Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4764
Revised: May 2, 2013
Accepted: May 16, 2013
Published online: August 7, 2013
Processing time: 161 Days and 19.2 Hours
AIM: To compare quality of life (QoL) outcomes in Chinese patients after curative laparoscopic vs open surgery for rectal cancer.
METHODS: Eligible Chinese patients with rectal cancer undergoing curative laparoscopic or open sphincter-preserving resection between July 2006 and July 2008 were enrolled in this prospective study. The QoL outcomes were assessed longitudinally using the validated Chinese versions of the European Organization for Research and Treatment of Cancer QLQ-C30 and QLQ-CR38 questionnaires before surgery and at 4, 8, and 12 mo after surgery. The QoL scores at the different time points were compared between the laparoscopic and open groups. A higher score on a functional scale indicated better functioning, whereas a higher score on a symptom scale indicated a higher degree of symptoms.
RESULTS: Seventy-four patients (49 laparoscopic and 25 open) were enrolled. The two groups of patients were comparable in terms of sociodemographic data, types of surgery, tumor staging, and baseline mean QoL scores. There was no significant decrease from baseline in global QoL for the laparoscopic group at different time points, whereas the global QoL was worse compared to baseline beginning at 4 mo but returned to baseline by 12 mo for the open group (P = 0.019, Friedman test). Compared to the open group, the laparoscopic group had significantly better physical (89.9 ± 1.4 vs 79.2 ± 3.7, P = 0.016), role (85.0 ± 3.4 vs 63.3 ± 6.9, P = 0.005), and cognitive (73.5 ± 3.4 vs 50.7 ± 6.2, P = 0.002) functioning at 8 mo, fewer micturition problems at 4-8 mo (4 mo: 32.3 ± 4.7 vs 54.7 ± 7.1, P = 0.011; 8 mo: 22.8 ± 4.0 vs 40.7 ± 6.9, P = 0.020), and fewer male sexual problems from 8 mo onward (20.0 ± 8.5 vs 76.7 ± 14.5, P = 0.013). At 12 mo after surgery, no significant differences were observed in any functional or symptom scale between the two groups, with the exception of male sexual problems, which remained worse in the open group (29.2 ± 11.3 vs 80.0 ± 9.7, P = 0.026).
CONCLUSION: Laparoscopic sphincter-preserving resection for rectal cancer is associated with better preservation of QoL and fewer male sexual problems when compared with open surgery in Chinese patients. These findings, however, should be interpreted with caution because of the small sample size of the study.
Core tip: This prospective nonrandomized study demonstrates that laparoscopic sphincter-preserving resection for rectal cancer is associated with better preservation of quality of life (QoL) and fewer male sexual problems when compared with open surgery in Chinese patients in the first postoperative year. Our study has several strengths. First, our study only focused on Chinese patients undergoing curative sphincter-preserving rectal resection, thus minimizing the impact of other potential confounders on the QoL assessment. Second, all our questionnaires were administered by a single research assistant and were completed by the patients during clinic visits. Therefore, we achieved 100% compliance at different time points.
-
Citation: Ng SSM, Leung WW, Wong CYN, Hon SSF, Mak TWC, Ngo DKY, Lee JFY. Quality of life after laparoscopic
vs open sphincter-preserving resection for rectal cancer. World J Gastroenterol 2013; 19(29): 4764-4773 - URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4764
Accumulating evidence from recent randomized trials indicates that laparoscopic surgery for rectal cancer is associated with clear short-term benefits and similar tumor clearance when compared with open surgery[1-5]. Researchers currently are particularly eager to know whether the long-term oncologic results are also comparable between the two approaches for rectal cancer[6]. Indeed, long-term survival has always been regarded as the most important study endpoint in these clinical trials[7]. However, functional outcomes and quality of life (QoL) must not be ignored in the quest for surgical and oncologic excellence.
Notably, up to 30% of rectal cancer survivors will develop urinary and sexual dysfunctions after surgery attributable to inadvertent injury of the pelvic autonomic nerves[8]. Bowel dysfunction and fecal incontinence are also not uncommon after sphincter-preserving rectal surgery and radiotherapy[9,10]. These functional hazards will have a significant negative impact on the patients’ functioning and QoL for the remainder of his/her life[11].
Therefore, in addition to traditional study endpoints such as postoperative recovery, morbidity, and survival, functional results and QoL have recently become important outcome parameters for defining surgical performance in clinical trials[12]. Within the context of medical and healthcare research, QoL is the patient’s subjective perception of the impact of his/her disease and its treatments on his/her physical, psychological, and social functioning and general well-being[13]. Health-related QoL after cancer surgery can be assessed by standardized instruments such as the questionnaires developed by the European Organization for Research and Treatment of Cancer (EORTC), which contain multidimensional generic and disease-specific domains; the EORTC QLQ-C30 and QLQ-CR38 are the most commonly used questionnaires in colorectal cancer trials[14,15].
The magnified vision and less traumatic surgery offered by the laparoscopic approach may allow better preservation of the pelvic autonomic nerves[4,5], and presumably, functional outcomes following laparoscopic surgery for rectal cancer may be better compared to open surgery. However, conflicting results have been reported in the literature[2,16]; some studies have even reported a higher incidence of sexual dysfunction after laparoscopic rectal surgery[17,18]. Furthermore, it is also unclear whether the short-term and long-term clinical benefits associated with the laparoscopic approach will translate into better QoL outcomes for patients with rectal cancer. To date, few studies have specifically compared QoL outcomes between laparoscopic and open surgery for rectal cancer[19]. We therefore conducted this prospective study to compare QoL outcomes in Chinese patients after curative laparoscopic vs open sphincter-preserving surgery for rectal cancer. Changes in QoL over time were also longitudinally assessed and compared between the two groups.
Between July 2006 and July 2008, eligible Chinese patients with rectal cancer undergoing curative laparoscopic or open sphincter-preserving resection at our hospital were enrolled in this prospective study. The study was approved ethically by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (CRE-2005.259). All patients provided written informed consent. We excluded the following patients: patients who presented with recurrent disease, patients who required multivisceral en bloc resections, patients who required conversion from laparoscopic to open surgery, patients with intestinal obstruction or perforation, and patients with known dementia or cognitive dysfunction.
The operative approach (laparoscopic or open resection) was decided by the operating surgeon after considering the tumor characteristics and the patient’s preference. All operations were performed by surgeons experienced in both laparoscopic and open colorectal surgery. Our laparoscopic techniques for resection of rectal cancer were previously described[20,21]. For mid and low rectal cancer located 5-12 cm from the anal verge, sphincter-preserving total mesorectal excision with protective loop ileostomy was performed. All patients in this study underwent ileostomy closure within 7 mo after the primary surgery. Adjuvant therapy was administered to patients with pathologic stage II or III disease. Clinical parameters including patient sociodemographic data, types of surgery, tumor staging, and short-term clinical outcomes were prospectively recorded.
After surgery, all patients were followed-up regularly at 4-mo intervals for clinical examination and carcinoembryonic antigen testing. All patients were free of recurrence during the study period.
Patient QoL was assessed using the QLQ-C30 and QLQ-CR38 questionnaires developed by the EORTC[14,15]. The clinical validity and reliability of the Chinese versions of both QLQ-C30 and QLQ-CR38 have been confirmed[22-24]. QLQ-C30 is a generic questionnaire for the assessment of QoL in cancer patients[14]. It includes 30 items, 24 of which are combined to form a global QoL scale, five functional scales (physical, role, emotional, cognitive, and social), and three symptom scales (fatigue, nausea/vomiting, and pain). The other six single items evaluate dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties. QLQ-CR38 is a specific questionnaire module specifically designed for assessment of QoL in patients with colorectal cancer[15]. It consists of 38 items covering symptoms and side effects related to different colorectal cancer treatment modalities. The module contains four functional scales (body image, sexual functioning, sexual enjoyment, and future perspective) and eight symptom scales/items (micturition problems, chemotherapy side effects, gastrointestinal tract symptoms, male sexual problems, female sexual problems, defecation problems, stoma-related problems, and weight loss).
The questionnaires were scored according to the EORTC Scoring Manual[25]. Each item has four response alternatives (scoring 1-4), “not at all”, “a little”, “quite a bit”, and “very much”, except for the global QoL scale, which has seven alternatives (scoring 1-7) from “very poor” to “excellent”. All questionnaire responses and scores were linearly transformed to a 0-100 scale. A higher score on the global QoL and functional scales represented a higher level of QoL and functioning, whereas a higher score on the symptom scales/items represented a higher degree of symptoms or dysfunction.
All questionnaires were administered by a single research assistant and completed by the patients before surgery and at 4, 8 and 12 mo after surgery (during clinic visits). Every effort was made to avoid missing data during questionnaire administration.
QoL scores were presented as the mean ± SD. For longitudinal assessment of changes of QoL scores over time, the Friedman test was used to identify overall significant differences between QoL scores at the four different time points (before surgery and at 4, 8 and 12 mo after surgery) for each variable. When the overall P value indicated statistical significance (i.e., P < 0.05), a post-hoc Wilcoxon signed-rank test for paired data using a simplified Bonferroni correction was used to compare pairs of QoL scores (with P < 0.0083 considered significant for six pair-wise comparisons). Cross-sectionally, to test for differences in QoL scores between the laparoscopic and open groups at different time points, the Mann-Whitney U test was used. The baseline characteristics of the two groups of patients were compared using the χ2 test (or Fisher’s exact test when appropriate), Student’s t test, and the Mann-Whitney U test for categorical, parametric, and non-parametric data, respectively. A P value of less than 0.05 was considered statistically significant, whereas a difference in mean QoL scores of more than 10 points was regarded as clinically significant[26]. Using a 5% significance level, a total sample size of 75 (50 laparoscopic and 25 open) would have a power of 80% to detect a minimum difference of 10 points in mean QoL scores between the two groups.
Between July 2006 and July 2008, 74 patients were enrolled in this study: 49 patients underwent laparoscopic surgery, and 25 patients underwent open surgery. The two groups of patients were comparable in terms of sociodemographic data, types of surgery, tumor staging, and the proportion of patients who received adjuvant therapy (Table 1). The overall short-term morbidity rates of the laparoscopic and open groups were 34.7% and 52%, respectively (P = 0.152, χ2 test). Transient urinary retention and septic complications (including chest infection, wound infection, and urinary tract infection) occurred more frequently in the open group. No patient in this study required reoperation for postoperative complications. With the exception of higher baseline symptom scores for insomnia in the open group, there was no significant difference in baseline mean QoL scores for any of the functional or symptom scales between the two groups (Table 2).
| Laparoscopic group | Open group | P value | |
| Number of patients | 49 | 25 | / |
| Age (yr, mean ± SD) | 65.6 ± 11.3 | 66.7 ± 12.4 | 0.7051 |
| Sex (male/female) | 30/19 | 15/10 | 0.9192 |
| Body mass index (kg/m2, mean ± SD) | 22.4 ± 3.4 | 21.4 ± 3.5 | 0.2101 |
| Number of patients with comorbidities | 27 (55.1) | 13 (52) | 0.8002 |
| Marital status (married/divorced/widow/single) | 36/5/6/2 | 18/3/4/0 | 0.7422 |
| Education level (under primary/primary/secondary/tertiary or above) | 10/19/16/4 | 8/10/6/1 | 0.6232 |
| Tumor location in rectum (upper/middle/lower) | 26/15/8 | 12/10/3 | 0.6972 |
| Types of surgery (AR/LAR with TME) | 24/25 | 12/13 | 0.9362 |
| Number of patients with temporary ileostomy | 25 (51) | 13 (52) | 0.9362 |
| Number of patients with complications | 17 (34.7) | 13 (52) | 0.1522 |
| Subclinical anastomotic leak | 0 | 1 | |
| Anastomotic bleeding | 1 | 0 | |
| Chest infection | 1 | 3 | |
| Wound infection | 2 | 5 | |
| Urinary tract infection | 2 | 5 | |
| Urinary retention | 4 | 5 | |
| Prolonged ileus | 7 | 3 | |
| Others | 1 | 3 | |
| Reoperation | 0 | 0 | |
| AJCC staging (I/II/III) | 3/26/20 | 2/13/10 | 0.9552 |
| Adjuvant chemotherapy | 20 (40.8) | 10 (40) | 0.9462 |
| Adjuvant radiotherapy | 11 (22.4) | 7 (28) | 0.5992 |
| Baseline | 4 mo | 8 mo | 12 mo | |||||||||
| Lap | Open | P value | Lap | Open | P value | Lap | Open | P value | Lap | Open | P value | |
| EORTC QLQ-C30 | ||||||||||||
| Functional scales | ||||||||||||
| Global QoL | 72.4 (3.4) | 68.3 (5.2) | 0.443 | 65.8 (3.6) | 47.3 (5.9) | 0.009 | 73.6 (3.8) | 52.0 (5.9) | 0.003 | 71.1 (3.4) | 61.0 (7.0) | 0.371 |
| Physical | 94.7 (1.3) | 91.5 (2.6) | 0.255 | 86.4 (2.3) | 77.6 (4.1) | 0.056 | 89.9 (1.4) | 79.2 (3.7) | 0.016 | 87.1 (2.6) | 81.3 (4.0) | 0.149 |
| Role | 88.8 (3.0) | 92.7 (3.1) | 0.660 | 75.9 (4.5) | 67.3 (6.6) | 0.155 | 85.0 (3.4) | 63.3 (6.9) | 0.005 | 82.7 (3.9) | 71.3 (7.1) | 0.129 |
| Emotional | 71.1 (4.1) | 66.0 (5.4) | 0.404 | 76.0 (4.0) | 82.0 (4.9) | 0.401 | 79.8 (3.4) | 71.3 (6.3) | 0.379 | 79.1 (3.2) | 71.0 (6.3) | 0.579 |
| Cognitive | 71.1 (4.2) | 66.7 (5.5) | 0.415 | 67.7 (3.6) | 59.3 (5.8) | 0.284 | 73.5 (3.4) | 50.7 (6.2) | 0.002 | 61.9 (4.2) | 60.0 (6.7) | 0.940 |
| Social | 82.7 (3.2) | 88.0 (4.4) | 0.292 | 73.5 (3.1) | 64.7 (5.7) | 0.268 | 76.9 (3.8) | 62.7 (7.1) | 0.110 | 76.5 (4.0) | 62.7 (7.4) | 0.124 |
| Symptom scales/items | ||||||||||||
| Fatigue | 16.6 (2.8) | 14.2 (2.9) | 0.859 | 17.7 (2.6) | 27.6 (4.3) | 0.042 | 13.8 (2.7) | 28.9 (5.9) | 0.027 | 12.0 (2.2) | 15.6 (4.0) | 0.520 |
| Nausea/vomiting | 0 (0) | 0.7 (0.7) | 0.162 | 3.1 (1.3) | 6.7 (3.7) | 0.931 | 0 (0) | 2.7 (2.1) | 0.046 | 0 (0) | 1.3 (1.3) | 0.162 |
| Pain | 14.3 (3.2) | 16.7 (4.5) | 0.772 | 20.1 (2.7) | 25.3 (5.2) | 0.534 | 16.7 (2.7) | 27.3 (5.2) | 0.089 | 15.6 (2.9) | 19.3 (5.4) | 0.755 |
| Dyspnea | 4.1 (1.6) | 2.7 (1.8) | 0.581 | 7.5 (2.2) | 16 (5.5) | 0.208 | 2.7 (1.3) | 12 (6.0) | 0.252 | 4.1 (2.1) | 12.0 (5.0) | 0.065 |
| Insomnia | 33.3 (5.1) | 58.7 (8.5) | 0.011 | 32.0 (5.1) | 46.7 (8.2) | 0.141 | 27.2 (4.8) | 45.3 (7.9) | 0.052 | 30.6 (5.1) | 46.7 (8.4) | 0.129 |
| Appetite loss | 8.8 (2.7) | 6.7 (4.3) | 0.313 | 7.5 (2.2) | 14.7 (6.4) | 0.794 | 6.8 (2.2) | 17.3 (4.8) | 0.035 | 7.5 (3.0) | 10.7 (4.2) | 0.330 |
| Constipation | 18.4 (4.6) | 29.3 (8.0) | 0.292 | 11.6 (4.2) | 12.0 (4.7) | 0.525 | 15.0 (4.4) | 6.7 (2.7) | 0.508 | 18.4 (4.5) | 10.7 (5.3) | 0.205 |
| Diarrhea | 19.7 (4.4) | 17.3 (6.1) | 0.759 | 23.1 (4.9) | 30.7 (7.2) | 0.394 | 15.0 (4.2) | 29.3 (8.2) | 0.156 | 15.0 (4.1) | 21.3 (6.6) | 0.379 |
| Financial difficulties | 13.6 (4.0) | 14.7 (4.7) | 0.426 | 24.5 (4.3) | 37.3 (6.5) | 0.066 | 23.1 (5.2) | 42.7 (7.1) | 0.010 | 13.6 (3.6) | 20.0 (6.7) | 0.580 |
| EORTC QLQ-CR38 | ||||||||||||
| Functional scales | ||||||||||||
| Body image | 93.9 (1.9) | 93.3 (2.6) | 0.945 | 81.0 (4.2) | 82.7 (6.0) | 0.699 | 81.2 (3.9) | 85.3 (4.8) | 0.834 | 87.8 (3.7) | 84.4 (5.6) | 0.526 |
| Sexual functioning | 18.7 (3.9) | 14.0 (5.3) | 0.268 | 18.7 (4.1) | 8.7 (5.1) | 0.069 | 19.0 (4.2) | 16.0 (5.7) | 0.630 | 19.0 (4.3) | 17.3 (5.7) | 0.807 |
| Future perspective | 54.4 (4.9) | 64.0 (7.2) | 0.272 | 54.4 (4.4) | 45.3 (6.0) | 0.309 | 44.2 (5.1) | 42.7 (7.8) | 0.779 | 44.2 (4.9) | 44.0 (6.9) | 0.995 |
| Symptom scales/items | ||||||||||||
| Micturition problems | 37.8 (4.4) | 38.7 (6.9) | 0.907 | 32.3 (4.7) | 54.7 (7.1) | 0.011 | 22.8 (4.0) | 40.7 (6.9) | 0.020 | 31.6 (4.5) | 41.3 (6.9) | 0.246 |
| Chemotherapy side effects1 | 16.7 (4.1) | 10.0 (2.6) | 0.530 | 41.1 (7.3) | 44.4 (11.1) | 0.846 | 20.6 (4.7) | 28.9 (7.8) | 0.422 | 8.9 (3.3) | 17.8 (6.0) | 0.214 |
| Gastrointestinal tract symptoms | 20.7 (2.2) | 19.5 (2.8) | 0.817 | 16.6 (1.9) | 18.1 (2.8) | 0.656 | 15.9 (1.9) | 24.8 (3.3) | 0.022 | 18.0 (2.2) | 16.3 (3.3) | 0.546 |
| Defecation problems2 | 22.2 (2.3) | 15.5 (3.5) | 0.133 | 24.3 (3.6) | 21.4 (3.0) | 0.821 | 11.8 (2.5) | 23.0 (4.9) | 0.062 | 13.2 (2.9) | 16.3 (3.3) | 0.235 |
| Weight loss | 27.9 (4.7) | 38.7 (8.3) | 0.376 | 8.8 (3.2) | 24.0 (7.6) | 0.079 | 6.1 (2.1) | 17.3 (6.1) | 0.094 | 6.1 (2.1) | 10.7 (4.2) | 0.383 |
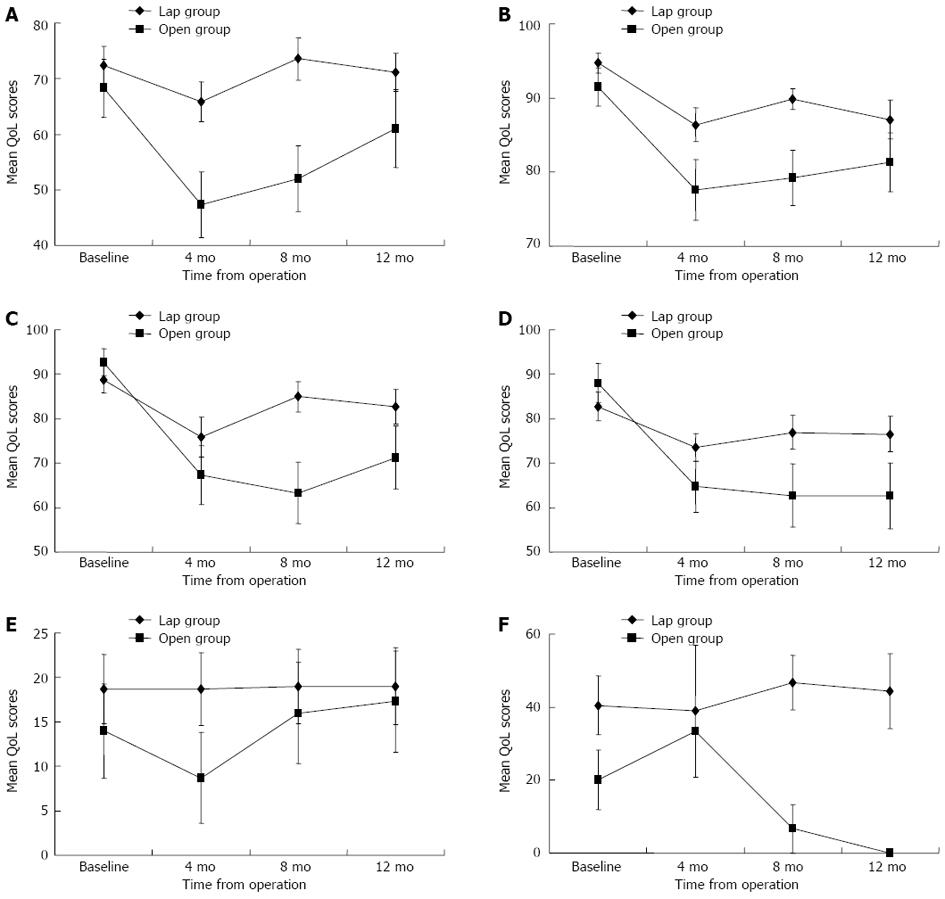
There was no significant decrease from baseline in global QoL scores for the laparoscopic group at the evaluated time points; the statistically significant difference detected with the Friedman test (P = 0.044) was due to an increase in global QoL scores from 4 to 8 mo (P = 0.031, post-hoc Wilcoxon signed-rank test) (Figure 1A). For the open group, the global QoL was worse than at baseline from 4 mo onward but gradually returned to baseline by 12 mo (P = 0.019, Friedman test; a significant decrease occurred between baseline and 4 mo, P = 0.004, post-hoc Wilcoxon sign-ranked test) (Figure 1A). Both the laparoscopic and open groups showed a significant decrease in physical functioning from 4 to 12 mo postoperatively (P < 0.001, Friedman test) (Figure 1B). Role functioning and social functioning were significantly worse than at baseline from 4 to 12 mo for the open group but remained the same as at baseline for the laparoscopic group (Figure 1C and D). There was no change from baseline in emotional functioning for either group. Cognitive functioning fluctuated over time for the laparoscopic group (P = 0.035, Friedman test) but remained the same as at baseline for the open group.
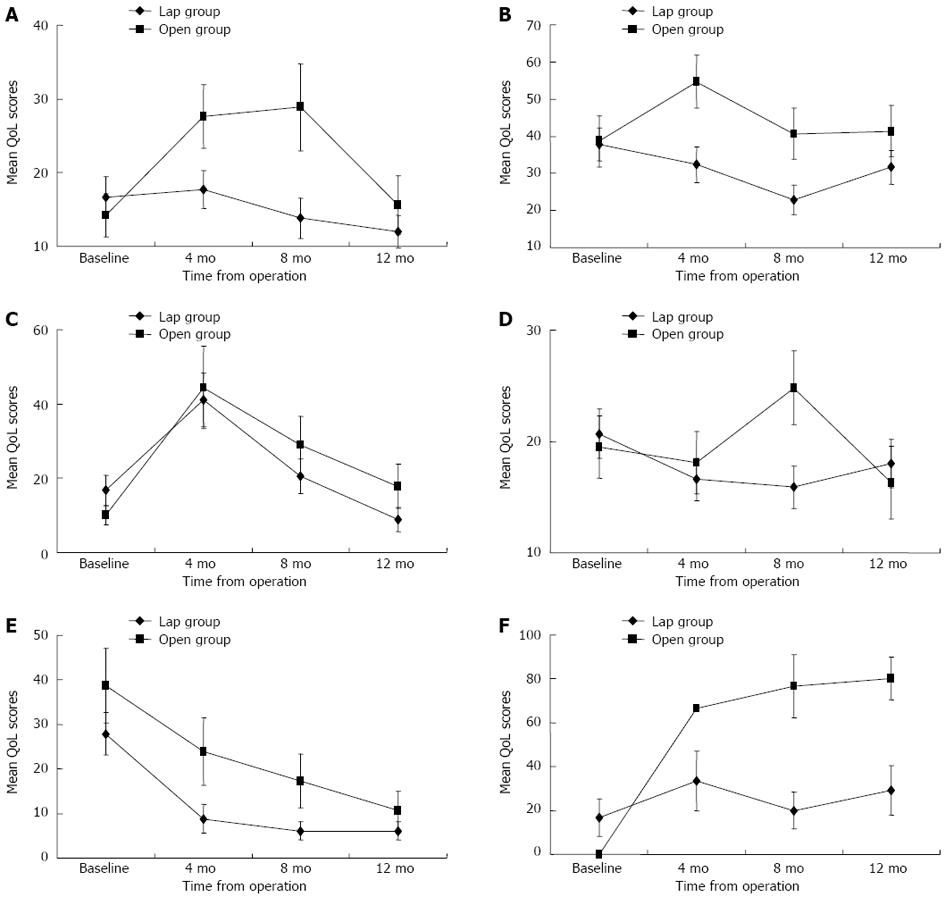
There was no significant change from baseline in fatigue scores for the laparoscopic group; more fatigue was reported in the open group beginning at 4 mo, but it returned to the baseline level by 12 mo (P = 0.003, Friedman test; a significant increase occurred between baseline and 4 mo, P = 0.004, post-hoc Wilcoxon sign-ranked test) (Figure 2A). The 4-mo to 12-mo symptom scores remained similar to those at baseline for nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, and diarrhea for both groups. More financial difficulties were reported at 4 mo postoperatively, but this returned to baseline levels by 12 mo for both groups.
Compared to the open group, the laparoscopic group had significantly better global QoL at 4 and 8 mo, better physical, role, and cognitive functioning at 8 mo, less fatigue at 4 and 8 mo, and less nausea/vomiting, appetite loss, and financial difficulties at 8 mo (Table 2). However, at 12 mo after surgery, no significant differences were observed in any of the EORTC QLQ-C30 functional or symptom scales between the two groups.
There was no significant change from baseline in body image for the open group; for the laparoscopic group, body image was significantly worse compared to baseline beginning at 4 mo but returned to baseline levels by 12 mo (P = 0.002, Friedman test). Sexual functioning remained the same as at baseline for both groups (Figure 1E), but the overall scores for sexual functioning were low (Table 2), indicating that the majority of patients were sexually inactive. There was a trend toward worsening of future perspective scores over time for the laparoscopic (P = 0.074, Friedman test) and open (P = 0.094, Friedman test) groups, but the change was statistically insignificant.
Improvement in micturition problems was noted in the laparoscopic group (P = 0.031, Friedman test; a decrease in symptom scores primarily occurred between baseline and 8 mo, P = 0.019, post-hoc Wilcoxon sign-ranked test), but there was no significant change from baseline for the open group (Figure 2B). More problems with chemotherapy side effects were reported at 4 mo postoperatively, but returned to baseline levels by 12 mo for patients receiving chemotherapy in both groups (Figure 2C). There was no significant change from baseline in gastrointestinal tract symptoms for either group (Figure 2D). Defecation problems significantly decreased from 4 to 8 mo for patients without stoma in the laparoscopic group (P = 0.003, Friedman test; P = 0.003, post-hoc Wilcoxon sign-ranked test), but they remained the same as at baseline for the open group. A significant improvement in weight loss over time was observed in both groups (Figure 2E). Compared to the open group, the laparoscopic group had significantly fewer micturition problems at 4 and 8 mo and fewer gastrointestinal symptoms at 8 mo (Table 2).
Sexual enjoyment and sexual problems were not evaluated in the female patients in this study because they were all sexually inactive. Altogether, 19 male patients (14 in the laparoscopic group and 5 in the open group) who had been sexually active before surgery were assessed for changes in QoL related to sexual activities (Table 3). Sexual enjoyment and male sexual problems remained relatively stable at different time points for the laparoscopic group, but less sexual enjoyment and more sexual problems were reported from 4 to 12 mo for the open group (Figures 1F and 2F). Compared to the laparoscopic group, the open group had significantly more sexual problems at 8 mo (P = 0.013, Mann-Whitney U test) and 12 mo (P = 0.026, Mann-Whitney U test) postoperatively.
| Baseline | 4 mo | 8 mo | 12 mo | |||||||||
| Lap | Open | P value | Lap | Open | P value | Lap | Open | P value | Lap | Open | P value | |
| Number of men who have been sexually active | 14 | 5 | / | 6 | 1 | / | 10 | 5 | / | 12 | 5 | / |
| Sexual enjoyment (functional scale) | 40.5 (8.0) | 20.0 (8.2) | 0.164 | 38.9 (18.1) | 33.3 (/) | 1.000 | 46.7 (7.4) | 6.7 (6.7) | 0.004 | 44.4 (10.3) | 0 (0.0) | 0.019 |
| Male sexual problems (symptom scale) | 16.7 (8.4) | 0 (0) | 0.194 | 33.3 (13.6) | 66.7 (/) | 0.309 | 20.0 (8.5) | 76.7 (14.5) | 0.013 | 29.2 (11.3) | 80.0 (9.7) | 0.026 |
At 12 mo after surgery, no significant differences were observed in any of the EORTC QLQ-CR38 functional or symptom scales between the two groups, with the exception of male sexual enjoyment and sexual problems, which remained worse in the open group.
This prospective study was specifically designed to compare QoL outcomes in Chinese patients after curative laparoscopic vs open sphincter-preserving resection for rectal cancer. Our study has several strengths. First, although nonrandomized, the baseline characteristics and sociodemographic data of the two groups of patients were similar, and a fair comparison could therefore be made. The social backgrounds of patients, such as marital status and education level, which may impact QoL after surgery[27], have seldom been provided by other studies comparing QoL after laparoscopic and open rectal surgery[1-3,28,29]. Second, other studies have included metastatic cases and abdominoperineal resection in their QoL analysis[1,3,28], whereas our study only focused on Chinese patients undergoing curative sphincter-preserving rectal resection, thus minimizing the impact of other potential confounders on the QoL assessment. Third, all our questionnaires were administered by a single research assistant and were completed by the patients during clinic visits. Therefore, we achieved 100% compliance at different time points, a figure that was not achieved by other studies in which the questionnaires were collected by mail[28,29]. Full compliance with the questionnaires is essential to ensure a precise longitudinal assessment of QoL changes.
Our results showed that laparoscopic sphincter-preserving resection for rectal cancer was associated with better preservation of QoL and fewer male sexual problems when compared with the open approach in the first year after surgery. Other benefits of the laparoscopic approach, such as better physical functioning and fewer micturition and gastrointestinal problems, were evident only in the short term. These findings are in accordance with those reported by Braga et al[3], who found that QoL after laparoscopic surgery for rectal cancer was better than the open approach only in the first year after surgery. Li et al[28] also reported transient QoL benefits in the early postoperative period after laparoscopic rectal surgery when compared with the open approach, but the overall QoL of the two groups was similar at a 1-year follow-up. Better QoL in the laparoscopic arm was also reported by the comparison of open vs laparoscopic surgery for mid and low rectal cancer after neoadjuvant chemoradiotherapy (COREAN) trial at 3 mo, but 1-year data were not provided[2].
On longitudinal assessment, the QoL scores of most of the functional and symptom scales of the laparoscopic group in our study remained relatively stable at different time points, whereas most of the QoL scores in the open group predominantly showed deterioration at 4-8 mo but gradually recovered by 1 year after surgery. This explains why significant differences in QoL scores between the laparoscopic and open groups in our study were primarily observed at 8 mo after surgery.
We have previously reported better short-term clinical outcomes and less long-term morbidity among patients undergoing laparoscopic surgery for rectal cancer when compared with the open approach[7,20,21]; this may partly account for the better short-term QoL associated with the laparoscopic arm in our study. However, in addition to the healthcare experience, patients’ expectations also play an important role in the determination of QoL. According to the dynamic model proposed by Carr et al[30], QoL is typically impacted when the health experience falls short of expectations. The better preservation of QoL in the laparoscopic group over time may imply that most of their initial positive expectations of laparoscopic surgery (the treatment that they had chosen) were met by their postoperative experience (the clinical benefits) and that the “expectation-experience homeostasis” remained unchanged throughout the entire assessment period. However, discrepancies between preoperative expectations and the postoperative experience may explain the initial deterioration of QoL in the open group, and a period of adaptation and alteration of expectations may have been needed to reestablish “homeostasis” by 1 year after surgery.
Interestingly, the same argument can also be used to explain the paradoxical finding of a worse functional scale of body image after laparoscopic surgery in our study. Patients in the laparoscopic group might have had high expectations regarding the initial cosmetic results. However, when they realized that the final cosmetic outcome (a 5-cm incisional wound over the left iliac fossa and an ileostomy over the right iliac fossa) did not meet their preoperative expectations, a significant impact on QoL with respect to body image occurred. Conversely, patients in the open group who did not have high expectations regarding the cosmetic results might not have experienced a significant change in the functional scale of body image after surgery.
Urinary and sexual dysfunctions are recognized complications after rectal cancer surgery, which may have a negative impact on QoL[11]. In our study, the laparoscopic group had significantly fewer micturition problems at 4-8 mo after surgery when compared with the open group, but the benefit disappeared at 1 year. The COREAN trial also reported fewer micturition problems in the laparoscopic group when compared with the open group at 3 mo after surgery[2]. This benefit of less urinary dysfunction is believed to be the result of better preservation of the autonomic nerves and less traumatic surgery, attributable to the magnified view provided by laparoscopic surgery[2,4,5]. However, when the transient neuropraxia of the pelvic autonomic nerves in the open group has fully recovered, this benefit will disappear.
In our study, male sexual enjoyment and male sexual problems were the only two QoL scales that remained worse in the open group when compared with the laparoscopic group at 1 year after surgery. Yang et al[29] also reported fewer male sexual problems and better sexual functioning at 12-18 mo after laparoscopic total mesorectal excision for low rectal cancer when compared with open surgery; better sexual enjoyment in the laparoscopic group was even observed after 24 mo postoperatively. By contrast, a nonsignificant trend for worse sexual function in males after laparoscopic surgery for rectal cancer was reported by the United Kingdom Medical Research Council trial of conventional vs laparoscopic-assisted surgery in colorectal cancer (CLASICC)[16]. Interestingly, the design of the CLASICC trial required that every participating surgeon had undertaken at least 20 laparoscopic resections, and most of the surgeons were likely still on their learning curve[1,31]. Although the laparoscopic approach can provide a clear, magnified view in the deep pelvis, the risk of autonomic nerve injury will still be substantial if the rectal dissection is performed by an inexperienced surgeon.
Similar to the study by Yang et al[29], our study is limited by its nonrandomized design, and the risk of selection bias is inevitable. Furthermore, the number of sexually active men recruited and analyzed was small, and therefore, a very strong conclusion regarding sexual function after laparoscopic vs open surgery for rectal cancer could not be drawn. Nevertheless, based on our findings, we may still conclude that laparoscopic sphincter-preserving resection for rectal cancer is associated with better preservation of QoL and fewer male sexual problems when compared with the open approach in the first year after surgery. Further large-scale, multicenter, randomized trials, including the American College of Surgeons Oncology Group Z6051 trial and the European COLOR II trial[32,33], will more definitively evaluate whether laparoscopic surgery truly provides better QoL and reduces urosexual dysfunction in patients with rectal cancer.
In conclusion, this prospective nonrandomized study demonstrates that laparoscopic sphincter-preserving resection for rectal cancer is associated with better preservation of quality of life and fewer male sexual problems when compared with open surgery in Chinese patients. These findings, however, should be interpreted with caution because of the small sample size of the study.
Most colorectal surgeons are only concerned about the surgical and oncologic safety of laparoscopic surgery for rectal cancer in comparison with the open approach, and many have ignored the importance of functional outcomes and quality of life (QoL). Furthermore, few studies have evaluated QoL outcomes in Chinese patients after laparoscopic surgery for rectal cancer. Authors therefore conducted a prospective study to compare QoL outcomes in Chinese patients after curative laparoscopic vs open sphincter-preserving resection for rectal cancer.
The magnified vision and less traumatic surgery offered by the laparoscopic approach may allow better preservation of the pelvic autonomic nerves, and presumably, functional outcomes following laparoscopic surgery for rectal cancer may be better compared to open surgery. However, conflicting results have been reported in the literature; some studies have even reported a higher incidence of sexual dysfunction after laparoscopic rectal surgery. Furthermore, it is also unclear whether the short-term and long-term clinical benefits associated with the laparoscopic approach will translate into better QoL outcomes for patients with rectal cancer.
This study has several strengths. First, although nonrandomized, the baseline characteristics and sociodemographic data of the two groups of patients were similar, and a fair comparison could therefore be made. Second, other studies have included metastatic cases and abdominoperineal resection in their QoL analysis, whereas the study only focused on Chinese patients undergoing curative sphincter-preserving rectal resection, thus minimizing the impact of other potential confounders on the QoL assessment. Third, all their questionnaires were administered by a single research assistant and were completed by the patients during clinic visits. As a result, authors achieved 100% compliance at different time points, a figure that was not achieved by other studies in which the questionnaires were collected by mail.
Their prospective nonrandomized study, albeit small in sample size, demonstrates that laparoscopic sphincter-preserving resection for rectal cancer is associated with better preservation of quality of life and fewer male sexual problems when compared with open surgery in Chinese patients. Further large-scale, multicenter, randomized trials, including the American College of Surgeons Oncology Group Z6051 trial and the European COLOR II trial, will more definitively evaluate whether laparoscopic surgery truly provides better QoL and reduces urosexual dysfunction in patients with rectal cancer.
Within the context of medical and healthcare research, QoL is the patient’s subjective perception of the impact of his/her disease and its treatments on his/her physical, psychological, and social functioning and general well-being. Health-related QoL after cancer surgery can be assessed by standardized instruments such as the questionnaires developed by the European Organization for Research and Treatment of Cancer (EORTC), which contain multidimensional generic and disease-specific domains; the EORTC QLQ-C30 and QLQ-CR38 are the most commonly used questionnaires in colorectal cancer trials.
The external validity of this study is limited by the fact that only Chinese patients with low body mass index were included, and patients with stage IV disease were excluded.
P- Reviewer Rutegard J S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [PubMed] |
| 2. | Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 739] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 3. | Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50:464-471. [PubMed] |
| 4. | Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc. 2004;18:1211-1215. [PubMed] |
| 6. | Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 7. | Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Hon SS. Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum. 2009;52:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Morino M, Parini U, Allaix ME, Monasterolo G, Brachet Contul R, Garrone C. Male sexual and urinary function after laparoscopic total mesorectal excision. Surg Endosc. 2009;23:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Lange MM, den Dulk M, Bossema ER, Maas CP, Peeters KC, Rutten HJ, Klein Kranenbarg E, Marijnen CA, van de Velde CJ. Risk factors for faecal incontinence after rectal cancer treatment. Br J Surg. 2007;94:1278-1284. [PubMed] |
| 10. | Canda AE, Terzi C, Gorken IB, Oztop I, Sokmen S, Fuzun M. Effects of preoperative chemoradiotherapy on anal sphincter functions and quality of life in rectal cancer patients. Int J Colorectal Dis. 2010;25:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Vironen JH, Kairaluoma M, Aalto AM, Kellokumpu IH. Impact of functional results on quality of life after rectal cancer surgery. Dis Colon Rectum. 2006;49:568-578. [PubMed] |
| 12. | Gujral S, Avery KN, Blazeby JM. Quality of life after surgery for colorectal cancer: clinical implications of results from randomised trials. Support Care Cancer. 2008;16:127-132. [PubMed] |
| 13. | Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol. 1996;14:671-679. [PubMed] |
| 14. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [PubMed] |
| 15. | Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer. 1999;35:238-247. [PubMed] |
| 16. | Jayne DG, Brown JM, Thorpe H, Walker J, Quirke P, Guillou PJ. Bladder and sexual function following resection for rectal cancer in a randomized clinical trial of laparoscopic versus open technique. Br J Surg. 2005;92:1124-1132. [PubMed] |
| 17. | Quah HM, Jayne DG, Eu KW, Seow-Choen F. Bladder and sexual dysfunction following laparoscopically assisted and conventional open mesorectal resection for cancer. Br J Surg. 2002;89:1551-1556. [PubMed] |
| 18. | Breukink SO, van der Zaag-Loonen HJ, Bouma EM, Pierie JP, Hoff C, Wiggers T, Meijerink WJ. Prospective evaluation of quality of life and sexual functioning after laparoscopic total mesorectal excision. Dis Colon Rectum. 2007;50:147-155. [PubMed] |
| 19. | Bartels SA, Vlug MS, Ubbink DT, Bemelman WA. Quality of life after laparoscopic and open colorectal surgery: a systematic review. World J Gastroenterol. 2010;16:5035-5041. [PubMed] |
| 20. | Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187-1192. [PubMed] |
| 21. | Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Teoh AY, Leung WW. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15:2418-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Zhao H, Kanda K. Translation and validation of the standard Chinese version of the EORTC QLQ-C30. Qual Life Res. 2000;9:129-137. [PubMed] |
| 23. | Chie WC, Yang CH, Hsu C, Yang PC. Quality of life of lung cancer patients: validation of the Taiwan Chinese version of the EORTC QLQ-C30 and QLQ-LC13. Qual Life Res. 2004;13:257-262. [PubMed] |
| 24. | Law CC, Tak Lam WW, Fu YT, Wong KH, Sprangers MA, Fielding R. Validation of the Chinese version of the EORTC colorectal cancer-specific quality-of-life questionnaire module (QLQ-CR38). J Pain Symptom Manage. 2008;35:203-213. [PubMed] |
| 25. | Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and treatment of Cancer 2001; . |
| 26. | Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139-144. [PubMed] |
| 27. | Sideris L, Zenasni F, Vernerey D, Dauchy S, Lasser P, Pignon JP, Elias D, Di Palma M, Pocard M. Quality of life of patients operated on for low rectal cancer: impact of the type of surgery and patients’ characteristics. Dis Colon Rectum. 2005;48:2180-2191. [PubMed] |
| 28. | Li J, Chen R, Xu YQ, Wang XC, Zheng S, Zhang SZ, Ding KF. Impact of a laparoscopic resection on the quality of life in rectal cancer patients: results of 135 patients. Surg Today. 2010;40:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Yang L, Yu YY, Zhou ZG, Li Y, Xu B, Song JM, Liu HY, Jiang X. Quality of life outcomes following laparoscopic total mesorectal excision for low rectal cancers: a clinical control study. Eur J Surg Oncol. 2007;33:575-579. [PubMed] |
| 30. | Carr AJ, Gibson B, Robinson PG. Measuring quality of life: Is quality of life determined by expectations or experience? BMJ. 2001;322:1240-1243. [PubMed] |
| 31. | Tekkis PP, Senagore AJ, Delaney CP, Fazio VW. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242:83-91. [PubMed] |
| 32. | Soop M, Nelson H. Laparoscopic-assisted proctectomy for rectal cancer: on trial. Ann Surg Oncol. 2008;15:2357-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Buunen M, Bonjer HJ, Hop WC, Haglind E, Kurlberg G, Rosenberg J, Lacy AM, Cuesta MA, D’Hoore A, Fürst A. COLOR II. A randomized clinical trial comparing laparoscopic and open surgery for rectal cancer. Dan Med Bull. 2009;56:89-91. [PubMed] |