Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6277
Revised: July 30, 2012
Accepted: August 3, 2012
Published online: November 21, 2012
AIM: To evaluate the durability in hepatitis B e antigen (HBeAg) positive chronic hepatitis B patients who discontinued antiviral treatment.
METHODS: A total of 48 HBeAg positive chronic hepatitis B patients who were administered nucleoside analogues and maintained virological response for ≥ 6 mo [hepatitis B virus (HBV) DNA < 300 copies/mL and HBeAg seroconversion] before cessation of treatment were enrolled between February 2007 and January 2010. The criteria for the cessation of the antiviral treatment were defined as follows: (1) achievement of virological response; and (2) duration of consolidation therapy (≥ 6 mo). After treatment cessation, the patients were followed up at 3-6 mo intervals. The primary endpoint was serologic and virologic recurrence rates after withdrawal of antiviral treatment. Serologic recurrence was defined as reappearance of HBeAg positivity after HBeAg seroconversion. Virologic recurrence was defined as an increase in HBV-DNA level > 104 copies/mL after HBeAg seroconversion with previously undetectable HBV-DNA level.
RESULTS: During the median follow-up period of 18.2 mo (range: 5.1-47.5 mo) after cessation of antiviral treatment, the cumulative serological recurrence rate was 15 % at 12 mo. The median duration between the cessation of antiviral treatment and serologic recurrence was 7.2 mo (range: 1.2-10.9 mo). Of the 48 patients with HBeAg positive chronic hepatitis, 20 (41.6%) showed virological recurrence. The cumulative virologic recurrence rates at 12 mo after discontinuing the antiviral agent were 41%. The median duration between off-treatment and virologic recurrence was 7.6 mo (range: 4.3-27.1 mo). The mean age of the virological recurrence group was older than that of the non-recurrence group (46.7 ± 12.1 years vs 38.8 ± 12.7 years, respectively; P = 0.022). Age (> 40 years) and the duration of consolidation treatment (≥ 15 mo) were significant predictive factors for offtreatment durability in the multivariate analysis [P = 0.049, relative risk (RR) 0.31, 95% CI (0.096-0.998) and P = 0.005, RR 11.29, 95% CI (2.054-65.12), respectively]. Patients with age (≤ 40 years) who received consolidation treatment (≥ 15 mo) significantly showed durability in HBeAg positive chronic hepatitis B patients (P = 0.014). These results suggest that additional treatment for more than 15 mo after HBeAg seroconversion in patients who are ≤ 40 years old may be beneficial in providing a sustained virological response.
CONCLUSION: Our data suggest that HBeAg seroconversion is an imperfect end point in antiviral treatment. Long-term consolidation treatment (≥ 15 mo) in younger patients is important for producing better prognosis in HBeAg positive chronic hepatitis B.
- Citation: Song MJ, Song DS, Kim HY, Yoo SH, Bae SH, Choi JY, Yoon SK, Paik YH, Lee JS, Lee HW, Kim HJ. Durability of viral response after off-treatment in HBeAg positive chronic hepatitis B. World J Gastroenterol 2012; 18(43): 6277-6283
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6277
Chronic hepatitis B virus (HBV) infection is a major problem in medical health care with approximately 400 million people infected worldwide[1,2]. Since lamivudine was introduced as an effective antiviral agent for chronic hepatitis B in the late 1990s, many types of nucleos(t)ide analogues (NUCs) have been developed to prevent the progression of cirrhosis and hepatocellular carcinoma[3-6]. To assess these effects of antiviral therapy, surrogate markers such as hepatitis B e antigen (HBeAg) seroconversion or HBsAg loss have been reported[7-12].
The current end-points of therapy are durable HBeAg seroconversion in HBeAg-positive patients[13]. International guidelines on HBV therapy suggest that finite duration of treatment with a NUC is a reasonable option, and recommend that treatment may be stopped after HBeAg seroconversion and an additional 6-12 mo of consolidation therapy in HBeAg-positive hepatitis[13,14]. However, it may be necessary to maintain long-term treatment for the ideal end-points such as HBsAg clearance or sustained virologic response in HBeAg-positive chronic hepatitis B due to a higher relapse rate[15,16].
The optimum treatment durations of NUCs also remain ill defined. Several studies on the durability of HBeAg seroconversion induced by lamivudine have produced contradictory results[16-19]. Reijnders et al[20] have reported that HBeAg seroconversion is an imperfect end point in antiviral treatment. Studies investigating the durability of the response to newer agents such as entecavir and clevudine are still lacking.
The durability of virological response (HBeAg seroconversion and undetectable HBV DNA) is important for developing a treatment strategy for chronic hepatitis B patients. In this study, we evaluated the off-treatment durability of response and the predictive factors for virologic recurrence in HBeAg positive chronic hepatitis B patients who achieved successful end point of therapy with nucleoside analogues such as entecavir and clevudine. Furthermore, we compared the durability of response between entecavir and clevudine.
This study included 48 Korean patients with chronic hepatitis B infection (HBsAg and HBeAg positivity for at least 6 mo) who were hospitalized at the Seoul St. Mary’s Hospital, Gangnam Severance Hospital, Inje University Ilsan Paik Hospital and Chung-Ang University Hospital between February 2007 and January 2010. All patients were treated with nucleoside analogue therapy such as clevudine and entecavir. When the therapy was initiated, the serum HBV DNA levels were at least 105 copies/mL and alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels were more than two times the upper limit of normal (ULN, 40 IU/L). There were no signs of either co-infection with hepatitis C virus or human immunodeficiency virus or hepatic decompensation such as ascites, variceal bleeding or hepatic encephalopathy, and treatment with (pegylated) interferon was discontinued at least 6 mo before the start of the NUC treatment.
The cessation criteria of the NUC treatment were defined as follows: (1) the achievement of a virological response; and (2) duration of consolidation therapy (≥ 6 mo). Virological response was defined as HBeAg seroconversion, HBV DNA < 300 copies/mL and normal ALT levels. The study was approved by the institutional Ethics Review Board and complied with the Declaration of Helsinki.
All patients were monitored at least every 3-6 mo during the antiviral treatment period. Biochemical (serum AST, ALT) and virological parameters (HBeAg, HBeAb status and quantitative HBV DNA) were assessed at every visit. Nucleoside analogue-treated patients who achieved virological response were evaluated every 3 mo. Consolidation therapy was continued for more than 6 mo after achieving virological response. In cases in which NUC treatment was discontinued, the patients were followed up at 3-6 mo intervals for a median period of 18.2 mo (range: 5.1-47.5 mo).
The primary endpoint was serologic and virologic recurrence rates after withdrawal of antiviral treatment. Serologic recurrence was defined as reappearance of HBeAg positivity after HBeAg seroconversion. Virologic recurrence was defined as an increase in HBV-DNA level > 104 copies/mL after HBeAg seroconversion with previously undetectable HBV-DNA level because a viral load of more than 10 000 copies/mL is associated with progression of liver disease[4,21].
The secondary endpoint was to evaluate the difference in the durability between entecavir and clevudine.
Serum HBeAg and HBeAb were measured with a radioimmunoassay according to the manufacturer’s protocol (Abbott Laboratories, Chicago, IL, United States). Serum HBV-DNA levels were measured with real-time PCR using Abott Realtime HBV Quantification Kit (Abott Molecular Inc., IL, United States).
Ethics approval was provided by the institutional Ethics Review Board, The Catholic University of Korea (KC11RIMI0560).
The continuous variables are expressed as the mean ± SE or median (range) where appropriate. Durability was calculated from the date of the antiviral treatment cessation to the date of virologic recurrence or censorship. The cumulative rates of virologic recurrence, HBeAg seroreversion were estimated with the Kaplan-Meier method. The Cox’s proportional hazard model was adopted to determine the predictive factors for relapse, among various variables including age, pretreatment ALT, AST level, pretreatment HBV DNA levels, consolidation treatment duration and total treatment duration. P < 0.05 was considered to be significant (SPSS 17, Chicago, IL).
The baseline characteristics of all patients are shown in Table 1. A total of 48 patients with chronic hepatitis B who were treated with a nucleoside analogue in multicenter were included in the analysis. Of the 48 patients, 29 (60.4%) were men and the mean age was 42.1 ± 12.9 years. The pretreatment ALT level and serum HBV DNA level were 198 IU/L (11-374 IU/L) and 8.8 log10 copies/mL (range: 5-10 log10 copies/mL), respectively. Overall, 31 of the 48 patients with HBeAg positive hepatitis were treated with entecavir, and 17 were treated with clevudine. A total of 41 (85.4%) patients were nucleoside analog treatment naïve, whereas 7 (14.6%) patients had received prior treatment with lamivudine. All patients who were treated with lamivudine did not have any evidence of lamivudine-resistance at the initiation of entecavir or clevudine treatment. The median duration of therapy was 26.1 mo (range: 9.4-47.4 mo). Patients were followed up for a median of 45.6 mo (range: 23.6-60.2 mo); 82 % (43/48) were followed up for more than 3 years.
| Baseline characteristics | Total (n = 48) |
| Age, yr (mean ± SD) | 42.1 ± 12.9 |
| Sex (male/female) | 29/19 |
| Pretreatment ALT level (IU/L) | 198 (11-374) |
| Pretreatment HBV DNA level (log10 copies/mL) | 8.5 (5.0-10) |
| Time to undetectable HBV (mo) | 7.7 (1.3-39.4) |
| Time to HBeAg seroconversion (mo) | 13.4 (0.8-39.4) |
| Consolidation treatment duration (mo) | 10.3 (6-37.9) |
| Total treatment duration (mo) | 26.1 (9.4-47.4) |
| Antiviral agent (entecavir/clevudine) | 31/17 |
| Previous NUC treatment (naïve/lamivudine) | 41/7 |
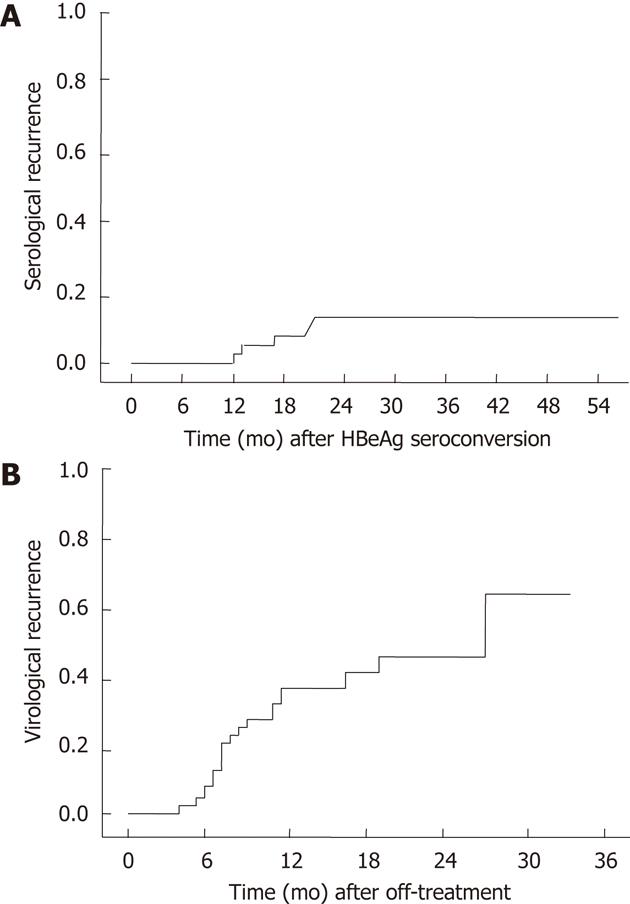
The median follow-up time after HBeAg seroconversion for 48 patients was 32.1 mo (range: 10.3-55.8 mo). Serologic recurrence occurred in 6 of 48 (12.5%) patients. The cumulative serologic recurrence rates at 6 mo, 12 mo and 18 mo after NUC induced HBeAg seroconversion were 0%, 7% and 15%, respectively. The median duration between the cessation of antiviral treatment and serologic recurrence was 7.2 mo (range: 1.2-10.9 mo). In 1 case, serologic recurrence occurred less than 6 mo after the cessation of NUC treatment, and in 5/6 (83%) cases, serologic recurrence occurred within 12 mo after off-treatment (Figure 1A).
Virologic recurrence occurred in 20 of 48 patients (41.6%) after HBeAg seroconversion. The cumulative virologic recurrence rates at 6 mo, 12 mo and 24 mo after discontinuing the antiviral agent were 38%, 41% and 60%, respectively (Figure 1B). Of these 20 patients, 6 reverted to chronic HBeAg-positive chronic hepatitis B, and 14 progressed to HBeAg negative chronic hepatitis B. One of 6 patients with HBeAg reversion (16.6%) showed HBV DNA levels of 10-100 copies/L. Six of 14 patients with HBeAg seronegativity (42.8%) showed HBV DNA levels of more than 100 copies/L (Table 2). The median duration between off-treatment and virologic recurrence was 7.6 mo (range: 4.3-27.1 mo) in HBeAg positive hepatitis. Seventeen cases (85%) of virologic recurrence occurred within 12 mo after off-treatment.
| Patient No. | Age (yr) | Sex | Serologic recurrence (HBeAg reversion) | Virological recurrence (copies/L) | ALT level at relapse (U/L) |
| 1 | 28 | Female | Yes | 97 600 | 139 |
| 2 | 24 | Female | Yes | 645 000 000 | 236 |
| 3 | 42 | Male | Yes | 120 759 | 71 |
| 4 | 46 | Male | Yes | 980 000 | 45 |
| 5 | 47 | Male | Yes | 254 251 078 | 89 |
| 6 | 48 | Female | Yes | 320 630 | 287 |
| 7 | 46 | Male | No | 42 900 | 42 |
| 8 | 32 | Female | No | 20 916 | 32 |
| 9 | 67 | Male | No | 10 083 | 38 |
| 10 | 42 | Male | No | 41 982 | 65 |
| 11 | 46 | Male | No | 86 000 | 40 |
| 12 | 66 | Female | No | 4 521 266 | 131 |
| 13 | 38 | Male | No | 587 000 | 43 |
| 14 | 67 | Female | No | 10 032 | 31 |
| 15 | 62 | Female | No | 637 898 | 28 |
| 16 | 50 | Male | No | 43 719 | 98 |
| 17 | 51 | Female | No | 32 033 | 101 |
| 18 | 42 | Male | No | 86 6197 | 243 |
| 19 | 53 | Male | No | 6 158 288 | 54 |
| 20 | 37 | Female | No | 116 000 000 | 436 |
In HBeAg positive chronic hepatitis, only 28 (58.3%) had a durable response, as defined by the continued absence of HBeAg and HBV DNA levels less than 10 copies/L, during a median follow-up period of 18.2 mo (range: 5.1-47.5 mo). In all patients, NUC therapy was discontinued after HBeAg seroconversion occurred and at least 6 mo of consolidation therapy. The clinical features and laboratory data including age, sex, pretreatment. ALT, HBV DNA titer, duration of consolidation treatment, total treatment duration, and duration of treatment before the HBV DNA levels were undetectable were compared between the virologic recurrence and the non-recurrence group (Table 3). The mean age of the recurrence group was older than that of the nonrecurrence group (46.7 ± 12.1 years vs 38.8 ± 12.7 years, respectively; P = 0.022).
| Virological recurrence(n = 20) | Virological non-recurrence(n = 28) | P value | |
| Age, yr (mean ± SD) | 46.7 ± 12.1 | 38.8 ± 12.7 | 0.022 |
| Sex (male/female) | 11/9 | 29/19 | 0.561 |
| Pretreatment ALT level (IU/L) | 199 (40-374) | 189 (11-364) | 0.491 |
| Pretreatment HBV DNA level (log10 copies/mL) | 7.7 (5.1-10) | 7 (5.0-9.1) | 0.125 |
| Time to undetectable HBV (mo) | 6.7 (2.3-39.4) | 9.1 (1.3-25.0) | 0.490 |
| Time to HBeAg seroconversion (mo) | 13.9 (0.8-39.4) | 12.7 (1.4-31.2) | 0.744 |
| Consolidation treatment duration (mo) | 10.3 (6-23.9) | 11.7 (6-37.9) | 0.517 |
| Total treatment duration (mo) | 25.6 (9.6-47.4) | 27.0 (9.4-43.3) | 0.358 |
| Antiviral agent (entecavir/clevudine) | 15/5 | 16/12 | 0.236 |
Univariate analysis was performed to determine the predictive factors of durability. The variables including age, sex, pretreatment ALT, level, pretreatment HBV DNA levels, time to undetectable HBV DNA, time to HBeAg seroconversion, consolidation treatment duration and total treatment duration. Age (≤ 40 years) and the duration of consolidation treatment (≥ 15 mo) were significant predictive factors for off-treatment durability (P = 0.017, P = 0.046, respectively). In the multivariate analysis using the Cox’s proportional hazard model, the factors of age (≤ 40 years) and prolonged-consolidation treatment ≥ 15 mo were also significant predictive factors of virological recurrence in HBeAg positive chronic hepatitis B patients [P = 0.049, 95% CI (0.096-0.998); P = 0.005, 95% CI (2.054-65.12)]. Serologic or virologic recurrence did not show a significant difference between clevudine and entecavir treatment (Table 4).
| Regression coefficent | Standard error | P value | RR (95% CI) | |
| Age ( ≤ 40 yr vs > 40 yr) | -1.17 | 0.596 | 0.049 | 0.310 (0.096-0.998) |
| Sex (male vs female) | -0.938 | 0.527 | 0.075 | 0.391 (0.139-1.099) |
| Pretreatment ALT level (< 2 × ULN vs≥ 2 × ULN) | -2.11 | 1.135 | 0.063 | 0.121 (0.013-1.121) |
| Pretreatment HBV DNA level (< 108 vs≥ 108) | -0.27 | 0.533 | 0.612 | 0.763 (0.268-2.170) |
| Time to undetectable HBV (< 6 mo vs≥ 6 mo) | -0.237 | 0.635 | 0.709 | 0.789 (0.227-2.739) |
| Time to HBeAg seroconversion (< 12 mo vs≥ 12 mo) | 1.417 | 0.846 | 0.094 | 4.126 (0.786-21.66) |
| Consolidation treatment (< 15 mo vs≥ 15 mo) | 2.425 | 0.87 | 0.005 | 11.299 (2.054-65.12) |
| Total treatment duration (< 24 mo vs≥ 24 mo) | 0.001 | 0.732 | 0.999 | 1.001 (0.238-4.207) |
| Antiviral agent (entecavir vs clevudine) | -1.529 | 0.861 | 0.076 | 0.217 (0.040-1.172) |
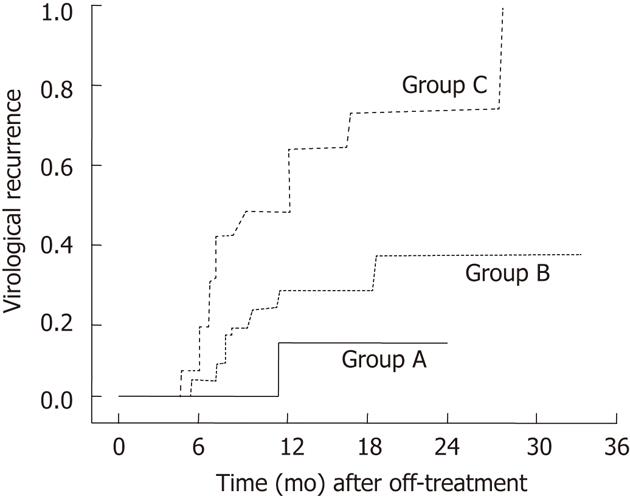
Using the two predictive factors including age (≤ 40 years) and prolonged-consolidation treatment ≥ 15 mo patients could be classified into 3 groups: patients who had both of the good predictors (age ≤ 40 years and consolidation treatment ≥ 15 mo, group A); patients with either ≥ 40 years old or underwent consolidation treatment ≥ 15 mo (group B), and patients with neither of the two good predictors (group C). Among these 3 groups, group C showed a significantly higher recurrence rate (Figure 2). These results suggest that additional treatment for more than 15 mo after HBeAg seroconversion in patients who are ≤ 40 years old may be beneficial in providing a sustained virological response.
The end points of chronic hepatitis B treatment, independent of HBeAg status, are the sustained suppression of HBV replication, the remission of liver disease and the prevention of hepatocellular carcinoma[22,23]. Clinical studies have reported that rapid and sustained suppression of viral load is an important factor in the prediction of the virological response. It was reported that entecavir had the potential for a very rapid and effective antiviral response and clevudine-induced viral response was durable after discontinuation of treatment[24]. It was hypothesized that entecavir would induce a more durable suppression of viral load compared with that of clevudine after discontinuation of treatment.
We studied the durability of off treatment for chronic HBV infection among entecavir-treated and clevudinetreated patients who achieved “virological response”. Current international guidelines suggest that treatment with NUC can be discontinued after 6-12 mo of consolidation therapy after HBeAg seroconversion[14].
Consistent with these recommendations, NUC treatment was discontinued in all patients after 6 mo of consolidation treatment. During follow-up after the discontinuation of NUC therapy, only 20 (41.6%) of the 48 patients showed a durable response, which was defined as HBeAg seroconversion and HBV DNA levels less than 10 copies/L. Moreover, a sustained response was achieved in patients who discontinued therapy after a consolidation therapy period of at least 15 mo regardless of the type of antiviral drug.
In our study, both serologic and virological recurrence after HBeAg seroconversion and undetectable HBV DNA were considered. Serologic recurrence showed 5 of 6 patients (83%) and virological recurrence occurred 17 of 20 patients (85%) within 12 mo after discontinuing the antiviral agent. This recurrence rate was consistent with a previous study[20]. These results showed longer treatment durations of nucleoside analogs may contribute to the suppression of viral replication and the clearance of infected hepatocytes, but HBV cannot be completely eradicated. Therefore, HBeAg seroconversion and consolidation therapy for more than 6 mo might be an imperfect end point in antiviral treatment.
Patients who were ≤ 40 years old also showed independently significant associations with a sustained response based on the multivariate analysis. Younger patients had a more sustained durability. This result was similar to that reported by Chien et al[25] who demonstrated that Taiwanese patients who were < 36 years of age had a more sustained response. This difference in durability based on age may be related to immune system activity[26]. It is assumed that younger patients were more likely to be sufficiently immunocompetent to maintain viral suppression, which contributed to a higher rate of off-treatment durability. While age appears to be directly related to durability in this study, further research is necessary to more clearly define the cutoff age.
This study has some limitations in a retrospective study. First, a limited number of patients were treated with either entecavir or clevudine monotherapy; Second, HBsAg loss in a more stable remission of chronic hepatitis B compared with HBeAg seroconversion was not analyzed because the follow-up period was relatively short. However, we intended to analyze the predictive factors of sustained virologic response in HBeAg positive chronic hepatitis B patients who discontinued successful antiviral therapy that was considered as high potency antiviral agent more than lamivudine. Our results might suggest that the high recurrence rates after achieving sustained virologic response (HBeAg seroconversion and undetectable HBV DNA) showed no difference of durability regardless of the type of oral high potency antiviral agent.
In conclusion, this study shows that HBeAg seroconversion and consolidation therapy for more than 6 mo are an imperfect end point in antiviral treatment. Longterm consolidation treatment (≥ 15 mo) in younger patients is important to produce better prognosis in HBeAg positive chronic hepatitis B.
The durability of the virologic response is important to plan the strategy of treatment in chronic hepatitis B. The present study evaluated the off-treatment durability of response and the predictive factors for virologic recurrence in hepatitis B e antigen (HBeAg) positive chronic hepatitis B patients who achieved the successful end point of nucleotide analogue therapy.
This study showed that HBeAg seroconversion is an imperfect end point in antiviral treatment. Long-term consolidation treatment (≥ 15 mo) in younger patients is important for producing better prognosis in HBeAg positive chronic hepatitis B.
The high recurrence rates after achieving sustained virologic response (HBeAg seroconversion and undetectable hepatitis B virus DNA) showed no difference of durability regardless of the type of oral high potency antiviral agent.
The study results suggest that long-term consolidation treatment (≥ 15 mo) after achieving virological response is important for maintaining better sustained virological response in HBeAg positive chronic hepatitis B.
The criteria for the cessation of treatment were defined as follows: (1) achievement of virological response; and (2) duration of consolidation therapy (≥ 6 mo). Virological response defined HBeAg seroconversion, HBV DNA < 300 copies/mL and normal alanine aminotransferase levels. Virologic recurrence was defined as an increase of HBV-DNA level > 10 copies/L after HBeAg seroconversion with previously undetectable HBV-DNA level because a viral load of more than 10 copies/L is associated with progression of liver disease.
This study can be considered as a preliminary report of the comparasion between entecavir and clevudine therapy in chronic HBV in relation to the behaviour of HBeAg positive and viral load. It was well designed and conducted but a continuation of this study must be done with a greatest number of patients under a longest time of therapy before discontinuation.
Peer reviewers: Heitor Rosa, Professor, Department of Gastroenterology and Hepatology, Federal University School of Medicine, Goiania, GO 74093-080, Brazil; George Papatheodoridis, MD, Associate Professor in Medicine and Gastroenterology, 2nd Department of Internal Medicine, Athens University Medical School, Hippokration General Hospital of Athens, 114 Vas. Sophias Ave., 11527 Athens, Greece
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225-1241. [PubMed] [Cited in This Article: ] |
| 2. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [PubMed] [Cited in This Article: ] |
| 3. | Doong SL, Tsai CH, Schinazi RF, Liotta DC, Cheng YC. Inhibition of the replication of hepatitis B virus in vitro by 2’,3’-dideoxy-3’-thiacytidine and related analogues. Proc Natl Acad Sci U S A. 1991;88:8495-8499. [PubMed] [Cited in This Article: ] |
| 4. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] [Cited in This Article: ] |
| 5. | Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49:S4-S12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Dienstag JL. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology. 2009;49:S112-S121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Bortolotti F, Guido M, Bartolacci S, Cadrobbi P, Crivellaro C, Noventa F, Morsica G, Moriondo M, Gatta A. Chronic hepatitis B in children after e antigen seroclearance: final report of a 29-year longitudinal study. Hepatology. 2006;43:556-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Fattovich G, Rugge M, Brollo L, Pontisso P, Noventa F, Guido M, Alberti A, Realdi G. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology. 1986;6:167-172. [PubMed] [Cited in This Article: ] |
| 9. | Hoofnagle JH, Dusheiko GM, Seeff LB, Jones EA, Waggoner JG, Bales ZB. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med. 1981;94:744-748. [PubMed] [Cited in This Article: ] |
| 10. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [PubMed] [Cited in This Article: ] |
| 11. | Lok AS, Lai CL, Wu PC, Leung EK, Lam TS. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology. 1987;92:1839-1843. [PubMed] [Cited in This Article: ] |
| 12. | McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759-768. [PubMed] [Cited in This Article: ] |
| 13. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1794] [Cited by in F6Publishing: 1739] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 14. | EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [PubMed] [Cited in This Article: ] |
| 15. | Lee KM, Cho SW, Kim SW, Kim HJ, Hahm KB, Kim JH. Effect of virological response on post-treatment durability of lamivudine-induced HBeAg seroconversion. J Viral Hepat. 2002;9:208-212. [PubMed] [Cited in This Article: ] |
| 16. | Song BC, Suh DJ, Lee HC, Chung YH, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology. 2000;32:803-806. [PubMed] [Cited in This Article: ] |
| 17. | Dienstag JL, Schiff ER, Mitchell M, Casey DE, Gitlin N, Lissoos T, Gelb LD, Condreay L, Crowther L, Rubin M. Extended lamivudine retreatment for chronic hepatitis B: maintenance of viral suppression after discontinuation of therapy. Hepatology. 1999;30:1082-1087. [PubMed] [Cited in This Article: ] |
| 18. | Dienstag JL, Cianciara J, Karayalcin S, Kowdley KV, Willems B, Plisek S, Woessner M, Gardner S, Schiff E. Durability of serologic response after lamivudine treatment of chronic hepatitis B. Hepatology. 2003;37:748-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Yoon SK, Jang JW, Kim CW, Bae SH, Choi JY, Choi SW, Lee YS, Lee CD, Chung KW, Sun HS. Long-term results of lamivudine monotherapy in Korean patients with HBeAg-positive chronic hepatitis B: response and relapse rates, and factors related to durability of HBeAg seroconversion. Intervirology. 2005;48:341-349. [PubMed] [Cited in This Article: ] |
| 20. | Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139:491-498. [PubMed] [Cited in This Article: ] |
| 21. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [PubMed] [Cited in This Article: ] |
| 22. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H, Wright TL. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006;4:936-962. [PubMed] [Cited in This Article: ] |
| 23. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [PubMed] [Cited in This Article: ] |
| 24. | Lee HS, Yoo BC, Lee KS, Kim JH, Um SH, Ryu SH, Lee YS, Kim YS, Yoo K, Han JY. Clevudine-induced viral response, associated with continued reduction of HBsAg titer, was durable after the withdrawal of therapy. J Gastroenterol. 2011;46:410-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Chien RN, Yeh CT, Tsai SL, Chu CM, Liaw YF. Determinants for sustained HBeAg response to lamivudine therapy. Hepatology. 2003;38:1267-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |