Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.7908
Peer-review started: April 6, 2016
First decision: May 12, 2016
Revised: July 18, 2016
Accepted: August 10, 2016
Article in press: August 10, 2016
Published online: September 21, 2016
Processing time: 161 Days and 16.4 Hours
Iron deficiency anemia (IDA) is associated with a number of pathological gastrointestinal conditions other than inflammatory bowel disease, and also with liver disorders. Different factors such as chronic bleeding, malabsorption and inflammation may contribute to IDA. Although patients with symptoms of anemia are frequently referred to gastroenterologists, the approach to diagnosis and selection of treatment as well as follow-up measures is not standardized and suboptimal. Iron deficiency, even without anemia, can substantially impact physical and cognitive function and reduce quality of life. Therefore, regular iron status assessment and awareness of the clinical consequences of impaired iron status are critical. While the range of options for treatment of IDA is increasing due to the availability of effective and well-tolerated parenteral iron preparations, a comprehensive overview of IDA and its therapy in patients with gastrointestinal conditions is currently lacking. Furthermore, definitions and assessment of iron status lack harmonization and there is a paucity of expert guidelines on this topic. This review summarizes current thinking concerning IDA as a common co-morbidity in specific gastrointestinal and liver disorders, and thus encourages a more unified treatment approach to anemia and iron deficiency, while offering gastroenterologists guidance on treatment options for IDA in everyday clinical practice.
Core tip: Iron deficiency anemia (IDA) frequently originates in the gastrointestinal (GI) tract and is a common cause of patient referral to gastroenterologists. Guidelines for the management of IDA in GI conditions are lacking. Symptoms such as fatigue and impaired exercise capacity should prompt a diagnostic work-up for anemia (hemoglobin), iron status (transferrin saturation, ferritin) and inflammation (C-reactive protein). Treatment of IDA should aim to restore normal hemoglobin levels, red cell indices and iron status. Intravenous administration is the preferred iron treatment in patients with chronic GI bleeding, patients being unresponsive or intolerant to oral iron and patients requiring rapid hemoglobin correction.
- Citation: Stein J, Connor S, Virgin G, Ong DEH, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol 2016; 22(35): 7908-7925
- URL: https://www.wjgnet.com/1007-9327/full/v22/i35/7908.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i35.7908
Iron deficiency anemia (IDA) is a common complication in routine clinical practice that frequently originates in the gastrointestinal (GI) tract[1-3]. Patients with IDA are therefore often referred to gastroenterologists for further examination and/or treatment. IDA associated with GI disorders can substantially reduce quality of life, contribute to fatigue, and may even lead to hospitalization[4,5].
In contrast to the well-documented inflammatory bowel disease (IBD)-associated IDA[6,7], prevalence data for IDA associated with other pathological conditions of the GI tract are sparse (Table 1). Guidelines for the diagnosis and management of anemia and iron deficiency are available for IBD[8], but not for other GI conditions. Overall, there are three main pathological contributors to IDA, namely chronic bleeding, malabsorption and inflammation[9-13]. However, other factors, such as poor or selective diet, as well as iron malabsorption (e.g., due to decreased gastric pH) should not be neglected in patients referred for IDA assessment. This applies particularly to elderly patients[14-18].
| Conditions | Anemia or IDA prevalence | Predominant pathological contributors to anemia | Association with anemia and ID | ||
| Bleeding | Malabsorption | Inflammation | |||
| Nonvariceal upper GI bleeding[33] | 80% | √ | > 80% of patients admitted to hospital with nonvariceal AUGIB were anemic at the time of discharge | ||
| Celiac disease[87-89] | 32%-69% | √ | √ | Well-established relationship between celiac disease and IDA | |
| Most widely cited cause of IDA is abnormal iron absorption, but bleeding and inflammation are also known contributory factors | |||||
| Intestinal parasitic infections[151] | 33%-61% | √ | √ | T. trichiura and hookworm infections are closely associated with IDA | |
| GI cancers[107,108,111,117,121-120] | 50%-60% | √ | √ | CRC: IDA associated with greater tumor diameter and with cancers of the right side of the colon | |
| Polyps: IDA much more common with malignant polyps than benign polyps | |||||
| GIST: Most frequent presentation is GI bleeding, which can result in anemia. In pediatric GIST, anemia is the most frequent clinical finding | |||||
| Gastric cancers: 6.8-fold relative risk of gastric cancer in patients with Pernicious anemia | |||||
| Small bowel malignancies: Anemia among most common presenting symptoms | |||||
| Esophageal cancers: Patients with Fanconi anemia at increased risk | |||||
| Esophagitis and hiatal hernia[23-26] | 8%-42% | √ | Gastric bleeding from hernia is an established cause of IDA | ||
| Even in absence of visible lesions, large hernia may be a possible cause of IDA with unexplained etiology | |||||
| Bariatric surgery[77,196] | 10%-40% | √ | ID and anemia are well-known risks after bariatric procedures, but causes are multifactorial and vary depending on exact procedure and patient population | ||
| Intestinal failure[101-103] | 30%-37% | √ | √ | Intestinal failure is associated with ID due to malabsorption, GI blood loss, and multiple surgery | |
| Diverticular disease[144] | 25% | √ | One of the most common causes of lower GI bleeding leading to IDA | ||
| Increasing prevalence due to rise in elderly population | |||||
| Restorative proctocolectomy[153] | 6%-21% | √ | √ | IDA due to mucosal bleeding and impaired iron absorption in patients developing symptomatic or asymptomatic pouchitis | |
| NSAID-associated fecal blood loss[1] | 10%-15% | √ | Even low dose aspirin and non-aspirin-NSAIDs increase mean fecal blood loss 2-4-fold compared with normal | ||
| Angiodysplasia[1] | 5% | √ | Most common cause of lower GI bleeding in the elderly | ||
| Gastric antral vascular ectasia (GAVE)[1,48,55] | 1%-2% | √ | Chronic, slow bleeding is typically associated with IDA | ||
| Gastritis[57,66] | NA | √ | √ | H. pylori infection suggested to play important role in development of IDA | |
| Peptic ulcer[197] | NA | √ | √ | H. pylori infection and IDA as above. Additionally, bleeding from ulcer | |
| Chronic hepatitis and liver conditions with GI bleeding[155] | 75% | √ | Chronic liver disease can be complicated by anemia, particularly due to bleeding | ||
| Non-alcoholic fatty liver disease (NAFLD)[171] | NA | √ | One-third of adult NAFLD subjects are reported to be iron deficient, defined by a TSAT < 20% | ||
Despite the increasing availability of effective and well-tolerated parenteral iron preparations for the treatment of IDA[19,20], a comprehensive overview of treatment approaches of IDA in GI conditions is currently lacking. Furthermore, definitions of IDA are inconsistent across clinical studies and publications, with the terms “iron deficiency”, “iron deficiency anemia” and “anemia” being used almost interchangeably. In addition, the diagnostic markers and cut-off levels used to define iron deficiency vary widely. While anemia is clearly defined according to the World Health Organization as a hemoglobin (Hb) level < 12 g/dL in women (< 11 g/dL in pregnant women) and < 13 g/dL in men, the situation is ambiguous for iron deficiency. Commonly, serum ferritin levels below 15-100 ng/mL (depending on the presence of concomitant inflammation) and transferrin saturation (TSAT) below 16%-20% are considered indicative of iron deficiency[1,7,8].
These aspects complicate the interpretation and comparability of data and highlight the need for standardization of definitions and proper assessment of iron status across different GI and liver diseases. Recently published reviews on IDA discuss IDA in general[21,22] but give only little attention to the fragmented yet consistent evidence that IDA is a common issue in most GI conditions. The aim of this review is therefore to illustrate how IDA represents a common co-morbidity in these disorders, and to encourage a more unified treatment approach to GI condition-associated anemia and iron deficiency (ID).
Relevant articles were identified by screening the PubMed database for articles on IDA or ID in the context of GI or liver disease and associated illnesses. Data reported in abstract form only were identified by manual search through abstracts from major congresses in the field. In addition, the authors’ own literature databases were screened for suitable publications. The results were filtered for articles with information on anemia prevalence and/or anemia management. The last search was conducted in June 2015.
Gastric bleeding from Cameron lesions in large diaphragmatic or hiatal hernia is an established cause of IDA[23,24]. However, axial and paraesophageal hernia without Cameron lesions can also be associated with IDA[25]. The reported incidence of IDA for all types of hernia ranges from 8% to 42%, with an average of 20%[26].
Hiatal hernia increases the risk of IDA independent of comorbid esophagitis[27]. Suggested causes of hernia-related IDA are mechanical trauma plus esophagitis, erosions or gastro-esophageal acid reflux[25,26].
Notably, the absence of endoscopic evidence of erosions in the majority of patients with hernia-related IDA does not exclude their causal role[26]. Therefore, even if no lesions are visible during endoscopy, larger hiatal hernia should still be considered as a possible cause of IDA with unexplained etiology.
Surgery in combination with proton pump inhibitor (PPI) therapy is evidently no better than PPI therapy alone in treating and preventing the recurrence of IDA, even in the case of larger hiatal hernia[26].
Acute upper gastrointestinal bleeding (AUGIB) is a common disorder associated with a high mortality rate of 3% to 15%[28-30]. While peri-endoscopic management of AUGIB, including blood transfusions, has been well characterized and standardized[31,32], guidelines for the monitoring and treatment of IDA in patients after non-variceal AUGIB are still lacking.
Recently, a retrospective study showed that more than 80% of patients admitted to hospital with non-variceal AUGIB were anemic at the time of discharge[33]. Of these, only 16% received a recommendation to begin oral iron supplementation while intravenous iron was not even considered, demonstrating that post-discharge anemia is often disregarded.
Studies analyzing the clinical impact and risks associated with anemia after AUGIB are scarce. One study revealed that patients with hemoglobin (Hb) values < 10 g/dL after AUGIB had two-fold greater risks of re-bleeding and mortality than patients with Hb values ≥ 10 g/dL[34].
A double-blind, placebo-controlled trial, recently demonstrated that patients with IDA after non-variceal AUGIB clearly benefit from iron supplementation[9]. In this study, oral and intravenous iron appeared to be equally effective in raising Hb levels, probably since most patients were not iron deficient at enrolment. However, iron stores (measured as serum ferritin) were replenished most effectively with intravenous iron supplementation.
Regarding the transfusion of red blood cell concentrates (RBC), a recent study in patients with AUGIB (TRIGGER)[35] suggests that the Hb threshold for RBC transfusion can be safely lowered without adversely affecting clinical outcomes. This is in line with results in other indications such as cardiac surgery, critical care and hip surgery. Accordingly, restrictive Hb thresholds (< 8.0 g/dL) should be considered except for patients with ischemic heart disease as pre-existing comorbidity[35,36].
The administration of nonsteroidal anti-inflammatory drugs (NSAIDs) is known for its association with upper and lower GI injury[37-39]. This injury can include bleeding[40-42] which may be severe enough to result in hospitalization[43,44]. Even low dose aspirin as well as non-aspirin-NSAIDs increase mean fecal blood loss from roughly 0.5 mL/d to 1-2 mL/d (i.e., 0.5-1.0 mg iron loss/d)[42]. Among patients treated with aspirin doses ≥ 1800 mg/d, 31% had a blood loss of ≥ 5 mL/d (i.e., ≥ 2.5 mg iron loss/d). Although cyclooxygenase-2 (COX-2) inhibitors are associated with fewer GI injuries than traditional NSAIDs, long-term use of a COX-2 inhibitor may also induce GI injuries and require concomitant medication for associated anemia and small intestinal injuries[45]. Notably, routine endoscopic examination may not reveal NSAID-induced GI injuries. Therefore, capsule endoscopy is recommended to screen for GI injuries in patients taking NSAIDs and presenting with unexplained anemia or ID[41,45,46].
Portal hypertensive gastropathy (PHG) and gastric antral vascular ectasia (GAVE), although being distinct entities[47], can cause chronic gastrointestinal blood loss in patients with liver cirrhosis[48,49]. Most frequently found in association with liver cirrhosis, PHG can also occur in non-cirrhotic patients (e.g., splanchnic venous thrombosis)[50]. The management of PHG is based on reducing hepatic venous pressure gradients and iron replacement therapy and/or blood transfusions. Severe cases may require shunt procedures[51-53].
GAVE, first described in 1953[54] in a patient with chronic IDA, accounts for up to 4% of all non-variceal upper gastrointestinal bleedings. Although cirrhosis is found in up to 30% of GAVE patients and occurs in about 2% of patients awaiting liver transplantation[49,55,56], portal hypertension does not seem to play an important role in the development of GAVE. Treatment comprises, in general, endoscopic interventions (e.g., Argon plasma coagulation, Nd: YAG laser) and surgical procedures (e.g., antrectomy and Billroth I anastomosis) to manage lesions, and symptomatic therapy with iron supplementation or blood transfusions, depending on the severity of anemia[49-52].
Autoimmune gastritis (AIG) is implicated in 20%-30% of IDA cases that are refractory to oral iron[57,58].
AIG, first described by Faber in 1909 as achlorhydric gastric atrophy, is a chronic progressive inflammatory condition leading to the decrease or disappearance of parietal cells, which results in reduced or absent acid production (hypochlorhydria or achlorhydria)[59].The lack of gastric acidity has only recently been confirmed as key factor for impaired intestinal iron absorption[60]. IDA is more often associated with AIG than classical pernicious anemia and frequently precedes vitamin B12 deficiency (at least in fertile women)[61,62].
IDA is a recognized extragastric manifestation of Helicobacter pylori (H. pylori) infection[63]. Over 50% of patients with unexplained refractory IDA have active H. pylori infection[57,58]. Data, showing that H. pylori eradication reverses IDA, were confirmed by several observational and interventional trials, subsequently summarized in two meta-analyses of randomized controlled trials[57,64,65]. Accordingly, the Maastricht IV H. pylori consensus report[66] and other national and international guidelines[67-69] recommend H. pylori eradication for the treatment of IDA of unknown origin. Notably, Bismuth-based eradication therapy is more effective in terms of increasing hemoglobin and iron stores than first line PPI-based triple therapy in patients with IDA and H. pylori infection[63].
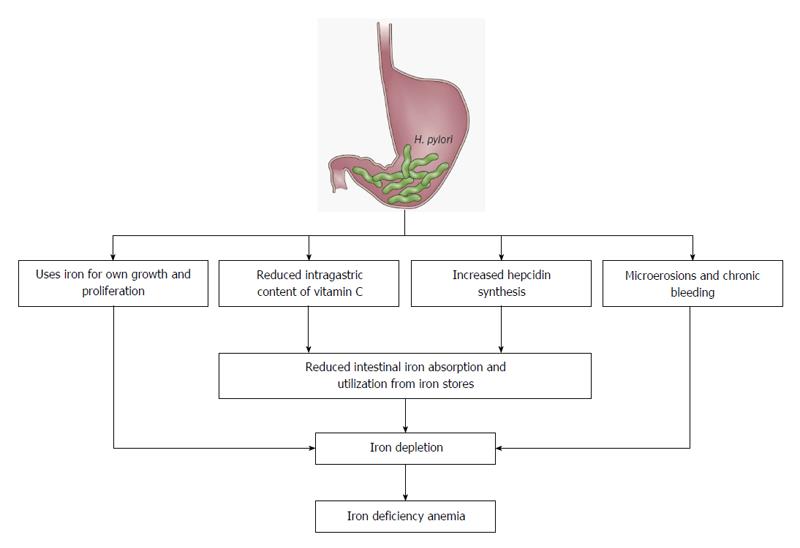
Discussed mechanisms underlying the pathogenesis of H. pylori-related IDA include occult chronic GI bleeding due to gastric mucosal microerosions, competition for dietary iron by the bacteria, reduced ascorbic acid concentration in the gastric juice, affecting the absorption of dietary iron and upregulation of proinflammatory cytokines and hepcidin, the key regulator of iron homeostasis (Figure 1)[57,70-72]. In one study, H. pylori strains retrieved from patients with IDA exhibited faster, iron-dependent cell growth and an enhanced iron uptake than strains from patients without IDA[73]. Furthermore, H. pylori accelerates the development of inflammation, dysplasia and adenocarcinoma (mediated by the H. pylori virulence factor cytotoxin-associated gene A [CagA]) in an ID environment[74-76]. CagA also facilitates H. pylori colonization through iron acquisition, indicating that CagA provides a survival advantage for H. pylori in this setting.
There is growing evidence of potentially severe, occasionally even life-threatening, nutritional and pharmacological consequences of bariatric surgery[77]. ID and IDA after bariatric procedures can result from intestinal bleeding (e.g., from marginal ulcers) or reduced iron absorption due to postoperative intolerance for red meat, diminished gastric acid secretion or exclusion of the duodenum from the alimentary canal[78,79].
The reported incidence of ID in patients following gastric bypass surgery ranges from 12% to 47%[77]. However, the interpretation of reported incidence rates is complicated by different definitions of ID and IDA, postsurgical follow-up periods, types of interventions and patient populations[80]. In a large, patient record review of 959 patients who underwent laparoscopic Roux-en-Y gastric bypass (RYGB) between 2001 and 2011, 51.3% were iron deficient and 6.7% required intravenous iron therapy[81]. Amongst these patients, the prevalence of ID is significantly higher in premenopausal women than in postmenopausal women and males (72% vs 35% and 20%). Comparing different types of surgery, a cross-sectional pilot study of 95 patients showed no significant difference in ID rates after RYGB or sleeve gastrectomy (30% vs 36.4%)[82].
Since oral iron substitution has been shown to be relatively ineffective following bariatric surgery, and tolerance to oral iron preparations is often poor, intravenous iron treatment has been put forward as a preferable option[77]. Some authors suggest that repeated doses of intravenous iron may be required over the course of a year[83].
Ferric carboxymaltose (FCM) showed promise for the treatment of IDA in five phase 3 clinical trials involving 281 patients who had undergone bariatric surgery[84]. FCM exhibited similar or improved efficacy in terms of increasing hemoglobin, ferritin and transferrin saturation (TSAT) values and a favorable safety profile compared with standard medical care (iron sucrose, ferric gluconate, iron dextran or oral iron). In addition, FCM offered the possibility for larger single-dose administrations at fewer visits compared to iron sucrose, ferric gluconate and oral iron[84].
Celiac disease is one of the most common chronic inflammatory conditions of the GI system, affecting about 1% of the population[85]. There is a well-established relationship between celiac disease and IDA[86]. Anemia is the most common presenting symptom of celiac disease, found in 32%-69% of adult patients[87-89]. Approximately 80% of anemic patients with celiac disease are also iron-deficient[88,89]. In 49% of anemic patients with celiac disease, ID was found to be the only detectable abnormality[87]. Conversely, among patients presenting with unexplained IDA, 5% have histologically-confirmed celiac disease[57,90,91].
Impaired iron absorption (due to villous atrophy of the intestinal mucosa) and blood loss are important pathological contributors to anemia in celiac disease[92]. Occult GI bleeding was detected in about half of patients with celiac disease adhering to a gluten-free diet[93]. In some patients, nutritional deficiencies may also be a (contributing) causative factor[94]. Inflammation is a major contributor to IDA, with interleukin (IL)-1, IL-6, IL-10, interferon (IFN)-γ and tumor necrosis factor (TNF)-α as inducers of hepcidin, the main regulator of iron homeostasis[92,95]. Accordingly, celiac disease-related IDA is refractory to oral iron treatment[57,92,96], and even after switching to a gluten-free diet, it takes 6-12 mo until most patients recover from anemia[97]. Notably, half of patients remain iron-deficient even after 1-2 years on a gluten-free diet. The slow or lacking recovery from ID may be due to the low absorption rate of nutritional iron (1-2 mg/d), which hinders the repletion of severely depleted iron stores, and the potentially low content of iron and other micronutrients in a gluten-free diet[98].
Therefore, patients with celiac disease clearly benefit from immediate intravenous iron treatment instead of switching to intravenous iron only after (foreseeable) non-response and/or intolerance to oral iron[96].
Intestinal failure (IF) results from obstruction, dysmotility, surgical resection, congenital defect, or disease-associated loss of absorption, and is characterized by the inability to maintain protein-energy, fluid, electrolyte, or micronutrient balance[99]. In patients suffering from IF, total parenteral nutrition (TPN) is a life-saving intervention until full or partial recovery of enteral nutrition (EN)[100]. Nevertheless, patients with IF are prone to ID as a result of malabsorption, gastrointestinal blood loss and multiple surgical procedures. Accordingly, ID is the most common micronutrient deficiency during and after transition from TPN to EN, with reported incidences of 60%-80% for ID, and 30%-37% for IDA[101-103]. A study from the Mayo Clinic, including 185 patients, showed that IDA developed much more rapidly in patients with fistula and bowel obstruction than in those with short bowel syndrome (SBS) and dysmotility[104]. Despite the high prevalence of ID, iron is not routinely added to parenteral nutrition formulations because of the risk of anaphylaxis and concerns about incompatibilities. Although data describing the compatibility of iron supplementation with parenteral formulations are conflicting[105], iron dextran has been found to be compatible with lipid-free solutions at an amino acid concentration > 2%[106]. A safer approach would prescribe intermittent infusion of therapeutic iron doses.
Anemia and IDA are common in patients with colorectal cancer (CRC), with a prevalence of 50%-60%[107-111]. Risk factors for anemia in patients with CRC are greater tumor diameter and cancer in the right side of the colon[108,112]. CRC is a cause of lower GI bleeding in 11% to 14% of cases[113], and malignant polyps are associated with greater blood loss and more frequent occurrence of IDA than benign polyps[114].
Anemia has also been described in the context of gastrointestinal stromal tumors (GIST), which are frequently associated with acute or chronic bleeding[115,116]. In pediatric GIST, anemia is the most frequent clinical finding (86% with symptomatic anemia)[117]. Notably, anemia is also one of the most frequent side effects of imatinib, the standard treatment for advanced/metastatic GIST, including small bowel cancers[118,119]. In addition to IDA, other specific forms of anemia such as pernicious anemia and Fanconi anemia are also increased in patients with gastric cancers (6.8-fold relative risk of pernicious anemia)[120], small bowel malignancies[121] and esophageal cancers[122].
Notably, ID (with or without anemia) is associated with an increased risk of GI malignancy 2 years after diagnosis of ID[123]. Therefore, unexplained IDA is an important measure for detection of GI malignancy[108]. In patients with advanced CRC, Hb levels < 11 g/dL are a poor prognostic factor[124] and prompt referral as well as investigation of IDA are recommended in patients with CRC[124-126] and cancers in general[127]. However, treatment options for IDA are not discussed, as the guidelines primarily focus on surgical follow-up for CRC.
Since CRC surgery may result in significant blood loss, perioperative allogeneic blood transfusion (ABT) has often been used in CRC patients[128,129]. However, ABT is associated with certain risks, such as an increased infective complication rate and increased disease recurrence[130-132]. Furthermore, ABT involves significant cost[133], and RBCs are an increasingly limited resource. Therefore, alternative options such as perioperative intravenous iron administration have been examined[19,128,129,134] and a multicenter randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron as preoperative anemia treatment in colorectal cancer patients is ongoing[135]. One randomized prospective placebo-controlled pilot study showed preoperative intravenous iron sucrose (total iron dose 600 mg) to have no effect on serum Hb concentration or the rate of blood transfusion in 62 patients scheduled for resection of suspected colorectal cancer[128]. However, the trial included only 11 patients with confirmed anemia, while 22 had a normal Hb, and in 29 patients, there was no recent record of anemia status at all. Furthermore, a Hb increase of 0.5 g/dL, defined as the primary endpoint, is clinically insignificant and unlikely to have an impact on perioperative transfusion requirements[136]. In general, a Spanish expert panel on alternatives to allogeneic blood transfusion suggests perioperative intravenous iron administration to anemic patients scheduled for gastrointestinal surgery[137]; yet overall, there are only few high-quality prospective studies of sufficient power[138].
Diverticular disease, one of the most common causes of lower GI bleeding[113,139], accounts for 30%-50% of massive lower GI bleeding cases[140]. Diverticulitis is a major healthcare problem which often requires surgical management to optimize patient outcomes[141]. Despite reports of IDA associated with clinical cases of diverticulitis[142,143], information on prevalence is lacking. In a study of 1124 cases of colonic diverticular disease seen at a hospital clinic during a 15-year period, 44 (3.9%) had diverticular hemorrhage and 25% of these patients had anemia (Hb < 12 g/dL)[144]. Anemia was most frequent in elderly patients (60 years and upwards) and those with acute bleeding.
Angiodysplasia is a poorly understood clinical condition involving fragile, thin-walled vascular malformations which are susceptible to rupture, and may thus cause severe GI bleeding[145]. Angiodysplasia accounts for up to 5% of cases of GI bleeding overall and up to 40% of obscure GI bleeding cases[146,147]. Angiodysplasia has been found to be present in 61% of patients over the age of 60, often with co-existing conditions[148]. Chronic angiodysplasia can be difficult to manage due to frequent rebleeding of multiple lesions clustered in different localizations of the GI tract and therefore frequently results in chronic IDA[149].
In the past, patients with angiodysplasia commonly had numerous and frequent blood transfusions and suffered end-organ damage due to refractory anemia[149]. Modern intravenous iron preparations can be considered a valuable treatment option if blood loss exceeds 10 mL/d (i.e., around 5 mg iron)[149].
Several studies have shown parasitic infections, especially T. trichiura and hookworm infections, to be closely associated with IDA[150-152]. Hookworm infections are associated with mucosal damage and endogenous loss of iron[151], while T. trichiura and E. histolytica cause bleeding and dysentery by invading the mucosa of the large intestine. Accordingly, intestinal parasitic infections are recognized as predictors of IDA.
A frequent complication of restorative proctocolectomy is pouchitis, which in turn is associated with IDA (6%-21% of patients with functional pouches) due to mucosal bleeding and impaired iron absorption[153]. Notably, pouchitis can be asymptomatic but still be associated with IDA, as can pouches in the abscence of pouchitis. In patients that are intolerant or unresponsive to oral iron, intravenous iron and erythropoiesis-stimulating agent (ESA) treatment can correct the anemia. Another deficiency, vitamin B12 deficiency, occurs in 25%-53% of pouch recipients (compared to 3%-40% in the general population), being also a frequent cause of anemia. In general, vitamin B12 deficiency can be resolved with oral cyanocobalamin[153,154], suggesting a post-procedural change in dietary habit as the main reason for this deficiency.
Among patients with chronic liver disease, 75% are anemic[155], mainly due to acute or chronic GI hemorrhage which may lead to iron deficiency as a consequence. Acute gastrointestinal hemorrhage is a potentially serious complication of portal hypertension and the second most common cause of mortality in patients with cirrhosis. The increased risk of bleeding in severe hepatocellular disease can result from impaired blood coagulation due to reduced synthesis of blood coagulation factors by hepatocytes, and lower thrombocyte numbers. Initial treatment aims to correct hypovolemia and restore stable hemodynamic function (e.g., gelatin-based colloids, solutions of human albumin or red blood cell transfusion)[155]. In addition, IDA caused by chronic blood loss may be treated with oral iron or intravenous iron in cases of advanced chronic liver disease.
Notably, anemia is frequently associated with both peginterferon (PEG-IFN) and ribavarin (RBV) in the treatment of chronic hepatitis C virus (HCV) infection, particularly when these drugs are administered in combination[156-158]. According to the WHO guidelines, grade 1 anemia (Hb 10-11 g/dL) has been reported in up to 30% and grade 2 (< 10 g/dL) in 9%-10% of cases. The addition of direct-acting anti-virals (DAAs) such as telaprevir (TVR) or boceprevir (BOC) as part of the more effective antiviral triple combination therapy has been shown to increase anemia by up to 20% compared to PEG-IFN/RBV in both treatment-naïve and -non-naïve patients[159-163]. Since this treatment-induced anemia is mainly due to hemolysis, dose reduction of RBV by up to 50% is recommended, followed by administration of recombinant erythropoietin[156,164-166]. Evaluation of inosine triphosphatase polymorphisms may help to predict the risk of anemia and response to treatment[156,167-169]. Second generation DAAs, including simeprevir (SMV), sofosbuvir (SOF), daclatasvir (DCV), and ledipasvir (LDV), approved in combination (e.g., SOF/SMV) as IFN-free regimens for the treatment of genotype 1 HCV infection, offer significantly greater cure rates and shorter treatment duration, and have been associated with lower incidence rates of anemia, ranging from 5% to 20%[170].
Non-alcoholic fatty liver disease (NAFLD) is becoming the most common liver disease worldwide (estimated prevalence of 25%-30%), with one-third of adult patients being iron deficient (TSAT < 20%)[171]. ID was significantly associated with female gender, obesity, increased BMI, lower alcohol consumption, non-white race and increased levels of IL-6 and IL-1ß. In contrast to patients with obesity-related, low-grade inflammation, serum hepcidin levels were low in NAFLD subjects with ID, reflecting an appropriate response of hepcidin signaling to ID. The authors concluded that initially, obesity-induced systemic inflammation may increase hepcidin levels and contribute to ID, but hepcidin is appropriately downregulated after ID is established[171]. Similar results of decreased intestinal iron absorption that are inversely associated with serum and urinary hepcidin levels have been reported in dysmetabolic iron overload syndrome (DIOS)[172] which is associated with half of NAFLD cases. Based on hepatic gene expression studies in pediatric patients with non-alcoholic steatohepatitis (NASH), it is hypothesized that, (1) a decreased level of transferrin receptor I in NASH patients is an indicator of reduced erythropoietic activity in the bone marrow, a typical feature of anemia of chronic inflammation; and (2) that elevated expression of transferrin and transferrin receptor II may result in the upregulation of hepcidin, leading to impaired duodenal iron absorption. In addition, the authors demonstrated that elevated serum ferritin levels do not reflect increased hepatic iron stores in patients with NASH, but are rather a consequence of hepatic and/or obesity-related inflammation[173].
While the origin of IDA is often multifactorial, a close relationship with various GI conditions has been established (Table 1)[1,2]. Nevertheless, management of patients with IDA often remains inadequate[1,4].
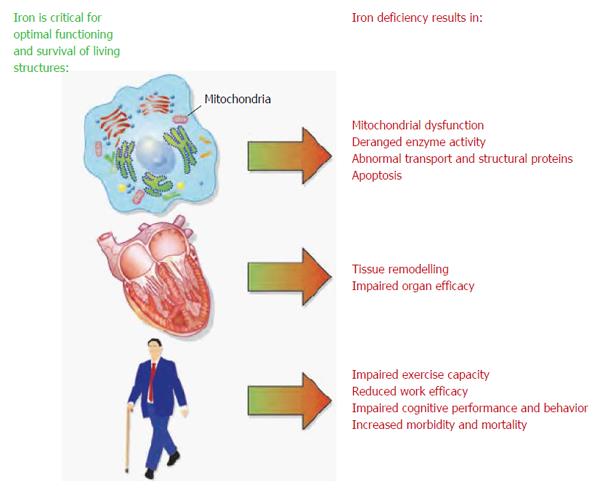
Even without anemia, ID can have a substantial impact on physical and cognitive function and quality of life (e.g., fatigue)[174-177] (Figure 2). This supports the need for regular assessment of iron status and consideration of the clinical consequences of any disturbance of iron status. Patients with typical GI symptoms, such as epigastric pain, change in bowel habit, weight loss, early satiety, or poor appetite, should be assessed for ID and anemia, since these symptoms are often associated with acute or chronic blood loss, malabsorption and/or chronic inflammation[5]. In the planning of treatment for ID/IDA and the selection of the iron administration route, the frequency and magnitude of blood loss as well as the known side effects of oral iron should be considered[4].
Although chronic GI bleeding and malabsorption in GI conditions are well-recognized causes of ID/IDA[5,12,13], other factors such as age and chronic inflammation that inhibit iron availability via increased hepcidin levels should also be borne in mind[2,15,179]. Normal iron homeostasis is based on two pillars: absorption of nutritional iron by enterocytes in the duodenum and upper jejunum (1-2 mg/d), and recycling of iron via phagocytosis of senescent red blood cells (20-25 mg/d)[179]. Absorbed or recycled iron is transiently stored in the monocytes/macrophages of the reticuloendothelial system, from where it is released via ferroportin, loaded on transferrin and transported to the bone marrow for erythropoiesis. Hepcidin is a key regulator of iron homeostasis, blocking the ferroportin-mediated release of iron from enterocytes and macrophages, and impairing the utilization of nutritional or supplemental oral iron. In patients with inflammation, iron release from the reticuloendothelial system is reduced to 44% of that measured in normal subjects[180].
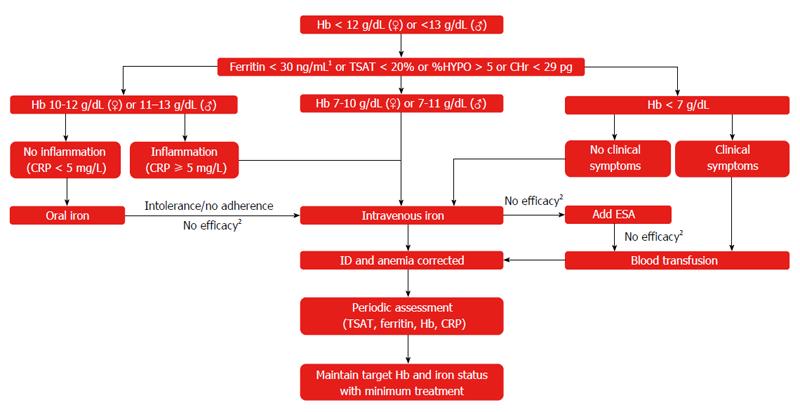
Diagnosis of anemia and ID involves standard laboratory tests (Hb, serum ferritin, TSAT) and blood counts, with low serum Hb or abnormal red blood cell indices usually being the initial finding in a routine complete blood count[5,181]. In some regions, anemic patients should be tested for hemoglobinopathies to exclude genetic reasons for anemia. Since little consensus exists among guidelines on different GI conditions as to the level of anemia that requires follow-up, it has been recommended that any degree of anemia should be further investigated for the presence of ID (Figure 3)[1].
In clinical practice, iron status is mainly assessed on the basis of serum ferritin levels[182]. However, serum ferritin is subject to gender differences and falsely elevated levels in populations with inflammatory reactions since it is also an acute-phase reactant[181]. Therefore, the diagnostic workup of anemic patients (i.e., men with Hb < 13 g/dL or non-pregnant women with Hb < 12 g/dL) should include CRP, to detect underlying inflammatory reactions (suggested cut-off 5 mg/L), and TSAT (suggested cut-off 20%), a marker of low iron availability that is less affected by inflammatory reactions[181,182].
Additional markers of ID include the percentage of hypochromic red cells (%HYPO, suggested cut-off 5%) and the hemoglobin content of reticulocytes (CHr, suggested cut-off 29 pg) as well as serum levels of soluble transferrin receptors (sTfR) and zinc protoporphyrin (ZPP)[17,181]. Since sTfR levels reflect the erythropoietic activity rather than the iron status, sTfR cannot be used in patients treated with ESAs.
Analogous to patients with IBD, iron replacement should also be initiated in non-IBD patients, once IDA is clearly ascertained or deemed likely based on assessed iron markers. Treatment options for IDA include oral and parenteral iron, erythropoiesis-stimulating agents and blood transfusions. There is widespread support for iron supplementation both for the correction of anemia and for replenishment of body iron stores[1]. Currently, the first-line approach for treating IDA is oral iron; usually, 200 mg iron is administered twice daily, but lower doses may be as effective and better tolerated[1]. However, the efficacy of oral iron may be limited when GI uptake is impaired (e.g., due to chronic inflammatory conditions, celiac disease or duodenal resection) or patient compliance is poor (e.g., due to gastrointestinal side effects such as nausea, flatulence and diarrhea[8]). Also large iron deficits that result from chronic or acute GI bleeding or perisurgical blood loss cannot be adequately and quickly counteracted with oral iron. Notably, oral iron can exacerbate existing symptoms of GI disease[4,7], and particularly oral ferrous salts lead to oxidative stress, as evidenced by increased levels of non-transferrin bound iron (NTBI)[183].
Intravenous iron has proven its efficacy and tolerability in a wide range of therapeutic areas, and is recommended in respective treatment guidelines[1,8,127,184-186]. In particular, parenteral iron is considered advisable for patients with GI conditions who cannot be treated adequately with oral iron supplements due to severe GI side effects, inadequate absorption, or anemia requiring urgent correction. Intravenous iron replacement facilitates faster correction of ID and avoids GI side effects by bypassing the GI tract. Although intravenous iron is more costly than oral treatment, administration by a medical professional ensures compliance and more reliable repletion of iron stores which in turn may prevent anemia recurrence and related treatment costs in the long term.
The underutilization of intravenous iron is largely based on past experience with high molecular weight iron dextran (HMWID) that is associated with anaphylactic reactions and therefore has been removed from the market in the United States and Europe. In recent years, safety of intravenous iron has been vastly improved by new, well-tolerated preparations[57,149,187]. A review issued by the United States Food and Drug Administration (FDA) studying serious adverse reactions across different intravenous compounds (iron sucrose, ferric gluconate and low molecular weight iron dextran) showed a cumulative rate of only < 1:200000[188,189]. In 2013, the European Medicines Agency (EMA) published an assessment report[190] concluding that the benefits of intravenous iron-containing medicinal products continue to outweigh the risks in the treatment of iron deficiency when the oral route is insufficient or poorly tolerated. Notably, the EMA removed the necessity of a test dose, yet trained staff and resuscitation facilities to manage anaphylactic or anaphylactoid reactions should be available when any intravenous iron product is administered.
Traditional calculation of iron deficits (iron doses) with the Ganzoni formula is error-prone, inconvenient and underestimates iron requirements[191]. Accordingly, a more simple fixed-dose regimen (of ferric carboxymaltose) based on Hb and body weight (Table 2) was tested in IBD patients, and found to be superior to the Ganzoni-calculated dosing (of iron sucrose) in terms of efficacy and compliance[192]. This novel dosing scheme can equally be utilized as a simple dosing guide for other patient groups and iron formulations that can be given at doses of 1000 mg per administration for efficient and rapid iron replenishment. Most clinical trial and observational data on high dose iron administration have been generated with ferric carboxymaltose and low molecular weight iron dextran. In cases of severe anemia, the iron dose should be increased by 500 mg.
| Degree of iron deficiency | Hemoglobin level (g/dL) | Iron deficit (mg) | |
| Body weight | Body weight | ||
| < 70 kg | ≥ 70 kg | ||
| Moderate | 10-12 (women) | 1000 | 1500 |
| 10-13 (men) | |||
| Severe | 7-10 | 1500 | 2000 |
| Critical | < 7 | 2000 | 2500 |
In response to parenteral iron administration, serum ferritin is greatly elevated for the first 8 wk after infusion. Therefore, ferritin should be monitored only after 8-12 wk, and in case of iron overload (TSAT > 50%), treatment should be adjusted accordingly.
Treatment response to intravenous iron replacement can be defined as an increase in hemoglobin levels of ≥ 2 g/dL within approximately 4-8 wk of infusion and restoration of appropriate iron availability (TSAT ≥ 30%). Patients who show limited or no response to intravenous iron therapy, especially those with anemia of chronic inflammation, should be considered for adjunctive treatment with ESAs (target Hb level ≤ 12 g/dL). Overall, intravenous iron replacement is increasingly recommended by gastroenterologists[1,5,8,149].
Regardless of the route chosen, iron therapy must continue after resolution of anemia until iron stores are completely replenished[4]. Once Hb levels and red cell indices have been normalized, they should be monitored at regular intervals[1]. The authors of the British Society of Gastroenterology guidelines propose assessments at 3-monthly intervals for one year, then after a further year, and immediately if symptoms of anemia reoccur[1].
Blood transfusions should be used only as a last option and as rescue treatment when faced with a life-threatening situation (e.g., in severe cases of acute bleeding)[4,5]. There is a wealth of evidence concerning post-operative mortality and morbidity following blood transfusion, even after transfusion of a single RBC unit[193-195]. Consequently, a restrictive approach to blood transfusion is warranted except for patients who present with ischemic heart disease as pre-existing comorbidity[35,36,195]. In patients with GI disease, transfusions should aim to restore Hb to a safe level, but not necessarily up to normal values, and iron supplementation should be given subsequently to replenish stores[1].
IDA is a common comorbidity in patients with GI or liver disorders. In general, the origin of IDA can be multifactorial, with bleeding, malabsorption and inflammation playing important roles in the context of different GI conditions. IDA can contribute substantially to the morbidity and mortality of the underlying disorder and even ID without anemia can reduce quality of life, exercise capacity and cognitive function. Therefore, effective treatment of ID and IDA as well as prevention of recurrence are necessary and may provide an important alleviation of the overall disease burden. The lack of guidelines on diagnosis and treatment of IDA in the field of GI disease results in suboptimal assessment and management of IDA.
The standard laboratory approach used to investigate IDA would benefit from inclusion of TSAT assessment, which is less affected by inflammatory reactions than the commonly-used acute-phase protein serum ferritin.
Oral iron, often selected as the initial treatment option, has considerable limitations in GI patients due to severe GI side effects, inadequate absorption and a slow course of action. Furthermore, patient compliance with oral iron therapy is often poor. If oral therapy fails or is inadvisable, intravenous iron replacement is a valuable option. Intravenous iron therapy is more efficient than oral iron, and faster at increasing Hb levels and replenishing iron stores. Iron therapy should be continued until iron stores are completely replenished. During subsequent follow-up visits for their GI disorder, patients should be routinely monitored for any signs of ID or IDA.
Medical writing support was provided by SFL Regulatory Affairs & Scientific Communication, Switzerland and funded by Vifor Pharma. In addition, the authors thank Janet Collins (ICCC Rhein-Main, Frankfurt, Germany) for fine-tuning and proofreading the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Maroni L, Sargsyants N, Strom SC S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 484] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 2. | Luman W, Ng KL. Audit of investigations in patients with iron deficiency anaemia. Singapore Med J. 2003;44:504-510. [PubMed] |
| 3. | Marignani M, Angeletti S, Filippi L, Danieli R, Schillaci O. Occult and obscure bleeding, iron deficiency anemia and other gastrointestinal stories (Review). Int J Mol Med. 2005;15:129-135. [PubMed] |
| 4. | Bayraktar UD, Bayraktar S. Treatment of iron deficiency anemia associated with gastrointestinal tract diseases. World J Gastroenterol. 2010;16:2720-2725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 2010;55:548-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 8. | Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, Gomollon F, Iqbal T, Katsanos K, Koutroubakis I. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 9. | Bager P, Dahlerup JF. Randomised clinical trial: oral vs. intravenous iron after upper gastrointestinal haemorrhage--a placebo-controlled study. Aliment Pharmacol Ther. 2014;39:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Fernández-Bañares F, Monzón H, Forné M. A short review of malabsorption and anemia. World J Gastroenterol. 2009;15:4644-4652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 11. | Gomollón F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659-4665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 126] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Niv E, Elis A, Zissin R, Naftali T, Novis B, Lishner M. Iron deficiency anemia in patients without gastrointestinal symptoms--a prospective study. Fam Pract. 2005;22:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Patterson RN, Johnston SD. Iron deficiency anaemia: are the British Society of Gastroenterology guidelines being adhered to? Postgrad Med J. 2003;79:226-228. [PubMed] |
| 14. | Busti F, Campostrini N, Martinelli N, Girelli D. Iron deficiency in the elderly population, revisited in the hepcidin era. Front Pharmacol. 2014;5:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Dharmarajan TS, Bullecer MLF, Pitchumoni CS. Anemia of gastrointestinal origin in the elderly. Pract Gastroenterol. 2015;26:22-36. |
| 16. | Ferguson A, Brydon WG, Brian H, Williams A, Mackie MJ. Use of whole gut perfusion to investigate gastrointestinal blood loss in patients with iron deficiency anaemia. Gut. 1996;38:120-124. [PubMed] |
| 17. | Stein J, Dignass AU. Anaemia in the Elderly IBD Patient. Curr Treat Options Gastroenterol. 2015;13:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Geisel T, Martin J, Schulze B, Schaefer R, Bach M, Virgin G, Stein J. An etiologic profile of anemia in 405 geriatric patients. Anemia. 2014;2014:932486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Gomollón F, Gisbert JP, García-Erce JA. Intravenous iron in digestive diseases: a clinical (re)view. Ther Adv Chronic Dis. 2010;1:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Koch TA, Myers J, Goodnough LT. Intravenous Iron Therapy in Patients with Iron Deficiency Anemia: Dosing Considerations. Anemia. 2015;2015:763576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 875] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 22. | Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 843] [Article Influence: 93.7] [Reference Citation Analysis (4)] |
| 23. | Cameron AJ. Incidence of iron deficiency anemia in patients with large diaphragmatic hernia. A controlled study. Mayo Clin Proc. 1976;51:767-769. [PubMed] |
| 24. | Cameron AJ, Higgins JA. Linear gastric erosion. A lesion associated with large diaphragmatic hernia and chronic blood loss anemia. Gastroenterology. 1986;91:338-342. [PubMed] |
| 25. | Carrott PW, Markar SR, Hong J, Kuppusamy MK, Koehler RP, Low DE. Iron-deficiency anemia is a common presenting issue with giant paraesophageal hernia and resolves following repair. J Gastrointest Surg. 2013;17:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Panzuto F, Di Giulio E, Capurso G, Baccini F, D’Ambra G, Delle Fave G, Annibale B. Large hiatal hernia in patients with iron deficiency anaemia: a prospective study on prevalence and treatment. Aliment Pharmacol Ther. 2004;19:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Ruhl CE, Everhart JE. Relationship of iron-deficiency anemia with esophagitis and hiatal hernia: hospital findings from a prospective, population-based study. Am J Gastroenterol. 2001;96:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Crooks CJ, West J, Card TR. Upper gastrointestinal haemorrhage and deprivation: a nationwide cohort study of health inequality in hospital admissions. Gut. 2012;61:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 295] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Jairath V, Kahan BC, Logan RF, Hearnshaw SA, Travis SP, Murphy MF, Palmer KR. Mortality from acute upper gastrointestinal bleeding in the United kingdom: does it display a “weekend effect”? Am J Gastroenterol. 2011;106:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (1)] |
| 32. | British Society of Gastroenterology Endoscopy Committee. Non-variceal upper gastrointestinal haemorrhage: guidelines. Gut. 2002;51 Suppl 4:iv1-iv6. [PubMed] |
| 33. | Bager P, Dahlerup JF. Lack of follow-up of anaemia after discharge from an upper gastrointestinal bleeding centre. Dan Med J. 2013;60:A4583. [PubMed] |
| 34. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [PubMed] |
| 35. | Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, Stanley AJ, Everett SM, Bailey AA, Dallal H. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 36. | Villanueva C. Gastrointestinal bleeding: Blood transfusion for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2015;12:432-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Fortun PJ, Hawkey CJ. Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol. 2007;23:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 38. | Hernández-Díaz S, Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093-2099. [PubMed] |
| 39. | Laine L, Smith R, Min K, Chen C, Dubois RW. Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2006;24:751-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [PubMed] |
| 41. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [PubMed] |
| 42. | Moore RA, Derry S, McQuay HJ. Faecal blood loss with aspirin, nonsteroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors: systematic review of randomized trials using autologous chromium-labelled erythrocytes. Arthritis Res Ther. 2008;10:R7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Lanas A, García-Rodríguez LA, Arroyo MT, Gomollón F, Feu F, González-Pérez A, Zapata E, Bástida G, Rodrigo L, Santolaria S. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 44. | Mamdani M, Rochon PA, Juurlink DN, Kopp A, Anderson GM, Naglie G, Austin PC, Laupacis A. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ. 2002;325:624. [PubMed] |
| 45. | Toyoda H, Tanabe N, Toyoda M, Toyoda N, Takei Y. Effect of the misoprostol-rebamipide combination on iron deficiency anemia in patients under long-term cyclooxygenase-2 selective inhibitor treatment for small bowel ulcers. Clin J Gastroenterol. 2012;5:155-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133-141. [PubMed] |
| 47. | Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion. 2008;77:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Nguyen H, Le C, Nguyen H. Gastric antral vascular ectasia (watermelon stomach)-an enigmatic and often-overlooked cause of gastrointestinal bleeding in the elderly. Perm J. 2009;13:46-49. [PubMed] |
| 49. | Johnson J, Derk CT. Gastric antral vascular ectasia in systemic sclerosis. Int J Rheumatol. 2011;2011:305238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Schouten JN, Verheij J, Seijo S. Idiopathic non-cirrhotic portal hypertension: a review. Orphanet J Rare Dis. 2015;10:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Patwardhan VR, Cardenas A. Review article: the management of portal hypertensive gastropathy and gastric antral vascular ectasia in cirrhosis. Aliment Pharmacol Ther. 2014;40:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 52. | Ripoll C, Garcia-Tsao G. Management of gastropathy and gastric vascular ectasia in portal hypertension. Clin Liver Dis. 2010;14:281-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Ripoll C, Garcia-Tsao G. The management of portal hypertensive gastropathy and gastric antral vascular ectasia. Dig Liver Dis. 2011;43:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | RIDER JA, KLOTZ AP, KIRSNER JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24:118-123. [PubMed] |
| 55. | Ghrénassia E, Avouac J, Khanna D, Derk CT, Distler O, Suliman YA, Airo P, Carreira PE, Foti R, Granel B. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol. 2014;41:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Dulai GS, Jensen DM, Kovacs TO, Gralnek IM, Jutabha R. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004;36:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Dickey W, Kenny BD, McMillan SA, Porter KG, McConnell JB. Gastric as well as duodenal biopsies may be useful in the investigation of iron deficiency anaemia. Scand J Gastroenterol. 1997;32:469-472. [PubMed] |
| 59. | Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10:529-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 60. | Betesh AL, Santa Ana CA, Cole JA, Fordtran JS. Is achlorhydria a cause of iron deficiency anemia? Am J Clin Nutr. 2015;102:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Hershko C, Ronson A, Souroujon M, Maschler I, Heyd J, Patz J. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood. 2006;107:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 62. | Hershko C, Ronson A. Iron deficiency, Helicobacter infection and gastritis. Acta Haematol. 2009;122:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Franceschi F, Zuccalà G, Roccarina D, Gasbarrini A. Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol. 2014;11:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter. 2008;13:323-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Yuan W, Li Yumin D, Yang L. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2010;45:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1587] [Article Influence: 122.1] [Reference Citation Analysis (5)] |
| 67. | Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 68. | Fischbach W, Malfertheiner P, Hoffmann JC, Bolten W, Bornschein J, Götze O, Höhne W, Kist M, Koletzko S, Labenz J. S3-guideline “helicobacter pylori and gastroduodenal ulcer disease” of the German society for digestive and metabolic diseases (DGVS) in cooperation with the German society for hygiene and microbiology, society for pediatric gastroenterology and nutrition e. V., German society for rheumatology, AWMF-registration-no. 021 / 001. Z Gastroenterol. 2009;47:1230-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 70. | Emiralioglu N, Yenicesu I, Sari S, Egritas O, Poyraz A, Pasaoglu OT, Celik B, Dalgic B. An insight into the relationships between prohepcidin, iron deficiency anemia, and interleukin-6 values in pediatric Helicobacter pylori gastritis. Eur J Pediatr. 2015;174:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Sato Y, Yoneyama O, Azumaya M, Takeuchi M, Sasaki SY, Yokoyama J, Shioji K, Kawauchi Y, Hashimoto S, Nishigaki Y. The relationship between iron deficiency in patients with Helicobacter pylori-infected nodular gastritis and the serum prohepcidin level. Helicobacter. 2015;20:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Azab SF, Esh AM. Serum hepcidin levels in Helicobacter pylori-infected children with iron-deficiency anemia: a case-control study. Ann Hematol. 2013;92:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Yokota S, Konno M, Mino E, Sato K, Takahashi M, Fujii N. Enhanced Fe ion-uptake activity in Helicobacter pylori strains isolated from patients with iron-deficiency anemia. Clin Infect Dis. 2008;46:e31-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 75. | Noto JM, Lee JY, Gaddy JA, Cover TL, Amieva MR, Peek RM. Regulation of Helicobacter pylori Virulence Within the Context of Iron Deficiency. J Infect Dis. 2015;211:1790-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 77. | Stein J, Stier C, Raab H, Weiner R. Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 78. | Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4:S73-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 79. | Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol. 2008;83:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 80. | ten Broeke R, Bravenboer B, Smulders FJ. Iron deficiency before and after bariatric surgery: the need for iron supplementation. Neth J Med. 2013;71:412-417. [PubMed] |
| 81. | Obinwanne KM, Fredrickson KA, Mathiason MA, Kallies KJ, Farnen JP, Kothari SN. Incidence, treatment, and outcomes of iron deficiency after laparoscopic Roux-en-Y gastric bypass: a 10-year analysis. J Am Coll Surg. 2014;218:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Alexandrou A, Armeni E, Kouskouni E, Tsoka E, Diamantis T, Lambrinoudaki I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus Roux-en-Y gastric bypass: a pilot study. Surg Obes Relat Dis. 2014;10:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 83. | Apovian CM, Cummings S, Anderson W, Borud L, Boyer K, Day K, Hatchigian E, Hodges B, Patti ME, Pettus M. Best practice updates for multidisciplinary care in weight loss surgery. Obesity (Silver Spring). 2009;17:871-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Malone M, Barish C, He A, Bregman D. Comparative review of the safety and efficacy of ferric carboxymaltose versus standard medical care for the treatment of iron deficiency anemia in bariatric and gastric surgery patients. Obes Surg. 2013;23:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Godfrey JD, Brantner TL, Brinjikji W, Christensen KN, Brogan DL, Van Dyke CT, Lahr BD, Larson JJ, Rubio-Tapia A, Melton LJ. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology. 2010;139:763-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 86. | Çekın AH, Çekın Y, Sezer C. Celiac disease prevalence in patients with iron deficiency anemia. Turk J Gastroenterol. 2012;23:490-495. [PubMed] |
| 87. | Unsworth DJ, Lock FJ, Harvey RF. Iron-deficiency anaemia in premenopausal women. Lancet. 1999;353:1100. [PubMed] |
| 88. | Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 89. | Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5:3975-3992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 90. | Corazza GR, Valentini RA, Andreani ML, D’Anchino M, Leva MT, Ginaldi L, De Feudis L, Quaglino D, Gasbarrini G. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand J Gastroenterol. 1995;30:153-156. [PubMed] |
| 91. | Howard MR, Turnbull AJ, Morley P, Hollier P, Webb R, Clarke A. A prospective study of the prevalence of undiagnosed coeliac disease in laboratory defined iron and folate deficiency. J Clin Pathol. 2002;55:754-757. [PubMed] |
| 92. | Hershko C, Patz J. Ironing out the mechanism of anemia in celiac disease. Haematologica. 2008;93:1761-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Fine KD. The prevalence of occult gastrointestinal bleeding in celiac sprue. N Engl J Med. 1996;334:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Martin J, Geisel T, Maresch C, Krieger K, Stein J. Inadequate nutrient intake in patients with celiac disease: results from a German dietary survey. Digestion. 2013;87:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 95. | Harper JW, Holleran SF, Ramakrishnan R, Bhagat G, Green PH. Anemia in celiac disease is multifactorial in etiology. Am J Hematol. 2007;82:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 96. | Maslovsky I. Intravenous iron in a primary-care clinic. Am J Hematol. 2005;78:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Annibale B, Severi C, Chistolini A, Antonelli G, Lahner E, Marcheggiano A, Iannoni C, Monarca B, Delle FG. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am J Gastroenterol. 2001;96:132-137. [PubMed] |
| 98. | Vici G, Belli L, Biondi M, Polzonetti V. Gluten free diet and nutrient deficiencies: A review. Clin Nutr. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 99. | Pironi L, Arends J, Baxter J, Bozzetti F, Peláez RB, Cuerda C, Forbes A, Gabe S, Gillanders L, Holst M. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 100. | Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130:S70-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 101. | Ubesie AC, Kocoshis SA, Mezoff AG, Henderson CJ, Helmrath MA, Cole CR. Multiple micronutrient deficiencies among patients with intestinal failure during and after transition to enteral nutrition. J Pediatr. 2013;163:1692-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Yang CF, Duro D, Zurakowski D, Lee M, Jaksic T, Duggan C. High prevalence of multiple micronutrient deficiencies in children with intestinal failure: a longitudinal study. J Pediatr. 2011;159:39-44.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Khaodhiar L, Keane-Ellison M, Tawa NE, Thibault A, Burke PA, Bistrian BR. Iron deficiency anemia in patients receiving home total parenteral nutrition. JPEN J Parenter Enteral Nutr. 2002;26:114-119. [PubMed] |
| 104. | Hwa YL, Rashtak S, Kelly DG, Murray JA. Iron Deficiency in Long-Term Parenteral Nutrition Therapy. JPEN J Parenter Enteral Nutr. 2016;40:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 105. | Allwood MC, Martin H, Greenwood M, Maunder M. Precipitation of trace elements in parenteral nutrition mixtures. Clin Nutr. 1998;17:223-226. [PubMed] |
| 106. | Robinson CA, Sawyer JE. Y-site compatibility of medications with parenteral nutrition. J Pediatr Pharmacol Ther. 2009;14:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 107. | Beale AL, Penney MD, Allison MC. The prevalence of iron deficiency among patients presenting with colorectal cancer. Colorectal Dis. 2005;7:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 108. | Ho CH, Yu YB, Wu PH. The prevalence of iron deficiency anemia and its clinical implications in patients with colorectal carcinoma. J Chin Med Assoc. 2008;71:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 109. | Edna TH, Karlsen V, Jullumstrø E, Lydersen S. Prevalence of anaemia at diagnosis of colorectal cancer: assessment of associated risk factors. Hepatogastroenterology. 2012;59:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 110. | Khanbhai M, Shah M, Cantanhede G, Ilyas S, Richards T. The problem of anaemia in patients with colorectal cancer. ISRN Hematol. 2014;2014:547914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Ludwig H, Müldür E, Endler G, Hübl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24:1886-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 112. | Del Giudice ME, Vella ET, Hey A, Simunovic M, Harris W, Levitt C. Systematic review of clinical features of suspected colorectal cancer in primary care. Can Fam Physician. 2014;60:e405-e415. [PubMed] |
| 113. | Wilkins T, Baird C, Pearson AN, Schade RR. Diverticular bleeding. Am Fam Physician. 2009;80:977-983. [PubMed] |
| 114. | Cappell MS. The pathophysiology, clinical presentation, and diagnosis of colon cancer and adenomatous polyps. Med Clin North Am. 2005;89:1-42, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 115. | Judson I, Demetri G. Advances in the treatment of gastrointestinal stromal tumours. Ann Oncol. 2007;18 Suppl 10:x20-x24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 117. | Kaemmer DA, Otto J, Lassay L, Steinau G, Klink C, Junge K, Klinge U, Schumpelick V. The Gist of literature on pediatric GIST: review of clinical presentation. J Pediatr Hematol Oncol. 2009;31:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 118. | Duffaud F, Even C, Ray-Coquard I, Bompas E, Khoa-Huynh T, Salas S, Cassier P, Dufresne A, Bonvalot S, Ducimetiere F. Recombinant erythropoietin for the anaemia of patients with advanced Gastrointestinal Stromal Tumours (GIST) receiving imatinib: an active agent only in non progressive patients. Clin Sarcoma Res. 2012;2:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Sodergren SC, White A, Efficace F, Sprangers M, Fitzsimmons D, Bottomley A, Johnson CD. Systematic review of the side effects associated with tyrosine kinase inhibitors used in the treatment of gastrointestinal stromal tumours on behalf of the EORTC Quality of Life Group. Crit Rev Oncol Hematol. 2014;91:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 120. | Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther. 2013;37:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 121. | Nakatani M, Fujiwara Y, Nagami Y, Sugimori S, Kameda N, Machida H, Okazaki H, Yamagami H, Tanigawa T, Watanabe K. The usefulness of double-balloon enteroscopy in gastrointestinal stromal tumors of the small bowel with obscure gastrointestinal bleeding. Intern Med. 2012;51:2675-2682. [PubMed] |
| 122. | Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 123. | Ioannou GN, Rockey DC, Bryson CL, Weiss NS. Iron deficiency and gastrointestinal malignancy: a population-based cohort study. Am J Med. 2002;113:276-280. [PubMed] |
| 124. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1104] [Article Influence: 84.9] [Reference Citation Analysis (1)] |
| 125. | Association of Coloproctology of Great Britain and Ireland. Guidelines for the Management of Colorectal Cancer. [last accessed 15 Feb 2015]. Available from: http://www.acpgbi.org.uk/resources/guidelines/guidelines-for-the-management-of-colorectal-cancer/. |
| 126. | Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and management of colorectal cancer. Available from: http://www.sign.ac.uk. |
| 127. | National Comprehensive Cancer Network Inc. NCCN Practice Guidelines in Oncology; Cancer and Chemotherapy-Induced Anemia - v.2.2015. Available from: http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf. |
| 128. | Edwards TJ, Noble EJ, Durran A, Mellor N, Hosie KB. Randomized clinical trial of preoperative intravenous iron sucrose to reduce blood transfusion in anaemic patients after colorectal cancer surgery. Br J Surg. 2009;96:1122-1128. [PubMed] |
| 129. | Muñoz M, Gómez-Ramírez S, Martín-Montañez E, Auerbach M. Perioperative anemia management in colorectal cancer patients: a pragmatic approach. World J Gastroenterol. 2014;20:1972-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 130. | Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;CD005033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 131. | Houbiers JG, van de Velde CJ, van de Watering LM, Hermans J, Schreuder S, Bijnen AB, Pahlplatz P, Schattenkerk ME, Wobbes T, de Vries JE. Transfusion of red cells is associated with increased incidence of bacterial infection after colorectal surgery: a prospective study. Transfusion. 1997;37:126-134. [PubMed] |
| 132. | Schiergens TS, Rentsch M, Kasparek MS, Frenes K, Jauch KW, Thasler WE. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum. 2015;58:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 133. | Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 562] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 134. | Keeler BD, Simpson JA, Ng S, Tselepis C, Iqbal T, Brookes MJ, Acheson AG. The feasibility and clinical efficacy of intravenous iron administration for preoperative anaemia in patients with colorectal cancer. Colorectal Dis. 2014;16:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 135. | Borstlap WA, Buskens CJ, Tytgat KM, Tuynman JB, Consten EC, Tolboom RC, Heuff G, van Geloven AA, van Wagensveld BA, C A Wientjes CA. Multicentre randomized controlled trial comparing ferric(III)carboxymaltose infusion with oral iron supplementation in the treatment of preoperative anaemia in colorectal cancer patients. BMC Surg. 2015;15:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 136. | Ranganathan P, Pramesh CS. Letter 1: Randomized clinical trial of preoperative intravenous iron sucrose to reduce blood transfusion in anaemic patients after colorectal cancer surgery (Br J Surg 2009; 96: 1122-1128). Br J Surg. 2010;97:297-298; author reply 299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 137. | Leal-Noval SR, Muñoz M, Asuero M, Contreras E, García-Erce JA, Llau JV, Moral V, Páramo JA, Quintana M. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 138. | Hallet J, Hanif A, Callum J, Pronina I, Wallace D, Yohanathan L, McLeod R, Coburn N. The impact of perioperative iron on the use of red blood cell transfusions in gastrointestinal surgery: a systematic review and meta-analysis. Transfus Med Rev. 2014;28:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 139. | Azzam N, Aljebreen AM, Alharbi O, Almadi MA. Prevalence and clinical features of colonic diverticulosis in a Middle Eastern population. World J Gastrointest Endosc. 2013;5:391-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | World Gastroenterology Organisation. World Gastroenterology Organisation Practice Guidelines: Diverticular Disease. Available from: http://www.worldgastroenterology.org/diverticular-disease.html. |
| 141. | Baxter NN. Emergency management of diverticulitis. Clin Colon Rectal Surg. 2004;17:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 142. | Al-Onaizi I, Al-Awadi F, Al-Dawood AL. Iron deficiency anaemia: an unusual complication of Meckel’s diverticulum. Med Princ Pract. 2002;11:214-217. [PubMed] |
| 143. | Sagar J, Kumar V, Shah DK. Meckel’s diverticulum: a systematic review. J R Soc Med. 2006;99:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 144. | Kubo A, Kagaya T, Nakagawa H. Studies on complications of diverticular disease of the colon. Jpn J Med. 1985;24:39-43. [PubMed] |
| 145. | Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD, Cutler DF. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 146. | Holleran G, Hall B, Hussey M, McNamara D. Small bowel angiodysplasia and novel disease associations: a cohort study. Scand J Gastroenterol. 2013;48:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 147. | Massyn MW, Khan SA. Heyde syndrome: a common diagnosis in older patients with severe aortic stenosis. Age Ageing. 2009;38:267-70; discussion 251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 148. | Aktas S, Kiyak M, Ozdil K, Kurtca I, Kibar S, Ahbab S, Karadeniz Y, Saler T. Gastrointestinal Tract Hemorrhage due to Angiodysplasia in Hutchinson Gilfort Progeria Syndrome. J Med Cases. 2013;4:576-578. |
| 149. | Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 150. | Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35-59. [PubMed] |
| 151. | Hesham MS, Edariah AB, Norhayati M. Intestinal parasitic infections and micronutrient deficiency: a review. Med J Malaysia. 2004;59:284-293. [PubMed] |
| 152. | Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, Gonzalez-Medina D, Barber R, Huynh C, Dicker D. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1071] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 153. | M’Koma AE, Wise PE, Schwartz DA, Muldoon RL, Herline AJ. Prevalence and outcome of anemia after restorative proctocolectomy: a clinical literature review. Dis Colon Rectum. 2009;52:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 154. | Coull DB, Tait RC, Anderson JH, McKee RF, Finlay IG. Vitamin B12 deficiency following restorative proctocolectomy. Colorectal Dis. 2007;9:562-566. [PubMed] |
| 155. | Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15:4653-4658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 156. | Hézode C. Management of anaemia and other treatment complications. Dig Liver Dis. 2013;45 Suppl 5:S337-S342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 157. | Ogawa E, Furusyo N, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A. Clinical milestones for the prediction of severe anemia by chronic hepatitis C patients receiving telaprevir-based triple therapy. J Hepatol. 2013;59:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 158. | Zeuzem S, DeMasi R, Baldini A, Coate B, Luo D, Mrus J, Witek J. Risk factors predictive of anemia development during telaprevir plus peginterferon/ribavirin therapy in treatment-experienced patients. J Hepatol. 2014;60:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 159. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] |
| 160. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [PubMed] |
| 161. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [PubMed] |
| 162. | Sulkowski MS, Poordad F, Manns MP, Bronowicki JP, Rajender Reddy K, Harrison SA, Afdhal NH, Sings HL, Pedicone LD, Koury KJ. Anemia during treatment with peginterferon Alfa-2b/ribavirin and boceprevir: Analysis from the serine protease inhibitor therapy 2 (SPRINT-2) trial. Hepatology. 2013;57:974-984. [PubMed] |
| 163. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [PubMed] |
| 164. | Mac Nicholas R, Norris S. Review article: optimizing SVR and management of the haematological side effects of peginterferon/ribavirin antiviral therapy for HCV - the role of epoetin, G-CSF and novel agents. Aliment Pharmacol Ther. 2010;31:929-937. [PubMed] |
| 165. | Poordad F, Lawitz E, Reddy KR, Afdhal NH, Hézode C, Zeuzem S, Lee SS, Calleja JL, Brown RS, Craxi A. Effects of ribavirin dose reduction vs erythropoietin for boceprevir-related anemia in patients with chronic hepatitis C virus genotype 1 infection--a randomized trial. Gastroenterology. 2013;145:1035-1044.e5. [PubMed] |
| 166. | Romero-Gómez M, Berenguer M, Molina E, Calleja JL. Management of anemia induced by triple therapy in patients with chronic hepatitis C: challenges, opportunities and recommendations. J Hepatol. 2013;59:1323-1330. [PubMed] |
| 167. | Clark PJ, Aghemo A, Degasperi E, Galmozzi E, Urban TJ, Vock DM, Patel K, Thompson AJ, Rumi MG, D’Ambrosio R. Inosine triphosphatase deficiency helps predict anaemia, anaemia management and response in chronic hepatitis C therapy. J Viral Hepat. 2013;20:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 168. | Jacobson IM, Kowdley KV, Kwo PY. Anemia management in the era of triple combination therapy for chronic HCV. Gastroenterol Hepatol (N Y). 2012;8:1-16. [PubMed] |
| 169. | Maan R, van der Meer AJ, Brouwer WP, Plompen EP, Sonneveld MJ, Roomer R, van der Eijk AA, Groothuismink ZM, Hansen BE, Veldt BJ. ITPA Polymorphisms Are Associated with Hematological Side Effects during Antiviral Therapy for Chronic HCV Infection. PLoS One. 2015;10:e0139317. [PubMed] |
| 170. | Suwanthawornkul T, Anothaisintawee T, Sobhonslidsuk A, Thakkinstian A, Teerawattananon Y. Efficacy of Second Generation Direct-Acting Antiviral Agents for Treatment Naïve Hepatitis C Genotype 1: A Systematic Review and Network Meta-Analysis. PLoS One. 2015;10:e0145953. [PubMed] |
| 171. | Siddique A, Nelson JE, Aouizerat B, Yeh MM, Kowdley KV. Iron deficiency in patients with nonalcoholic Fatty liver disease is associated with obesity, female gender, and low serum hepcidin. Clin Gastroenterol Hepatol. 2014;12:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 172. | Ruivard M, Lainé F, Ganz T, Olbina G, Westerman M, Nemeth E, Rambeau M, Mazur A, Gerbaud L, Tournilhac V. Iron absorption in dysmetabolic iron overload syndrome is decreased and correlates with increased plasma hepcidin. J Hepatol. 2009;50:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 173. | Moya D, Baker SS, Liu W, Garrick M, Kozielski R, Baker RD, Zhu L. Novel pathway for iron deficiency in pediatric non-alcoholic steatohepatitis. Clin Nutr. 2015;34:549-556. [PubMed] |
| 174. | Brownlie T, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437-443. [PubMed] |
| 175. | Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A, Gasche C. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women--PREFER a randomized, placebo-controlled study. PLoS One. 2014;9:e94217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 176. | Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118:3222-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 177. | Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778-787. [PubMed] |
| 178. | Jankowska EA, Von HS, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2012;34:816-829. |
| 179. | Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 180. | Fillet G, Beguin Y, Baldelli L. Model of reticuloendothelial iron metabolism in humans: abnormal behavior in idiopathic hemochromatosis and in inflammation. Blood. 1989;74:844-851. [PubMed] |
| 181. | Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1 Suppl 1:S4-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 182. | Stein J, Bager P, Befrits R, Gasche C, Gudehus M, Lerebours E, Magro F, Mearin F, Mitchell D, Oldenburg B. Anaemia management in patients with inflammatory bowel disease: routine practice across nine European countries. Eur J Gastroenterol Hepatol. 2013;25:1456-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 183. | Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 184. | Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, Fergusson DA, Gombotz H, Habler O, Monk TG. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (1)] |
| 185. | Jumaa AK. Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012;2 Suppl:279-335. |
| 186. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3411] [Cited by in RCA: 3548] [Article Influence: 272.9] [Reference Citation Analysis (0)] |
| 187. | Qunibi WY. The efficacy and safety of current intravenous iron preparations for the management of iron-deficiency anaemia: a review. Arzneimittelforschung. 2010;60:399-412. [PubMed] |
| 188. | Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 299] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 189. | Yessayan L, Sandhu A, Besarab A, Yessayan A, Frinak S, Zasuwa G, Yee J. Intravenous iron dextran as a component of anemia management in chronic kidney disease: a report of safety and efficacy. Int J Nephrol. 2013;2013:703038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 190. | European Medicines Agency (EMA). Assessment report for: Iron containing intravenous (IV) medicinal products. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500150771.pdf. |
| 191. | Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D’Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 192. | Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, Chopey IV, Gutzwiller FS, Riopel L, Gasche C. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 193. | Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 994] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 194. | Spahn DR, Moch H, Hofmann A, Isbister JP. Patient blood management: the pragmatic solution for the problems with blood transfusions. Anesthesiology. 2008;109:951-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 195. | Taylor RW, O’Brien J, Trottier SJ, Manganaro L, Cytron M, Lesko MF, Arnzen K, Cappadoro C, Fu M, Plisco MS. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302-238; quiz 2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 196. | Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Rebolledo A, Basfi-fer K, Csendes A, Papapietro K, Pizarro F. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |