.311 | |
| < 55 yr | 97 (216/223) | |
| 55-65 yr | 97 (369/382) | |
| > 65 yr | 94 (123/131) | |
| Fibrosis Tests | 0.489 | |
| Liver biopsy | 97 (306/317) | |
| Vibration-controlled transient elastography | 92 (23/25) | |
| FIBROSPECT II | 100 (3/3) | |
| Overall fibrosis stage (cumulative: biopsy/VCTE/FIBROSPECT II) | ||
| Stage 0 | 98 (44/45) | |
| Stage 1 | 95 (155/164) | 0.611 |
| Stage 2 | 98 (102/104) | |
| Stage 3 | 97 (28/29) | |
| Stage 4 | 100 (2/2) |
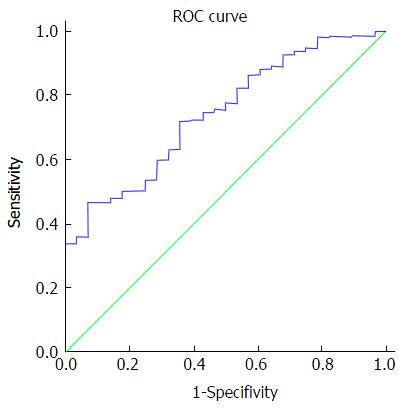
We found that HCV RNA viral load plays a role in predicting SVR with high accuracy - the area under a receiver operating characteristic (AUROC) was 0.743 (95%CI: 0.66-0.82) with a cutoff value of 2.2 million IU/mL, as depicted in Figure 3. A significantly lower SVR was observed among patients with HCV-RNA more than 2.2 million IU/mL (91% vs 98%, P < 0.001). Table 3 exhibits the odds ratios for SVR12 in multivariate logistic regression. We found that patients with HCV-RNA less than 2.2 million IU/mL were more likely to achieve SVR compared to those with more than 2.2 million IU/mL (OR = 0.22, 95%CI: 0.1-0.49, P < 0.001). Age groups, gender, ethnicity and genotype 1 subtype did not predict SVR. Non-cirrhotic state determined by clinical judgment based on simple, non-invasive tests was not associated with lower SVR (OR = 1.02, 95%CI: 0.48-2.17, P = 0.962).
| Characteristics | OR (95%CI) for SVR12 (n = 736) | P value |
| Age 55-65 yr (ref. < 55) | 0.92 (0.36-2.34) | 0.861 |
| Age > 65 yr (ref. < 55) | 0.5 (0.18-1.41) | 0.188 |
| Male (ref. female) | 0.47 (0.21-1.08) | 0.077 |
| African-American (ref. Caucasian) | 0.84 (0.11-6.57) | 0.868 |
| Hispanic (ref. Caucasian) | 2.07 (0.21-20.66) | 0.537 |
| Asian/Pacific Islander (ref. Caucasian) | 0.98 (0.16-8.28) | 0.983 |
| Non-cirrhotic state determined by clinical judgement (ref. Fibrosis Test: biopsy/VCTE/FIBROSPECT) | 1.02 (0.48-2.17) | 0.962 |
| HCV RNA ≥ 2200000 IU/mL (ref. < 2200000 IU/mL) | 0.22 (0.1-0.49) | < 0.001 |
| HCV genotype - subtype 1b (ref. 1a) | 2.19 (0.25-19.15) | 0.480 |
Table 4 reveals the safety analysis of the patients who received 8-wk LDV/SOF therapy. Three (0.5%) patients discontinued treatment due to intolerable adverse events: severe rheumatoid arthritis exacerbation, intractable nausea and declining renal function with glomerular filtration rate 22. One patient who developed drug-induced liver injury (DILI) from LED/SOF therapy with positive biopsy findings discontinued the treatment. Interestingly, one of two patients who were excluded from the study due to missing more than 7 d of therapy achieved SVR. This patient HCV-RNA was 625000 IU/mL. No death or significant morbidities were reported. Four (0.5%) patients experienced serious adverse events during therapy: 2 were hospitalized for observation to evaluate non-cardiac chest pain, 1 was hospitalized for DILI and 1 was due to emergency department admission for pneumonia. The most common minor adverse events were fatigue (14%), headache (13%), insomnia (5%), arthralgia/myalgia (4%) and nausea (4%).
| Adverse events | n (%) |
| No. adverse event, mean ± SD (Range) | 0.5 ± 0.7 (0-6) |
| Serious adverse events | 4 (0.5) |
| Hospital admissions | |
| Non-cardiac chest pain | 2 |
| Drug-induced liver injury | 1 |
| Pneumonia | 1 |
| Minor adverse events | |
| Fatigue | 104 (14) |
| Headache | 98 (13) |
| Insomnia | 35 (5) |
| Arthralgia/myalgia | 29 (4) |
| Nausea | 29 (4) |
| Cough | 15 (2) |
| Rash | 19 (3) |
| Dizziness | 12 (2) |
| Diarrhea | 14 (2) |
| Pruritus | 11 (1) |
| Irritability/anxiety | 10 (1) |
| Edema | 2 (< 0.5) |
| Discontinuation | 4 (0.5) |
| Drug-induced liver injury | 1 |
| Severe rheumatoid arthritis exacerbation | 1 |
| Intractable nausea | 1 |
| Decreased renal function during treatment (GFR < 30) | 1 |
| Death | 0 |
Our findings have validated that SVR rate of 8-wk LDV/SOF therapy in treatment naïve, non-cirrhotic, genotype 1 HCV patients with RNA < 6 million IU/mL is comparable with clinical trials and preliminary outcomes from small real-world studies[7-9]. We demonstrated that there is no difference in SVR between patients whose cirrhosis state was determined by fibrosis testing or clinical judgment. All patients had at least baseline ultrasound of the liver and blood tests such as transaminases levels, platelet count and International normalized ration. We calculated overall fibrosis stages on biopsy, VCTE and FIBROSPECT II and found no difference in SVR across fibrosis stages although very few patients had stage 0, 3 and 4. Our finding suggests that clinical judgment of non-cirrhotic state results in same outcome of SVR 96% compared to SVR of patients who had liver biopsy, VCTE or FIBROSPECT tests.
In our cohort, all patients had pre-treatment HCV-RNA < 6 million IU/mL. We divided to 2 subgroups containing RNA < 800000 IU/mL and > 800000 IU/mL. We found that patients with lower RNA < 800000 IU/mL achieved significantly higher SVR compared to patients with higher RNA in both univariate and multivariate analyses. This finding suggests that HCV viral load plays an important role in predicting SVR although the determination of the optimal cut-off value of HCV-RNA level to consider 8-wk therapy to achieve SVR is currently not available [14]. Our study highlights that HCV RNA 2.2 million IU/mL was associated significant impact on outcomes with AUROC 0.73. While female gender and Latino ethnicity achieved slightly higher SVRs, there is no statistical difference compared to male gender and other ethnicities. We found no difference in SVR rates between African-Americans and Caucasians in contrast to other studies which demonstrated the decreased likelihood of SVR in African-American population[12].
The wholesale acquisition cost for LDV/SOF combination drug in the United States is $1125 per pill. Cost of 8-wk course of therapy is $63000 and cost of 12-wk course is $94500 - net cost saving of $31500 per patient when 8-wk treatment is administered. Healthcare expenses can substantially be reduced by selecting 8-wk LDV/SOF therapy in treatment-naïve, non-cirrhotic genotype 1 HCV patients.
The strengths of our study are its real-world experience and an integrated healthcare model involving all clinical services. We were able to abstract data regarding all clinic/emergency department/urgent care visits, hospitalizations, telephone/electronic-mail encounters and all laboratory tests from the integrated EMR system. All providers used KPSC-Regional HCV treatment guidelines which is readily available on the EMR system for review. Treatment duration, reasons for discontinuation and medication compliance were clearly documented. All patients in the final analysis had good post-treatment follow ups with available SVR12 data. The limitation of our study is its retrospective nature.
In conclusion, our outcomes from real-world cohort validate high SVR rates in non-cirrhotic, treatment naïve HCV genotype 1 patients with HCV RNA < 6 million IU/mL who received 8-wk LDV/SOF therapy. There was no difference in SVR between patients whose non-cirrhotic state was determined by clinical judgment and patients who had fibrosis testing. HCV RNA less than 2.2 million IU/mL was associated with significantly higher SVR. LDV/SOF therapy is safe and well-tolerated with high adherence rates. Therefore, 8-wk LDV/SOF therapy can be used in selected subset of patients with chronic HCV genotype 1 infection who meet aforementioned clinical criteria. Further studies are in need to evaluate and validate HCV RNA cutoff value to achieve the optimal more than 95% of SVR.
Hepatitis C treatment has evolved from a 78-wk interferon monotherapy to 48-wk pegylated interferon plus ribavirin therapy and now 12-wk therapy with newer all-oral direct-acting antiviral (DAA) agents. DAA regimens have revolutionized the treatment of hepatitis C with their excellent sustained virologic response (SVR), tolerable side effect profiles and shorter duration of therapy. Although ION-3 trials and other real-world studies have revealed that 8-wk ledipasvir/sofosbuvir therapy is effective and has comparable sustained viral response outcomes for non-cirrhotic patients who have untreated genotype-1 hepatitis C virus (HCV) infection and HCV RNA < 6 million IU/mL, the current AASLD guidelines recommend 12-wk therapy. Eight-week therapy may provide significantly lower cost, better patient compliance and adherence.
The authors validated that SVR outcomes in 8-wk LED/SOF therapy was comparable with 12-wk therapy in this large, real-wold cohort.
This study highlights that HCV RNA 2.2 million IU/mL was associated significant impact on outcomes with AUROC 0.73. Patients with HCV RNA more than 2.2 millon IU/mL were observed to have significantly lower SVR (92% vs 98%, P < 0.001). They found no difference in SVR rates between African-Americans and Caucasians in contrast to other studies which demonstrated the decreased likelihood of SVR in African-American population.
This study validate other real-world studies which have shown that 8-wk therapy in selected subset of patients (non-cirrhotic, untreated, genotype-1 with HCV RNA < 6 million IU/mL) is effective and comparable to 12-wk therapy. We can apply these findings and amend changes in national guidelines regarding HCV treatment which can save significant amount of HCV treatment cost and boost patient compliance and adherence.
This study is good, and it's important knowledge for clinicians before treating HCV patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gatselis NK, Ratnasari N, Sirin G S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 2. | Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 556] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 3. | Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 312] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 4. | Jacobson IM, Davis GL, El-Serag H, Negro F, Trépo C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8:924-933; quiz e117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 6. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 911] [Cited by in F6Publishing: 906] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 7. | Lai JB, Witt MA, Pauly MP, Ready J, Allerton M, Seo S, Witt DJ. Eight- or 12-Week Treatment of Hepatitis C with Ledipasvir/Sofosbuvir: Real-World Experience in a Large Integrated Health System. Drugs. 2017;77:313-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Kowdley KV, Sundaram V, Jeon CY, Qureshi K, Latt NL, Sahota A, Lott S, Curry MP, Tsai N, Chaiyakunapruk N. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology. 2017;65:1094-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | Wilder JM, Jeffers LJ, Ravendhran N, Shiffman ML, Poulos J, Sulkowski MS, Gitlin N, Workowski K, Zhu Y, Yang JC. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: A retrospective analysis of phase 3 data. Hepatology. 2016;63:437-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | American Association for the Study of Liver Diseases; Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Available from: http://www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. [Cited in This Article: ] |
| 12. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 877] [Cited by in F6Publishing: 890] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 13. | Harvoni (ledipasvir and sofosbuvir) tablet product information. Foster City, CA: Gilead Sciences, Inc.; 2015. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205834s001lbl.pdf. [Cited in This Article: ] |
| 14. | O’Brien TR, Feld JJ, Kottilil S, Pfeiffer RM. No scientific basis to restrict 8 weeks of treatment with ledipasvir/sofosbuvir to patients with hepatitis C virus RNA & lt; 6,000,000 IU/mL. Hepatology. 2016;63:28-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |