Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.629
Peer-review started: March 23, 2014
First decision: April 2, 2014
Revised: May 12, 2014
Accepted: July 22, 2014
Article in press: July 22, 2014
Published online: January 14, 2015
Processing time: 303 Days and 1.5 Hours
AIM: To survey the detailed analyses for Helicobacter pylori (H. pylori) infection and gastric mucosal status in Myanmar.
METHODS: A total of 252 volunteers with dyspeptic symptoms (155 female and 97 male; mean age of 43.6 ± 14.2 years) was participated in Yangon and Mandalay. The status of H. pylori infection was determined based on 5 different tests including rapid urease test, culture, histology, immunohistochemistry and serology. Histological scores were evaluated according to the update Sydney system and the Operative Link for Gastritis Assessment system. Pepsinogen (PG) I and PG II were measured using enzyme-linked immunosorbent assays.
RESULTS: The overall prevalence of H. pylori infection was 48.0%. There was no relationship between age and infection rate. Even in young group (less than 29 years old), the H. pylori infection rate was relatively high (41.9%). The prevalence of H. pylori infection was significantly higher in Yangon than that of Mandalay. H. pylori infection was significantly associated with the presence of gastric mucosal atrophy. All 7 subjects with peptic ulcer were infected with H. pylori. Although H. pylori-positive subjects showed stronger gastritis than H. pylori-negative subjects, most cases had mild gastritis.
CONCLUSION: We revealed the prevalence of H. pylori infection in patients with dyspeptic symptoms in Myanmar. The H. pylori infection was a risk factor for peptic ulcer and stronger gastritis.
Core tip: The prevalence of Helicobacter pylori (H. pylori) infection in Myanmar has not been elucidated. Our study revealed that the overall prevalence of H. pylori infection was 48.0% in patients with dyspeptic symptoms. Even among young group (less than 29 years old), the H. pylori infection rate was relatively high (41.9%). Nevertheless, most cases showed mild gastritis, which suggests that the moderate of the incidence of gastric cancer might be attributed to the mild atrophy. All 7 subjects with peptic ulcer were infected with H. pylori.
-
Citation: Myint T, Shiota S, Vilaichone RK, Ni N, Aye TT, Matsuda M, Tran TTH, Uchida T, Mahachai V, Yamaoka Y. Prevalence of
Helicobacter pylori infection and atrophic gastritis in patients with dyspeptic symptoms in Myanmar. World J Gastroenterol 2015; 21(2): 629-636 - URL: https://www.wjgnet.com/1007-9327/full/v21/i2/629.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.629
Helicobacter pylori (H. pylori) infection is strongly related with the development of gastroduodenal diseases including peptic ulcer and functional dyspepsia[1]. Although H. pylori infection is also a major factor to development of gastric cancer[2], the difference of H. pylori infection rate is not enough to explain the difference of the incidence of gastric cancer in the world. For example, despite the high prevalence of H. pylori infection in India, the incidence of gastric cancer in India is much lower than in other countries, the so-called Asian enigmas[3]. In addition to host and environmental factors, as a part, the difference of the incidence of gastric cancer irrespective of H. pylori infection rate can be explained by the difference of virulence factors of H. pylori rather than H. pylori infection rate[4]. In fact, H. pylori strains isolated in India are Western-type strains, on the other hand, those of Chinese are East-Asian-type strains[5,6]. In Thailand, H. pylori strains isolated from Chinese-Thai showed East-Asian-type whereas those from Thai-Thai showed Western-type strains[7].
Myanmar is located in Southeast Asia bordered by China, Thailand, India, Laos and Bangladesh. The age-standardized incidence rate (ASR) of gastric cancer in Myanmar was reported to be 11.2/100000 per year[8] (http://globocan.iarc.fr/), which is higher than that of India and Thailand, and lower than that of China (6.1, 3.1 and 22.7/100000, respectively). To our knowledge, there is no previous study published focusing on the H. pylori infection in Myanmar. To understand the reason for higher incidence of gastric cancer in Myanmar than India or Thailand, it is important to elucidate of H. pylori infection rate in Myanmar. In addition, phylogeographic analyses with genomic difference of H. pylori strains can assume the migration of human populations[9]. Therefore, analyses of H. pylori strains isolated from Myanmar might be contributed to the exploration of human migration pattern in south Asian countries.
Furthermore, the gastric cancer risk can be assessed by the status of gastric atrophy[10]. Not only endoscopic and histological examination but also the measurements of serum pepsinogen (PG) I and PG I/II levels can be available to examine the status of gastric mucosal atrophy. A meta-analysis showed that a PG I level ≤ 70 ng/mL and a PG I/II ratio ≤ 3 had a sensitivity of 57%, specificity of 80%, positive predictive value of 15%, and negative predictive value of 83% in screening for atrophic gastritis to detect gastric cancer[11]. However, the proper cut-off value can be various according to the geographic difference.
In this study, we first disclosed the infection rate of H. pylori in Myanmar by multiple tests including rapid urease test, culture, histology, immunohistochemistry and serology. In addition, we examined the status of gastric mucosa based on histology and serology.
We consecutively recruited a total of 252 volunteers with dyspeptic symptoms (155 female and 97 male; mean age of 43.6 ± 14.2 years, range 13 to 85 years old) in our prospective study in 2012. The survey took place in the largest city, Yangon (n = 182) and the second largest city, Mandalay (n = 70). Subjects with a history of partial gastric resection were excluded. Total of 252 subjects were consisted of 43 at ≤ 29 years old, 65 at 30-39 years old, 56 at 40-49 years old, 55 at 50-59 years old, and 33 at ≥ 60 years old. Peripheral blood was collected from each subject after overnight fasting. Samples were collected into serum tubes and centrifuged within 1 h after collection. Separated sera were used for serological identification of H. pylori and measurement of the PG levels. All reagents for H. pylori cultures (e.g., disposable forceps, transport mediums) were brought from Thailand and Japan. We performed endoscopy on the same day with blood collection. Written informed consent was obtained from all participants, and the protocol was approved by the Ethics and Research Committee of University of Medicine (1), Myanmar, that of Mandalay General Hospital, that of Thammasat University Hospital as well as that of Oita University Faculty of Medicine, Japan.
During each endoscopy session, 4 gastric biopsy specimens were obtained (three from the lesser curvature of the antrum approximately three cm from the pyloric ring and one from the greater curvature of the corpus). Three specimens from the antrum were used for H. pylori culture, rapid urease test and histological examination. One specimen from the corpus was used for histological examination. Peptic ulcers and gastric cancer were identified by endoscopy. Gastritis was defined as H. pylori gastritis in the absence of peptic ulcer or gastric malignancy.
To maximize the diagnostic accuracy, 5 different methods were combined for the diagnosis of H. pylori infection including rapid urease test, culture, histology, immunohistochemistry, and serology. Subjects were considered to be H. pylori-negative when all 5 tests were negative, whereas H. pylori-positive status required at least one positive test result.
One biopsy specimen from the antrum was homogenized in saline and inoculated onto Mueller Hinton II Agar medium (Becton Dickinson, NJ, United States) supplemented with 7% horse blood without antibiotics. The plates were incubated for up to 10 days at 37 °C under microaerophilic conditions (10% O2, 5% CO2 and 85% N2). H. pylori was identified on the basis of colony morphology, Gram staining and positive reactions for oxidase, catalase, and urease. Isolated strains were stored at -80 °C in Brucella Broth (Difco, NJ, United States) containing 10% dimethylsulfoxide and 10% horse serum. For histology, all biopsy materials were fixed in 10% buffered formalin for 24 h, and then embedded in paraffin. Serial sections were stained with hematoxylin and eosin and with May-Giemsa stain. The degree of bacterial load was classified into four grades: 0, “normal”; 1, “mild”; 2, “moderate”; and 3, “marked” according to the updated Sydney system[12]. More than or equal of 1 grade of bacterial load was defined as H. pylori positive.
Anti-H. pylori IgG levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Eiken Co., Ltd., Tokyo, Japan) according to the manufacturer’s instructions. Serum PG I and PG II levels were measured using Pepsinogen ELISA (Eiken, Co. Ltd.) according to the manufacturer’s instructions. Individuals with a serum H. pylori antibody titer ≥ 10 U/mL were classified as H. pylori-positive according to the manufacturer’s instructions; those with PG I levels ≤ 70 ng/mL and a PG I/II ratio ≤ 3.0 were classified as PG-positive according to the Japanese guidelines[13].
Immunohistochemistry was performed as described previously[14]. Briefly, after antigen retrieval and inactivation of endogenous peroxidase activity, tissue sections were incubated with α-H. pylori antibody (DAKO, Denmark) overnight at 4 °C. After washing, the sections were incubated with biotinylated goat antirabbit IgG (Nichirei Co., Japan), followed by incubation with a solution of avidin-conjugated horseradish peroxidase (Vectastain Elite ABC kit; Vector Laboratories Inc., Burlingame, CA, United States). Peroxidase activity was detected using H2O2/diaminobenzidine substrate solution. For all cases, we performed Giemsa staining using a serial section to identify the presence of H. pylori. If the H. pylori identified by Giemsa staining was found to be positively immunostained, we judged the case as positive.
The degree of gastritis was classified using 4 grades: 0, normal; 1, mild; 2, moderate; and 3, marked according to the updated Sydney system[12]; samples of grade 1 or more were considered atrophy-positive according to a previous report[15]. In addition, on the basis of the topographic locations (antrum and corpus), the gastritis stage (the severity and topography of atrophy) was assessed according to the Operative Link on Gastritis Assessment (OLGA) system[16,17].
Data were analyzed using SPSS, version 19 (SPSS Inc., Chicago, IL, United States). Statistical evaluation was performed by the chi-square test to compare discrete variables and the Mann-Whitney U-test and the t-test to compare continuous variables. Differences in prevalence in each group were analyzed using the Mantel-Haenszel method. Spearman rank coefficients (r) were determined to evaluate the association between the severity of mucosal atrophy and PGs. Multiple backward stepwise logistic regression analyses were performed to examine the associations of atrophy with the main predictor variables, such as age, sex, H. pylori infection. For each variable, the odds ratio (OR) and 95%CI were calculated. A two-tailed P value < 0.05 was considered significant. Receiver operating curves (ROC) were used to calculate the best cut-off values for discriminating atrophic gastritis by PG I/II.
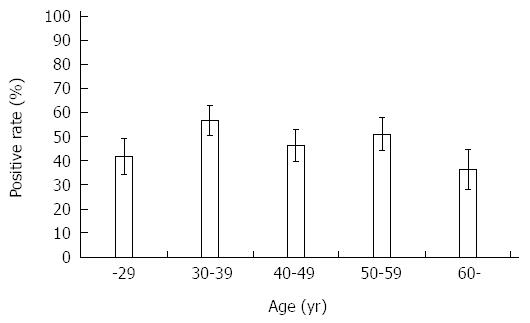
Table 1 showed H. pylori positive rate in each test. The results of histology and immunohistochemistry were identical. Among 5 tests, serological test showed higher positive rate compared with culture, although it did not reach a statistical significance (P = 0.07). When subjects were considered to be H. pylori positive in case at least one test showed positive, overall, the prevalence of H. pylori infection in Myanmar was 48.0% (121/252). Figure 1 shows the prevalence of H. pylori infection according to various range age groups. There was no statistical difference in the positive rate with age (P = 0.31). Even in younger age group, the prevalence of H. pylori infection was more than 40%. There was no difference of H. pylori infection rate between male and female (P = 0.43).
| Age (yr) | ||||||
| -29 | 30-39 | 40-49 | 50-59 | 60- | Total | |
| n | 43 | 65 | 56 | 55 | 33 | 252 |
| Serum | 16 (37.2) | 27 (41.5) | 21 (37.5) | 21 (38.2) | 8 (24.2) | 93 (36.9) |
| RUT | 9 (20.9) | 33 (50.8) | 17 (30.4) | 21 (38.2) | 6 (18.2) | 86 (34.1) |
| Culture | 9 (20.9) | 26 (40.0) | 18 (32.1) | 18 (32.7) | 3 (9.1) | 74 (29.4) |
| Histology | 11 (25.6) | 29 (44.6) | 20 (35.7) | 23 (41.8) | 7 (21.2) | 90 (35.7) |
| IHC | 11 (25.6) | 29 (44.6) | 20 (35.7) | 23 (41.8) | 7 (21.2) | 90 (35.7) |
| Final | 18 (41.9) | 37 (56.9) | 26 (46.4) | 28 (50.9) | 12 (36.4) | 121 (48.0) |
The prevalence of H. pylori infection differed among the 2 cities. The prevalence of H. pylori infection in Yangon was 41.9% (13/31) at ≤ 29 years old, 62.2% (28/45) at 30-39 years old, 55.8% (24/43) at 40-49 years old, 51.2% (21/41) at 50-59 years old, and 40.9% (9/22) at ≥ 60 years old. On the other hand, the prevalence of H. pylori infection in Mandalay was 41.7% (5/12) at ≤ 29 years old, 45.0% (9/20) at 30-39 years old, 15.4% (2/13) at 40-49 years old, 50.0% (7/14) at 50-59 years old, and 27.3% (3/11) at ≥ 60 years old. The overall prevalence of H. pylori infection in Yangon was significantly higher than that of Mandalay even when the age was adjusted by the Mantel-Haenszel method (52.2% vs 37.1%, P = 0.04).
In endoscopic diagnosis, gastritis was most common findings (233/252, 92.4%). Gastric and duodenal ulcer was found at 3 cases (1.1%) and 4 cases (1.5%), respectively. Gastric cancer was found in 3 cases (1.1%). Other diagnosis including submucosal tumor was found in 9 subjects. Among 233 subjects with gastritis, 109 (46.8%) were infected with H. pylori. On the other hand, all 7 subjects with peptic ulcer were infected with H. pylori, which was significantly higher than that of gastritis (100 vs 46.8%, P = 0.006). Among 3 subjects with gastric cancer, 2 subjects were infected with H. pylori.
According to the updated Sydney system, 114 subjects (45.3%) were grade 0 for atrophy in the antrum, 131 subjects (51.9%) had grade 1 and 7 subjects (2.7%) had grade 2. None had grade 3. In the corpus, 220 cases (87.3%) were grade 0 for atrophy in the corpus and 27 and 5 cases (10.7% and 1.9%, respectively) were of grades 1, and 2 for atrophy, respectively. Therefore, 138 subjects (54.7%) had gastric mucosal atrophy in the antrum, and 32 (12.6%) subjects had gastric mucosal atrophy in the corpus when samples of grade 1 or more were considered atrophy-positive. The OLGA system was also used to assess the staging of gastritis; 109 (43.2%) was stages 0 and stage I was found in 52.3% (132/252). Stage II was found in 3.9% (10/252). Stage III was found only 1 (0.3%) subject and Stage IV were not found. The differences of histological scores according to the status of H. pylori infection were shown in Table 2. The scores for activity, inflammation, and atrophy both in antrum and corpus were significantly higher in H. pylori-positive subjects than negative subjects (all P < 0.0001). The score for intestinal metaplasia in the antrum was significantly higher in H. pylori-positive subjects than negative subjects (P = 0.02). Intestinal metaplasia in the antrum was found in 11.5% (14/121) in H. pylori-positive and 3.8% (5/131) in -negative subjects; therefore, the prevalence of intestinal metaplasia in the antrum was significantly higher in H. pylori-positive subjects than that of negative subjects (P = 0.01). OLGA score was also significantly higher in H. pylori-positive subjects than negative subjects (0.84 ± 0.56 vs 0.40 ± 0.52, P < 0.0001).
| H. pylori (+) | H. pylori (-) | P value | |
| n | 121 | 131 | |
| Age | 42.5 ± 13.1 | 44.7 ± 15.2 | 0.22 |
| Male | 50 | 47 | 0.37 |
| PG I | 86.9 ± 72.0 | 75.8 ± 77.0 | 0.006 |
| PG II | 17.3 ± 11.6 | 9.8 ± 10.4 | < 0.001 |
| PG I/II | 5.3 ± 2.0 | 8.1 ± 2.6 | < 0.001 |
| PG-positive | 8 | 4 | 0.18 |
| Antrum | |||
| Activity | 1.22 ± 0.82 | 0.08 ± 0.26 | < 0.0001 |
| Inflammation | 1.53 ± 0.65 | 0.50 ± 0.54 | < 0.0001 |
| Atrophy | 0.78 ± 0.52 | 0.39 ± 0.50 | < 0.0001 |
| Intestinal metaplasia | 0.19 ± 0.59 | 0.05 ± 0.31 | 0.02 |
| Corpus | |||
| Activity | 0.74 ± 0.65 | 0.08 ± 0.29 | < 0.0001 |
| Inflammation | 0.99 ± 0.63 | 0.15 ± 0.42 | < 0.0001 |
| Atrophy | 0.25 ± 0.50 | 0.05 ± 0.25 | < 0.0001 |
| Intestinal metaplasia | 0.04 ± 0.32 | 0.04 ± 0.28 | 0.72 |
| OLGA score | 0.84 ± 0.56 | 0.40 ± 0.52 | < 0.0001 |
To evaluate predictive factors for the presence of atrophy, we performed a multivariate analysis. H. pylori infection was an independent risk factor for the presence of atrophy even after adjustment by age and gender (P < 0.0001, OR = 5.27, 95%CI: 3.02-9.18).
PG II was significantly higher in H. pylori-positive than -negative subjects (P < 0.001); whereas there was no difference of PG I among two group (Table 2). On the other hand, PG I/II was significantly lower in H. pylori-positive than -negative subjects (P < 0.001). When PG-positive was defined as the cutoff of PG I levels ≤ 70 ng/mL and a PG I/II ratio ≤ 3.0, the percentage of PG-positive was higher in H. pylori-positive subjects [6.6% (8/121)] than that of H. pylori-negative subjects [3.0% (4/131)] although it did not reach the statistical significance (P = 0.18).
The overall prevalence of the PG-positive was only 4.7% (12/252). PG-positive was also significantly correlated with the presence of atrophy (P = 0.012). Among the 12 PG-positive subjects, 11 (91.6%) had atrophy. On the other hand, 132 (55.0%) out of 240 PG-negative subjects showed the presence of atrophy. Therefore, it means that when PG has high positive predictive value for the presence of atrophy; however it show high false-negative rate. Next, we examined the correlations between the severity of gastric mucosal atrophy and PGs (Table 3). In case of the antrum, PG I and PG II were significantly correlated with the severity of atrophy (r = 0.13, P = 0.03 for PG I, r = 0.34, P < 0.001 for PG II). On the other hand, PG I/II was significantly inversely correlated with the severity of atrophy (r = -0.34, P < 0.001). In case of the corpus, there was no correlation between PG I and the severity of atrophy. PG II were also significantly correlated with the severity of atrophy in the corpus (r = 0.22, P < 0.001). PG I/II was also significantly inversely correlated with the severity of atrophy in the corpus (r = -0.37, P < 0.001). The correlation between OLGA score and the severity of atrophy was also examined. PG II were significantly correlated with the OLGA score (r = 0.35, P < 0.001). PG I/II was significantly inversely correlated with OLGA score (r = -0.39, P < 0.001). There was no correlation between PG I and the OLGA score.
| Grade | n | PG I | PG II | PG I/II | |
| Antrum | 0 | 114 | 76.2 ± 82.0 | 10.2 ± 10.2 | 7.7 ± 2.4 |
| 1 | 131 | 85.1 ± 68.9 | 15.7 ± 12.0 | 6.0 ± 2.7 | |
| 2 | 7 | 87.7 ± 57.4 | 20.9 ± 14.6 | 4.9 ± 2.2 | |
| 3 | 0 | NA | NA | NA | |
| Corpus | 0 | 220 | 82.3 ± 77.1 | 12.6 ± 11.4 | 7.1 ± 2.5 |
| 1 | 27 | 65.4 ± 40.5 | 17.6 ± 9.6 | 4.0 ± 2.2 | |
| 2 | 5 | 114.0 ± 105.1 | 23.9 ± 22.0 | 4.5 ± 2.4 | |
| 3 | 0 | NA | NA | NA | |
| OLGA | 0 | 109 | 77.2 ± 83.6 | 10.1 ± 10.3 | 7.8 ± 2.3 |
| I | 132 | 82.6 ± 66.3 | 15.3 ± 11.2 | 6.0 ± 2.7 | |
| II | 10 | 104.8 ± 84.1 | 24.8 ± 17.7 | 4.1 ± 1.8 | |
| III | 1 | 67.8 | 8.7 | 7.8 | |
| IV | 0 | NA | NA | NA |
When we used the cut-off value of PG I/II as ≤ 3.0 for more than stage I in the OLGA score, sensitivity and specificity were 8.3%, 99.0%, respectively. In case more than stage II in the OLGA score, they were 18.1% and 95.4%, respectively. Therefore, we calculated the best cut-off value of PG I/II from ROC curve. For more than stage I in OLGA score, the best cut-off value of PG I/II was 6.25 (sensitivity 62.9%, specificity 76.1%) [area under the ROC was 0.720 (95%CI: 0.657-0.782)]. For more than stage II in OLGA score, the best cut-off value of PG I/II was 5.35 (sensitivity 81.8%, specificity 67.2%) [area under the ROC was 0.750 (95% CI: 0.610-0.889)].
We revealed that the prevalence of H. pylori in patients with dyspeptic symptoms in Myanmar was 48.0% by different 5 tests. In contrast to developed countries, H. pylori infections occur earlier in life and with a higher frequency in the developing world[18]. For example, the prevalence of H. pylori infection was decreasing according to the improvement of sanitary condition[19]. The present study showed that high prevalence of H. pylori infection was detected even in younger age group (41.9% at ≤ 29 years old) in Myanmar. Sanitary conditions such as a full equipment rate of water and sewage are considered as important factor for H. pylori infection[18]. The percentage of improved sanitation facilities in 2011 was still 77% in Myanmar (UNICEF, http://www.unicef.org/), which might be the reason for constant infection rate in every age group. The improvement of sanitary condition might be decreased H. pylori infection rate in Myanmar in the future. In addition, we found that higher prevalence of H. pylori infection was found in the largest city, Yangon compared with the second largest city, Mandalay. The percentage of usage of pit latrine is higher in Mandalay than in Yangon (Myanmar Multiple Indicator Cluster Survey 2009-2010, UNICEF, http://www.unicef.org/myanmar). In addition, drinking water sources is more improved in Yangon than in Mandalay (http://www.unicef.org/myanmar). Therefore, it is difficult to explain the difference of H. pylori infection rate by the differences of sanitary condition. Unidentified genetic or host factors may result in them being less susceptible to H. pylori infection[20].
We found that 54.7% had mucosal atrophy in the antrum, and 12.6% subjects also had gastric mucosal atrophy in the corpus when samples of grade 1 or more were considered atrophy-positive. We previously reported that gastric mucosal atrophy was found in 91.9% in the antrum and 37.7% in the corpus in Bhutan where the incidence of gastric cancer is high (17.2 cases per 100000 population per year)[21]. Our study showed that another staging of gastritis (OLGA system) showed that most of case was stage 0-II in Myanmar. Only one subject showed stage III and none had stage IV. On the other hand, stage III and IV were found in approximately 40% in Japan where the incidence of gastric cancer is quite high[22]. Furthermore, although it was significantly higher in H. pylori-positive than that of -negative subjects, the score of intestinal metaplasia in the antrum was lower in Myanmar than that of Japan (0.19 ± 0.59 in Myanmar, 0.50 ± 0.07 in Japan)[23]. Milder gastritis might be related with a moderate incidence of gastric cancer in Myanmar in spite of high H. pylori infection rate.
In this study, when PG-positive was defined as the cutoff of PG I levels ≤ 70 ng/mL and a PG I/II ratio ≤ 3.0, 55.0% of PG-negative subjects showed the presence of atrophy in Myanmar. Therefore, PG show high false-negative rate in Myanmar. The serum PG level can be affected by the ethnic background. In fact, the prevalence of low PG levels was the highest in the Indian compared to the Chinese and Malay populations even after adjustment for gender and H. pylori prevalence[24]. This showed that the serum PG criterion cannot be used in the Indian population for gastric cancer screening[25]. Other factors, such as age, gender, height, body weight, body surface area, smoking, and drinking habits, might be related to PG I and PG II levels[26]. Therefore, different cutoff values used in different studies might affect the sensitivity and specificity of the results[27,28]. For example, in the Chinese population, the cutoff values for PG I and the PG I/II ratio used for the effective detection of atrophic gastritis were 82.3 ng/mL and 6.05, respectively[29]. In our study, we could not find any significant correlation between PG I and gastric mucosal atrophy in the corpus. On the other hand, PG I/II was significantly inversely correlated with the severity of atrophy both in the antrum and corpus. We found that the best cut-off value of PG I/II for more than stage I in OLGA score was 6.25 (sensitivity 62.9%, specificity 76.1%), and 5.35 (sensitivity 81.8%, specificity 67.2%) for more than stage II in OLGA score. Future studies are needed to define the optimal PG cutoff values for gastric cancer screening in Myanmar.
The difference of the incidence of gastric cancer between China, Myanmar, India, and Thailand might be explain the difference of virulence factors of H. pylori in addition to the host factor and diet. Indeed, virulence factors of H. pylori have been revealed to be the predictors of gastric atrophy, intestinal metaplasia and severe clinical outcomes[4]. For example, CagA is the most studied virulence factor of H. pylori[4]. Western-type CagA is predominant in India, on the other hand, East-Asian-type CagA is predominant in China. It has been reported that East-Asian-type CagA strains are more virulent than Western-type CagA[4]. VacA is the second most extensively studied H. pylori virulence factor[30]. vacA s1 or m1 H. pylori strains have an increased risk of peptic ulcer or gastric cancer compared with those with s2 or m2 strains[30]. The prevalence of vacA m1 genotype was 73% in Thailand and approximately 60% in India[5,7,31]. Interestingly, recent study revealed that although CagA was translocated into a host cell, it did not persist for a long period by autophagy in response to vacA m1 but not m2[32]. On the other hand, the CagA expression was persisted in the CD44v9-expressing human gastric cancer cells[32]. A study to investigate virulence factors of H. pylori strains in Myanmar is now in progress. The genetic diversity of H. pylori strains in addition to environmental and host factors might be associated with the difference of the incidence of gastric cancer in Myanmar.
Another important finding was that the prevalence of H. pylori in patients with peptic ulcer was significantly higher than that of gastritis which consistent with previous reports[33-35]. This suggests that H. pylori infection can be a risk factor for the development of peptic ulcer even in Myanmar. Furthermore, histological scores were higher in H. pylori-positive subjects than negative subjects consistent with other report[23]. Therefore, eradication therapy for H. pylori infection can be contributed to the decreasing peptic ulcer in Myanmar.
However, our study includes several limitations. We obtained the samples from the patients living in Yangon and Mandalay which are the largest and the second largest cities in Myanmar. In general, the prevalence of H. pylori infection is higher in country sides than that of cities due to the difference of environmental factors including sanitary condition[18]. Therefore, our results cannot be generalized in Myanmar. In addition, we included only the patients with dyspeptic symptoms but not general population. The percentage of female was also higher than that of male although there was no difference of H. pylori infection rate between male and female. In general, the dyspeptic symptom is more common in female than in male[36]. In addition, we used the ELISA kit manufactured by Eiken Company in Japan for serology. It based on a Japanese H. pylori strain for the detection of H. pylori infection[37,38]. H. pylori antibody titers varied greatly depending on the test kit used[13,39]. It might be preferable to develop a domestic ELISA kit using H. pylori strains obtained in Myanmar for future studies.
In conclusion, the prevalence of H. pylori infection in patients with dyspeptic symptoms in Myanmar was high in spite of moderate incidence of gastric cancer. On the other hand, most cases had mild gastritis. Strains isolated from Myanmar might be less virulent than those of East-Asian countries, but more virulent than those of India and Thailand. Furthermore, the presence of H. pylori was related with peptic ulcer and gastritis. Therefore, eradication therapy of H. pylori can contribute to decrease H. pylori-related diseases such as peptic ulcer and gastric cancer.
We thank Ms. Yoko Kudo for excellent technical assistance.
The age-standardized incidence rate of gastric cancer in Myanmar was reported to be 11.2/100000 per year, which is higher than that of India and Thailand, and lower than that of China (6.1, 3.1 and 22.7/100000, respectively). Although the Helicobacter pylori (H. pylori) infection is the most important factor for the development of gastric cancer, the prevalence of H. pylori infection in Myanmar have not been elucidated.
To understand the reason for higher incidence of gastric cancer in Myanmar than India or Thailand, it is important to elucidate of H. pylori infection rate in Myanmar. Furthermore, the gastric cancer risk can be assessed by the status of gastric atrophy. Not only endoscopic and histological examination but also the measurements of serum pepsinogen (PG) I and PG I/II levels can be available to examine the status of gastric mucosal atrophy. However, the proper cut-off value can be various according to the geographic difference.
The prevalence of H. pylori infection in patients with dyspeptic symptoms in Myanmar was high in spite of moderate incidence of gastric cancer. On the other hand, most cases had mild gastritis. Strains isolated from Myanmar might be less virulent than those of East-Asian countries, but more virulent than those of India and Thailand. Furthermore, the presence of H. pylori was related with peptic ulcer and gastritis.
Eradication therapy of H. pylori can contribute to decrease H. pylori-related diseases such as peptic ulcer and gastric cancer.
In this manuscript the authors evaluate the relation between H. pylori infection and atrophic gastritis in a Myanmar population. In agreement to literature data the study found a significant relation between two variables. The paper appears of clinical interest because these results are not previously reported in these geographic area.
P- Reviewer: Baik GH, De Francesco V, Gasbarrini H, Hagen SJ S- Editor: Ma N L- Editor: A E- Editor: Liu XM
| 1. | Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:205-214. [PubMed] |
| 4. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 5. | Mukhopadhyay AK, Kersulyte D, Jeong JY, Datta S, Ito Y, Chowdhury A, Chowdhury S, Santra A, Bhattacharya SK, Azuma T. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J Bacteriol. 2000;182:3219-3227. [PubMed] |
| 6. | Yamaoka Y, Orito E, Mizokami M, Gutierrez O, Saitou N, Kodama T, Osato MS, Kim JG, Ramirez FC, Mahachai V. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11827] [Article Influence: 844.8] [Reference Citation Analysis (4)] |
| 9. | Suzuki R, Shiota S, Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infect Genet Evol. 2012;12:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Sipponen P, Graham DY. Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol. 2007;42:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 13. | Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - “ABC method”. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:405-414. [PubMed] |
| 14. | Uchida T, Kanada R, Tsukamoto Y, Hijiya N, Matsuura K, Yano S, Yokoyama S, Kishida T, Kodama M, Murakami K. Immunohistochemical diagnosis of the cagA-gene genotype of Helicobacter pylori with anti-East Asian CagA-specific antibody. Cancer Sci. 2007;98:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Bornschein J, Selgrad M, Wex T, Kuester D, Malfertheiner P. Serological assessment of gastric mucosal atrophy in gastric cancer. BMC Gastroenterol. 2012;12:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Rugge M, Genta RM. Staging gastritis: an international proposal. Gastroenterology. 2005;129:1807-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, Graham DY. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 18. | Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16 Suppl 1:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 19. | Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol. 2013;7:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Lee YY, Mahendra Raj S, Graham DY. Helicobacter pylori infection--a boon or a bane: lessons from studies in a low-prevalence population. Helicobacter. 2013;18:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Shiota S, Mahachai V, Vilaichone RK, Ratanachu-ek T, Tshering L, Uchida T, Matsunari O, Yamaoka Y. Seroprevalence of Helicobacter pylori infection and gastric mucosal atrophy in Bhutan, a country with a high prevalence of gastric cancer. J Med Microbiol. 2013;62:1571-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Satoh K, Osawa H, Yoshizawa M, Nakano H, Hirasawa T, Kihira K, Sugano K. Assessment of atrophic gastritis using the OLGA system. Helicobacter. 2008;13:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Kodama M, Murakami K, Okimoto T, Sato R, Uchida M, Abe T, Shiota S, Nakagawa Y, Mizukami K, Fujioka T. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Ang TL, Fock KM, Dhamodaran S, Teo EK, Tan J. Racial differences in Helicobacter pylori, serum pepsinogen and gastric cancer incidence in an urban Asian population. J Gastroenterol Hepatol. 2005;20:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351-365. [PubMed] |
| 26. | Kim N, Jung HC. The role of serum pepsinogen in the detection of gastric cancer. Gut Liver. 2010;4:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 647] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 28. | Brenner H, Rothenbacher D, Weck MN. Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer. 2007;121:2782-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Cao Q, Ran ZH, Xiao SD. Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies. J Dig Dis. 2007;8:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Shiota S, Suzuki R, Yamaoka Y. The significance of virulence factors in Helicobacter pylori. J Dig Dis. 2013;14:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Chattopadhyay S, Datta S, Chowdhury A, Chowdhury S, Mukhopadhyay AK, Rajendran K, Bhattacharya SK, Berg DE, Nair GB. Virulence genes in Helicobacter pylori strains from West Bengal residents with overt H. pylori-associated disease and healthy volunteers. J Clin Microbiol. 2002;40:2622-2625. [PubMed] |
| 32. | Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1586] [Article Influence: 122.0] [Reference Citation Analysis (5)] |
| 34. | Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 532] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 35. | Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Flier SN, Rose S. Is functional dyspepsia of particular concern in women? A review of gender differences in epidemiology, pathophysiologic mechanisms, clinical presentation, and management. Am J Gastroenterol. 2006;101:S644-S653. [PubMed] |
| 37. | Matsuo K, Hamajima N, Suzuki T, Nakamura T, Matsuura A, Tominaga S. Better ROC Curves for a Regionally Developed Helicobacter Pylori Antibody Test. Asian Pac J Cancer Prev. 2001;2:155-156. [PubMed] |
| 38. | Fujioka T, Tokieda M. Validity of serum anti-Helicobacter pylori antibody using enzyme immunoassay for the diagnosis in eradication of Helicobacter pylori [in Japanese]. Jpn J Med Pharm Sci. 2000;43:573–579. |
| 39. | Burucoa C, Delchier JC, Courillon-Mallet A, de Korwin JD, Mégraud F, Zerbib F, Raymond J, Fauchère JL. Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter. 2013;18:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (1)] |