Published online Jun 14, 2016. doi: 10.3748/wjg.v22.i22.5276
Peer-review started: January 25, 2016
First decision: February 18, 2016
Revised: March 2, 2016
Accepted: March 30, 2016
Article in press: March 30, 2016
Published online: June 14, 2016
Processing time: 129 Days and 18.3 Hours
AIM: To evaluate the assessment of primary biliary cirrhosis degree by acoustic radiation force impulse imaging (ARFI) and hepatic fibrosis indicators.
METHODS: One hundred and twenty patients who developed liver cirrhosis secondary to primary biliary cirrhosis were selected as the observation group, with the degree of patient liver cirrhosis graded by Child-Pugh (CP) score. Sixty healthy individuals were selected as the control group. The four indicators of hepatic fibrosis were detected in all research objects, including hyaluronic acid (HA), laminin (LN), type III collagen (PC III), and type IV collagen (IV-C). The liver parenchyma hardness value (LS) was then measured by ARFI technique. LS and the four indicators of liver fibrosis (HA, LN, PC III, and IV-C) were observed in different grade CP scores. The diagnostic value of LS and the four indicators of liver fibrosis in determining liver cirrhosis degree with PBC, whether used alone or in combination, were analyzed by receiver operating characteristic (ROC) curve.
RESULTS: LS and the four indicators of liver fibrosis within the three classes (A, B, and C) of CP scores in the observation group were higher than in the control group, with C class > B class > A class; the differences were statistically significant (P < 0.01). Although AUC values of LS within the three classes of CP scores were higher than in the four indicators of liver fibrosis, sensitivity and specificity were unstable. The ROC curves of LS combined with the four indicators of liver fibrosis revealed that: AUC and sensitivity in all indicators combined in the A class of CP score were higher than in LS alone, albeit with slightly decreased specificity; AUC and specificity in all indicators combined in the B class of CP score were higher than in LS alone, with unchanged sensitivity; AUC values (0.967), sensitivity (97.4%), and specificity (90%) of all indicators combined in the C class of CP score were higher than in LS alone (0.936, 92.1%, 83.3%).
CONCLUSION: The diagnostic value of PBC cirrhosis degree in liver cirrhosis degree assessment by ARFI combined with the four indicators of serum liver fibrosis is of satisfactory effectiveness and has important clinical application value.
Core tip: One hundred and twenty patients who had developed liver cirrhosis from primary biliary cirrhosis were assessed by ARFI imaging and hepatic fibrosis index alongside sixty healthy individuals. The ROC curves of LS combined with four liver fibrosis indexes showed that the AUC values (0.967), sensitivity (97.4%), and specificity (90%) of all indexes combined in the C grade of CP score were higher than in those of LS alone (0.936, 92.1%, and 83.3%). The diagnostic value of PBC cirrhosis degree in liver cirrhosis degree assessment by ARFI combined with the four indicators of serum liver fibrosis is of satisfactory effectiveness and has important clinical application value.
- Citation: Zhang HC, Hu RF, Zhu T, Tong L, Zhang QQ. Primary biliary cirrhosis degree assessment by acoustic radiation force impulse imaging and hepatic fibrosis indicators. World J Gastroenterol 2016; 22(22): 5276-5284
- URL: https://www.wjgnet.com/1007-9327/full/v22/i22/5276.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i22.5276
Primary biliary cirrhosis (PBC) is a chronic cholestatic disease[1] that can develop into liver fibrosis, cirrhosis[2-4], and even lead to liver failure[5]. When a patient is already in the liver cirrhosis stage, accurate diagnosis, and assessment of the extent of liver cirrhosis is vital to the diagnosis, treatment, and prognosis of the disease[6]. Therefore, exploring a high value examination method to diagnose liver cirrhosis is very significant[7-9]. It has been reported that liver cirrhosis can be divided into three classes according to the Child-Pugh (CP) scoring criteria, and the accuracy of their assessment methods have been demonstrated[10-13]. Although liver biopsy is still currently the preferred diagnostic method for cirrhosis, the resulting trauma to the patient’s body leads to low acceptance[14-17]. Serum fibrosis indicators are a non-invasive examination method of cirrhosis diagnosis with a wide range of applications[18], however its accuracy in the assessment of cirrhosis degree remains to be studied[19]. Acoustic radiation force impulse imaging (ARFI) is a new ultrasound elastography technique[20] that can detect the hardness of the liver parenchyma for liver disease accurate assessment, and is non-invasive, simple, repeatable[21-23], and it can effectively compensate for the lack of liver biopsy and serum liver fibrosis markers. ARFI technology in China remains at the clinical development phase[24-26]. However, comparative studies of ARFI technology and other methods to assess the degree of liver cirrhosis and joint applications are few[27-30]. This study intends to use the CP score as a grading standard, as well as to observe the comparison of ARFI technology measured serum fibrosis markers alone and in combination with diagnostic accuracy to find a more satisfactory diagnostic method for PBC, with the aim of providing a theoretical basis for the clinical diagnosis and treatment of liver cirrhosis.
From January 2014 to September 2015, 120 patients with primary cholestatic cirrhosis that had developed to the stage of cirrhosis and were admitted to Huashan Hospital (Baoshan Branch Affiliated to Fudan University, Shanghai, China) were selected as the observation group. The patients consisted of 35 males and 85 females, with an average age of 56.33 ± 7.42 years. Patients were divided into different groups according to Child-Pugh score as follows: grade A, 39 cases; grade B, 43 cases; and grade C, 38 cases. Meanwhile, 60 healthy subjects were chosen as the control group, and consisted of 24 males and 36 females, with an average age of 54.27 ± 8.31 years. General information on the differences between these two groups was not statistically significant (P > 0.05). This study was approved by the ethics committee.
The degree of liver cirrhosis in patients was diagnosed based on symptoms, signs, CT, MRI, biochemical examination, and liver biopsy results.
(1) Diagnosed with PBC that has developed to liver cirrhosis; (2) healthy subjects with no hepatobiliary diseases; (3) independent and able to cooperate with the test; and (4) provided written informed consent.
(1) Patients with liver cancer or heart, lung, or other vital organs diseases; (2) disturbance of consciousness or mental illness; and (3) patients who provided written informed consent, but failed to cooperate with the test.
Patients were scored according to hepatic encephalopathy, peritoneal effusion, total bilirubin and albumin content, prolonged prothrombin time, and other conditions. Child-Pugh classification criteria (Table 1): class A, 5-6 points; class B, 7-9 points; and class C, ≥ 10 points.
| Indicator | Score | ||
| 1 point | 2 point | 3 point | |
| Hepatic encephalopathy (grade) | None | Slight | Occasional drowsiness |
| Ascites | None | Small amount of diuretics can be controlled | Numerous |
| Total bilirubin (μmol/L) | < 34 | 34-51 | > 51 |
| Albumin (g/L) | > 35 | 28-35 | < 28 |
| Prolonged prothrombin time(s) | < 4 | 4-6 | > 6 |
Liver fibrosis index detection: (1) After fasting, 5 ml of morning blood samples were collected from patients and kept at room temperature for approximately 30 min; (2) serum was separated and stored at -70 °C; and (3) four indexes of liver fibrosis were determined using fluorescence immunoassay: hyaluronic acid (HA), laminin (LN), procollagen III (PCIII), and collagen IV (IV-C).
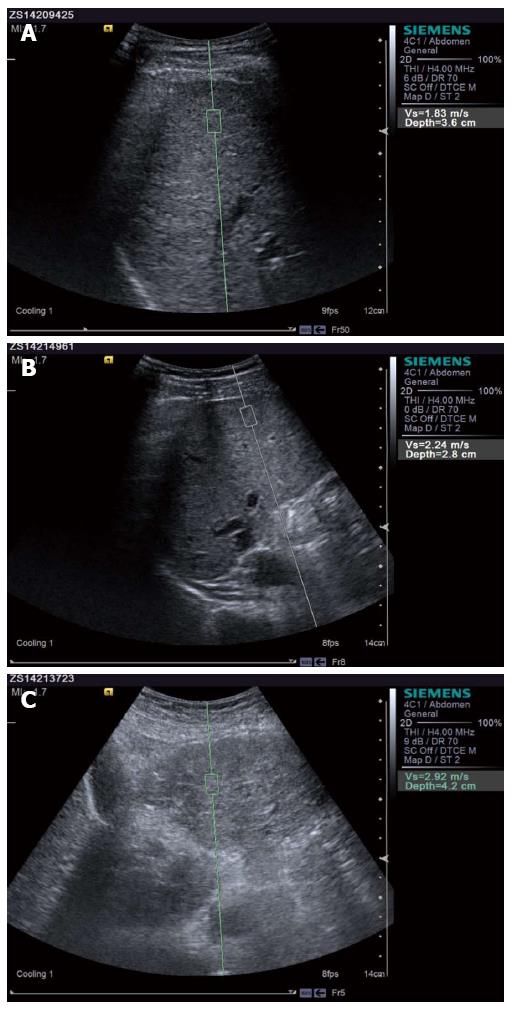
ARFI detection: Siemens ACUSON S2000 color ultrasound diagnostic apparatus was used to conduct ARFI detection. (1) After fasting, the patient was placed on the left lateral position with the right hand on the head, and the right lobe of the liver tissue was detected; (2) elastic sampling frame was perpendicular to the surface of the liver, with a depth of approximately 2-5 cm while avoiding the surrounding blood vessels, and the patient was asked to hold their breath; and (3) the update button was pressed, a high-strength low-frequency pulse was launched, and the transverse shear wave velocity (Vs) was received. Units were in m/s and the value was recorded. Measurements were repeated 10 times and Vs were averaged to determine liver parenchyma hardness LS value.
SPSS 17.0 statistical software was used for all data results. LS value and the four indicators of liver fibrosis were measurement data presented as mean ± SD, with groups compared using two independent samples t-test. To evaluate the diagnostic value of LS value and the four serum indicators for liver fibrosis detected by ARFI (HA, LN, PCIII, and IV-C) for PBC, receiver operating characteristic (ROC) curve analysis with the area under the ROC curve (AUC), sensitivity and specificity representations were used. P < 0.05 was considered statistically significant.
CP scores, LS values, and the four serum indicators for liver fibrosis in the three classes (A, B, and C) of patients in the observation group were significantly higher than controls; the difference was statistically significant (P < 0.01). In the observation group, CP scores in the three classes of patients, LS values (Figure 1), and the four serum indicators for liver fibrosis revealed that class C > class B > class A; differences were statistically significant (P < 0.01), as shown in Table 2.
| Item | Control group (n = 60) | Observation group | ||
| A class (n = 39) | B class (n = 43) | C class (n = 38) | ||
| LS value (m/s) | 1.03 ± 0.03 | 1.90 ± 0.07a | 2.31 ± 0.02a | 2.92 ± 0.17a |
| HA (ng/mL) | 54.96 ± 21.13 | 431.01 ± 118.04a | 619.03 ± 164.28a | 857.13 ± 192.05a |
| LN (ng/mL) | 79.11 ± 15.37 | 116.14 ± 18.77a | 153.42 ± 36.25a | 211.09 ± 30.18a |
| PCIII (ng/mL) | 89.91 ± 18.76 | 142.51 ± 30.07a | 227.93 ± 69.11a | 367.39 ± 99.21a |
| IV-C (ng/mL) | 51.32 ± 9.27 | 104.58 ± 42.17a | 168.99 ± 32.14a | 193.36 ± 30.22a |
ROC curve analysis of LS values and the four diagnostic indicators of liver fibrosis of CP rates in different cirrhosis grades and each index of the AUC showed: grade C > grade B > grade A, as well as that the sensitivity and specificity were different (Table 3). Comparison of results of CP levels of LS values and the four indicators of liver fibrosis in the ROC curve are as follows:
| Item | A Class | B Class | C Class | ||||||
| AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | |
| LS value | 0.852 | 57.9% | 93.3% | 0.911 | 97.4% | 75.0% | 0.936 | 92.1% | 83.3% |
| HA | 0.694 | 97.4% | 55.0% | 0.852 | 97.4% | 65.0% | 0.888 | 63.2% | 96.7% |
| LN | 0.707 | 97.4% | 43.3% | 0.746 | 46.2% | 96.7% | 0.828 | 97.4% | 58.3% |
| PCIII | 0.741 | 57.9% | 86.7% | 0.823 | 53.8% | 96.7% | 0.871 | 86.8% | 75.0% |
| IV-C | 0.688 | 78.9% | 56.7% | 0.785 | 97.4% | 51.7% | 0.889 | 94.7% | 73.3% |
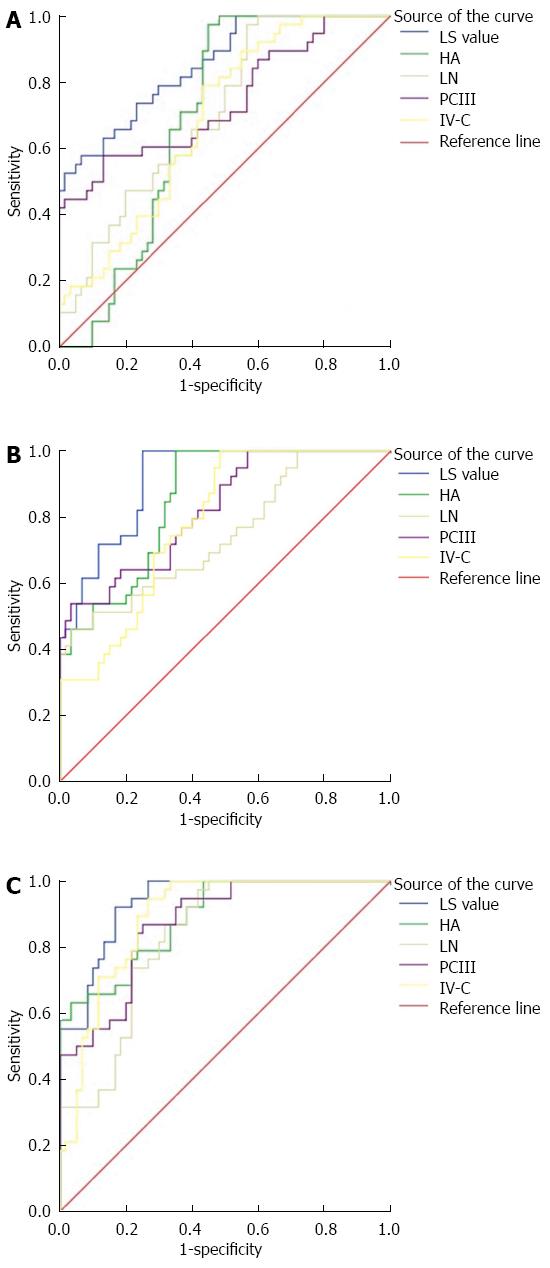
In CP score grade A, LS values in the AUC and the specificity were high compared with serum liver fibrosis, albeit with lower sensitivity (Figure 2A).
In grade B, the AUC value of LS and specificity were high compared with HA and IV-C, but with lower sensitivity; AUC and sensitivity were high compared with LN and PCIII, but with lower specificity (Figure 2B).
In grade C, AUC values of LS, sensitivity, and specificity were high compared with PCIII; AUC and sensitivity were high compared with HA, but with lower specificity; AUC and specificity were high compared with LN and IV-C, but with lower sensitivity (Figure 2C).
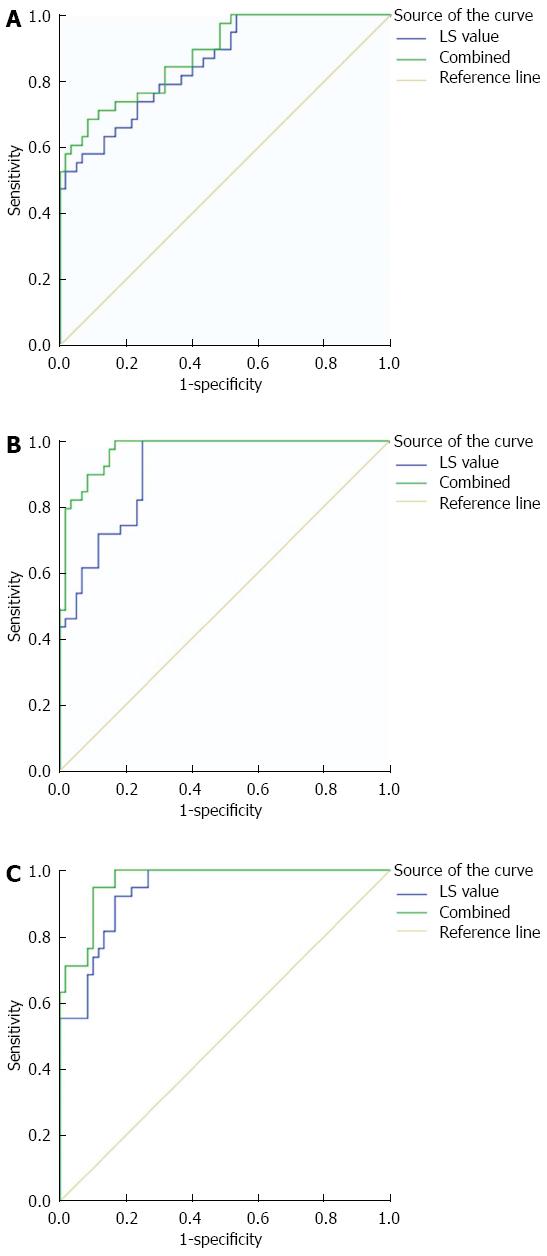
LS values detected by ARFI in the observation group combined with the four indicators of serum liver fibrosis in the ROC curve show the following (Table 4): in each indicator of CP score grade A, the AUC and sensitivity were higher than the LS value detected by ARFI alone, although its specificity decreased slightly (Figure 3A); in CP score grade B, the AUC and sensitivity were higher than LS detected by ARFI alone, with sensitivity being constant (Figure 3B); in CP score grade C, the AUC, sensitivity, and specificity were higher than the LS values detected by ARFI alone (Figure 3C).
| LS value | Combination | |||||
| AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | |
| A Class | 0.852 | 57.9% | 93.3% | 0.881 | 68.4% | 91.7% |
| B Class | 0.911 | 97.4% | 75.0% | 0.973 | 97.4% | 85.0% |
| C Class | 0.936 | 92.1% | 83.3% | 0.967 | 97.4% | 90.0% |
Cholestatic liver cirrhosis is a chronic liver disease with a long and gradual progression to liver cirrhosis[31-34]. An accurate assessment of early liver cirrhosis can effectively prevent further liver damage that can result in liver failure[35-37]; this has great significance for the diagnosis, treatment, and prognosis of chronic liver disease[38-40]. In this study, by comparing the diagnostic values of AFRI detected LS values and the four indicators (HA, LN, PCIII, and IV-C) of serum liver fibrosis alone or in combination, we aimed to accurately and effectively explore this examination method for the assessment of cirrhosis degree.
Liver stiffness increases as chronic liver disease develops to liver fibrosis and cirrhosis. In this study, the LS value results and four indicators of serum liver fibrosis in the observation group showed class C > class B > class A trends; the level of indicators were significantly higher. LS values and the four indicators of serum liver fibrosis of cirrhotic patients were higher than in the control group; this increased as liver cirrhosis degree increased. This also proves that ARFI-detected LS values and the four indicators of serum liver fibrosis can reflect changes in the degree of cirrhosis. Studies have reported[41] that AFRI-detected LS values increased as the degree of hepatic fibrosis increased; this can be widely used in patients with chronic liver disease. In recent years, this research has garnered more attention. The four serum fibrosis indicators for liver damage can be assessed via changes in each indicator, and thus can effectively diagnose cirrhosis. However, its detection accuracy for liver cirrhosis degree remains as yet unconfirmed[42].
In the ROC curve analysis of LS value and the four indicators of liver fibrosis, we found the following: LS value and the four indicators of liver fibrosis in the AUC are present in class C > class B > class A trends, and that the diagnostic accuracy of each indicator can increase with increased liver cirrhosis degree (i.e., each indicator can assess the degree of cirrhosis). While each indicator for the diagnostic value of different grades of liver cirrhosis are different, a comparison of results from the ROC curves show that the CP score of the three classes in the AUC were higher than in the four indicators of liver fibrosis, but that its sensitivity and specificity were unstable. CP score class A: LS values were higher than that of serum-specific liver fibrosis, but with lower sensitivity; CP score class B: LS values and specificity were higher than HA and IV-C, but with lower sensitivity (sensitivity was higher than LN and PCIII, but with lower specificity); CP score class C: LS values and sensitivity were higher than HA, but with lower specificity (specifically was higher than LN and IV-C, but with relatively lower sensitivity). The results show that the diagnostic value of LS values is high compared to the four indicators of liver fibrosis and that it has high diagnostic accuracy, although its diagnostic sensitivity and specificity is unstable. The sensitivity of the four indicators of liver fibrosis for the diagnosis of cirrhosis degree is strong, but its specificity and overall diagnostic value are insufficient. Detection of the four indicators of serum liver fibrosis can effectively diagnose cirrhosis, but lacks specificity in the accurate assessment of cirrhosis degree; thus, its technical support requires improvement[43]. The most commonly used method for the clinical diagnosis of cirrhosis is liver biopsy. However, due to its invasiveness, it has low acceptance limitations and causes more distress in clinical diagnosis and treatment to a certain extent[44-46]. On the other hand, ARFI ultrasound is a non-invasive detection technology. The degree of liver fibrosis can be determined by detecting LS value, which can compensate for the weakness of liver biopsy in detecting liver fibrosis[47].
In the observation group, the results of the ROC curve analysis of LS value combined with the four indicators of serum liver fibrosis revealed that the CP score of the three classes combined with the diagnosis of AUC values were higher than ARFI-detected LS values alone, with sensitivity and specificity also improving. CP score class A: combined diagnosis sensitivity was higher than the LS value, albeit with slightly decreased specificity; CP score class B: the combined diagnostic specificity value was higher than the LS value, but sensitivity remained unchanged and there was no reduction; CP score class C: combined diagnostic sensitivity and specificity values were higher than the LS value. These results show that combined diagnosis improves the diagnostic accuracy of single-use LS values, and that diagnostic sensitivity and specificity can be guaranteed. The combined diagnostic value of LS values is high compared to the four indicators of liver fibrosis. It also proves that the LS value combined with the four indicators of serum liver fibrosis in the diagnosis of cirrhosis degree is higher than the diagnostic value of each indicator alone.
Requirements for AFRI examination in patients were stringent. This may be due to insufficient coordination between doctors and patients, which affects the accuracy of the examination[48-50]. The detection operation for the four indicators of serum liver fibrosis is relatively simple, but also has its own shortcomings. Combined diagnosis can therefore play a complementary role and help improve diagnostic accuracy. Furthermore, LS values and indicators of liver fibrosis by way of motion detection can assist doctors in understanding the condition of a patient’s
liver disease, which is of great significance in the diagnosis and prognosis of liver cirrhosis.
In summary, the clinical diagnostic value of AFRI-detected LS value for determining liver cirrhosis degree is high compared to the four indicators of serum liver fibrosis. The diagnostic value of two combined diagnostics was more satisfactory compared to the indicators alone. Thus, detection by AFRI technology combined with the four indicators of serum liver fibrosis may serve as a powerful tool for determining liver cirrhosis degree, which has important clinical value and is worthy of wide promotion.
Primary biliary cirrhosis (PBC) is a chronic cholestatic disease that may develop into liver fibrosis, cirrhosis, and even lead to liver failure. When the patient is already in the liver cirrhosis stage, the accurate diagnosis and assessment of the extent of liver cirrhosis is vital in the diagnosis, treatment, and prognosis of the disease. Therefore, exploring a high value examination method to diagnose liver cirrhosis is very significant.
It has been reported that liver cirrhosis can be divided into three classes according to the Child-Pugh (CP) scoring criteria, and the accuracy of their assessment methods have been demonstrated. Although liver biopsy is still currently the preferred diagnostic method for cirrhosis, the resulting trauma to the patient’s body leads to low acceptance. Serum fibrosis indicators are a non-invasive examination method of cirrhosis diagnosis with a wide range of applications; however its accuracy in the assessment of cirrhosis degree remains to be studied. Acoustic radiation force impulse imaging (ARFI) is a new ultrasound elastography technique that can detect the hardness of the liver parenchyma for liver disease accurate assessment, and is non-invasive, simple, repeatable, and can effectively compensate for the lack of liver biopsy and serum liver fibrosis markers.
The clinical diagnostic value of AFRI-detected LS value for determining the degree of liver cirrhosis is high compared to the four indicators of serum liver fibrosis (HA, LN, PCIII, and IV-C). The diagnostic value of two combined diagnostics was more satisfactory compared to the indicators alone. Thus, detection by AFRI technology combined with the four indicators of serum liver fibrosis may serve as a powerful tool for determining liver cirrhosis degree, which has important clinical value and is worthy of wide promotion.
The diagnostic value of cirrhosis degree with PBC through liver cirrhosis degree assessment by ARFI combined with the four indicators of serum liver fibrosis is more satisfactory compared to the indicators alone and has important clinical application value. Results have shown that the higher the LS value, the higher the degree of liver fibrosis. This also confirmed that the diagnostic value of LS value was higher than that of the four indicators of liver fibrosis and, despite high diagnostic accuracy, that the diagnostic sensitivity and specificity were not stable. Further, the diagnosis value of liver cirrhosis degree for LS value combined with the four serum liver fibrosis was higher than each index alone.
The diagnostic value of cirrhosis degree with PBC through liver cirrhosis degree assessment by ARFI combined with the four indicators of serum liver fibrosis is more satisfactory compared to the indicators alone and has important clinical application value. The combination of AFRI and serum liver fibrosis four indicators can be used as a powerful tool to evaluate the degree of cirrhosis. It has important clinical application value and is worthy of clinical application.
P- Reviewer: Adhoute X, Itzel T S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Zhang DN
| 1. | Carbone M, Bufton S, Jones DE, Neuberger JM. Reply to: “Fatigue and liver transplantation in patients with primary biliary cirrhosis”. J Hepatol. 2014;60:1328-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Zhang XX, Wang LF, Jin L, Li YY, Hao SL, Shi YC, Zeng QL, Li ZW, Zhang Z, Lau GK. Primary biliary cirrhosis-associated hepatocellular carcinoma in Chinese patients: incidence and risk factors. World J Gastroenterol. 2015;21:3554-3563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Carrion AF, Bhamidimarri KR. Liver transplant for cholestatic liver diseases. Clin Liver Dis. 2013;17:345-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Genda T, Ichida T, Sakisaka S, Sata M, Tanaka E, Inui A, Egawa H, Umeshita K, Furukawa H, Kawasaki S. Waiting list mortality of patients with primary biliary cirrhosis in the Japanese transplant allocation system. J Gastroenterol. 2014;49:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Inamine T, Higa S, Noguchi F, Kondo S, Omagari K, Yatsuhashi H, Tsukamoto K, Nakamura M. Association of genes involved in bile acid synthesis with the progression of primary biliary cirrhosis in Japanese patients. J Gastroenterol. 2013;48:1160-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 7. | Karlsen TH, Vesterhus M, Boberg KM. Review article: controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther. 2014;39:282-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Campos CF, Paiva DD, Perazzo H, Moreira PS, Areco LF, Terra C, Perez R, Figueiredo FA. An inexpensive and worldwide available digital image analysis technique for histological fibrosis quantification in chronic hepatitis C. J Viral Hepat. 2014;21:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Choi YR, Lee JM, Yoon JH, Han JK, Choi BI. Comparison of magnetic resonance elastography and gadoxetate disodium-enhanced magnetic resonance imaging for the evaluation of hepatic fibrosis. Invest Radiol. 2013;48:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Choi TW, Kim HC, Lee JH, Yu SJ, Kang B, Hur S, Lee M, Jae HJ, Chung JW. The Safety and Clinical Outcomes of Chemoembolization in Child-Pugh Class C Patients with Hepatocellular Carcinomas. Korean J Radiol. 2015;16:1283-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yamamoto N, Okano K, Oshima M, Akamoto S, Fujiwara M, Tani J, Miyoshi H, Yoneyama H, Masaki T, Suzuki Y. Laparoscopic splenectomy for patients with liver cirrhosis: Improvement of liver function in patients with Child-Pugh class B. Surgery. 2015;158:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Lutz P, Pfarr K, Nischalke HD, Krämer B, Goeser F, Glässner A, Wolter F, Kokordelis P, Nattermann J, Sauerbruch T. The ratio of calprotectin to total protein as a diagnostic and prognostic marker for spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. Clin Chem Lab Med. 2015;53:2031-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Kaplan DE, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, Knott A, Pedrosa M, Pocha C, Mehta R. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin Gastroenterol Hepatol. 2015;13:2333-41.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Chung SR, Lee SS, Kim N, Yu ES, Kim E, Kühn B, Kim IS. Intravoxel incoherent motion MRI for liver fibrosis assessment: a pilot study. Acta Radiol. 2015;56:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Park HJ, Lee MW, Lee MH, Hwang J, Kang TW, Lim S, Rhim H, Lim HK. Fusion imaging-guided percutaneous biopsy of focal hepatic lesions with poor conspicuity on conventional sonography. J Ultrasound Med. 2013;32:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ribeiro RT, Marinho RT, Sanches JM. Classification and staging of chronic liver disease from multimodal data. IEEE Trans Biomed Eng. 2013;60:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Short SS, Papillon S, Hunter CJ, Stanley P, Kerkar N, Wang L, Azen C, Wang K. Percutaneous liver biopsy: pathologic diagnosis and complications in children. J Pediatr Gastroenterol Nutr. 2013;57:644-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Lupsor-Platon M, Badea R. Noninvasive assessment of alcoholic liver disease using unidimensional transient elastography (Fibroscan(®)). World J Gastroenterol. 2015;21:11914-11923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Erdogan S, Dogan HO, Sezer S, Uysal S, Ozhamam E, Kayacetin S, Koca Y. The diagnostic value of non-invasive tests for the evaluation of liver fibrosis in chronic hepatitis B patients. Scand J Clin Lab Invest. 2013;73:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Frossard JL, Giostra E, Rubbia-Brandt L, Hadengue A, Spahr L. The role of transient elastography in the detection of liver disease in patients with chronic pancreatitis. Liver Int. 2013;33:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Berzigotti A, Castera L. Update on ultrasound imaging of liver fibrosis. J Hepatol. 2013;59:180-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Balleyguier C, Canale S, Ben Hassen W, Vielh P, Bayou EH, Mathieu MC, Uzan C, Bourgier C, Dromain C. Breast elasticity: principles, technique, results: an update and overview of commercially available software. Eur J Radiol. 2013;82:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Behrens CB, Langholz JH, Eiler J, Jenewein R, Naehrlich L, Fuchs K, Harth S, Krombach GA, Alzen GF. A pilot study of the characterization of hepatic tissue strain in children with cystic-fibrosis-associated liver disease (CFLD) by acoustic radiation force impulse imaging. Pediatr Radiol. 2013;43:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Cassinotto C, Lapuyade B, Aït-Ali A, Vergniol J, Gaye D, Foucher J, Bailacq-Auder C, Chermak F, Le Bail B, de Lédinghen V. Liver fibrosis: noninvasive assessment with acoustic radiation force impulse elastography--comparison with FibroScan M and XL probes and FibroTest in patients with chronic liver disease. Radiology. 2013;269:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Fierbinteanu Braticevici C, Sporea I, Panaitescu E, Tribus L. Value of acoustic radiation force impulse imaging elastography for non-invasive evaluation of patients with nonalcoholic fatty liver disease. Ultrasound Med Biol. 2013;39:1942-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 27. | Goertz RS, Sturm J, Zopf S, Wildner D, Neurath MF, Strobel D. Outcome analysis of liver stiffness by ARFI (acoustic radiation force impulse) elastometry in patients with chronic viral hepatitis B and C. Clin Radiol. 2014;69:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Lammert C, Nguyen DL, Juran BD, Schlicht E, Larson JJ, Atkinson EJ, Lazaridis KN. Questionnaire based assessment of risk factors for primary biliary cirrhosis. Dig Liver Dis. 2013;45:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Landi A, Weismuller TJ, Lankisch TO, Santer DM, Tyrrell DL, Manns MP, Houghton M. Differential serum levels of eosinophilic eotaxins in primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis. J Interferon Cytokine Res. 2014;34:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Mason AL, Montano-Loza AJ, Saxinger L. Letter: biochemical response to combination anti-retroviral therapy in patients with primary biliary cirrhosis. Aliment Pharmacol Ther. 2014;39:236-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Popov Y. Mouse model of primary biliary cirrhosis with progressive fibrosis: are we there yet? Hepatology. 2013;57:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Hanquinet S, Courvoisier D, Kanavaki A, Dhouib A, Anooshiravani M. Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children. Pediatr Radiol. 2013;43:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Bota S, Sporea I, Sirli R, Popescu A, Danila M, Jurchis A, Gradinaru-Tascau O. Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of Acoustic Radiation Force Impulse (ARFI) elastography--analysis of a cohort of 1,031 subjects. Eur J Radiol. 2014;83:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Floreani A, Farinati F. Risk factors associated with hepatocellular carcinoma in primary biliary cirrhosis. Hepatology. 2013;58:1520-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 36. | Fontanilla T, Cañas T, Macia A, Alfageme M, Gutierrez Junquera C, Malalana A, Luz Cilleruelo M, Roman E, Miralles M. Normal values of liver shear wave velocity in healthy children assessed by acoustic radiation force impulse imaging using a convex probe and a linear probe. Ultrasound Med Biol. 2014;40:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Bota S, Bob F, Sporea I, Şirli R, Popescu A. Factors that influence kidney shear wave speed assessed by acoustic radiation force impulse elastography in patients without kidney pathology. Ultrasound Med Biol. 2015;41:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Angulo P. Strengthening the bones in primary biliary cirrhosis. Hepatology. 2013;58:1871-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Fujimoto K, Kato M, Kudo M, Yada N, Shiina T, Ueshima K, Yamada Y, Ishida T, Azuma M, Yamasaki M. Novel image analysis method using ultrasound elastography for noninvasive evaluation of hepatic fibrosis in patients with chronic hepatitis C. Oncology. 2013;84 Suppl 1:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Suh CH, Kim SY, Kim KW, Lim YS, Lee SJ, Lee MG, Lee J, Lee SG, Yu E. Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology. 2014;271:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Gara N, Zhao X, Kleiner DE, Liang TJ, Hoofnagle JH, Ghany MG. Discordance among transient elastography, aspartate aminotransferase to platelet ratio index, and histologic assessments of liver fibrosis in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2013;11:303-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Isgro G, Calvaruso V, Andreana L, Luong TV, Garcovich M, Manousou P, Alibrandi A, Maimone S, Marelli L, Davies N. The relationship between transient elastography and histological collagen proportionate area for assessing fibrosis in chronic viral hepatitis. J Gastroenterol. 2013;48:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Raszeja-Wyszomirska J, Wunsch E, Kempinska-Podhorodecka A, Smyk DS, Bogdanos DP, Milkiewicz M, Milkiewicz P. TRAF1-C5 affects quality of life in patients with primary biliary cirrhosis. Clin Dev Immunol. 2013;2013:510547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Singh S, Kim WR, Talwalkar JA. Predicting clinical outcomes with elastography in primary biliary cirrhosis: one step closer? Gastroenterology. 2013;144:851-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Verloh N, Utpatel K, Haimerl M, Zeman F, Fellner C, Fichtner-Feigl S, Teufel A, Stroszczynski C, Evert M, Wiggermann P. Liver fibrosis and Gd-EOB-DTPA-enhanced MRI: A histopathologic correlation. Sci Rep. 2015;5:15408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Xie L, Chen X, Guo Q, Dong Y, Guang Y, Zhang X. Real-time elastography for diagnosis of liver fibrosis in chronic hepatitis B. J Ultrasound Med. 2012;31:1053-1060. [PubMed] |
| 47. | Friedrich-Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, Schneider MD, Herrmann E, Petersen J, Schulze F. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Karlas T, Neuschulz M, Oltmanns A, Wirtz H, Keim V, Wiegand J. ARFI and transient elastography for characterization of cystic fibrosis related liver disease: first longitudinal follow-up data in adult patients. J Cyst Fibros. 2013;12:826-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Park H, Park JY, Kim do Y, Ahn SH, Chon CY, Han KH, Kim SU. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J Gastroenterol. 2013;19:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Schiavon Lde L, Narciso-Schiavon JL, de Carvalho-Filho RJ. Non-invasive diagnosis of liver fibrosis in chronic hepatitis C. World J Gastroenterol. 2014;20:2854-2866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |