Published online Jan 14, 2012. doi: 10.3748/wjg.v18.i2.175
Revised: June 17, 2011
Accepted: June 24, 2011
Published online: January 14, 2012
AIM: To determine the role of circulating tumor cells (CTCs) in prediction of the overall survival of patients with advanced malignant biliary tract obstruction.
METHODS: We investigated the prognostic value of CTCs by examining two markers, cytokeratin (CK) 19 and human telomerase reverse transcriptase (hTERT) mRNA, in 40 patients diagnosed with advanced malignant biliary tract diseases. Quantitative real-time reverse transcription polymerase chain reaction was used to detect CK19 and hTERT mRNA in the peripheral blood of these patients. Overall survival was analyzed using the Kaplan-Meier method and Cox regression modeling.
RESULTS: Positive CK19 and hTERT mRNA expression was detected in 45% and 60%, respectively, of the 40 patients. Univariable analysis indicated that positive CK19 mRNA expression was significantly associated with worse overall survival (P = 0.009). Multivariable analysis determined that positive CK19 mRNA expression, patient’s age and serum bilirubin were each independently associated with overall survival.
CONCLUSION: CK19 mRNA expression levels in peripheral blood appear to provide a valuable marker to predict the overall survival of patients with advanced malignant biliary tract obstruction.
- Citation: Leelawat K, Narong S, Udomchaiprasertkul W, Wannaprasert J, Treepongkaruna SA, Subwongcharoen S, Ratanashu-ek T. Prognostic relevance of circulating CK19 mRNA in advanced malignant biliary tract diseases. World J Gastroenterol 2012; 18(2): 175-181
- URL: https://www.wjgnet.com/1007-9327/full/v18/i2/175.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i2.175
Malignant biliary tract obstruction is a condition that can result from tumors of the biliary tract, ampulla of vater, duodenum or head of the pancreas. In Thailand, cholangiocarcinoma (CCA) is the most common cause of malignant biliary tract obstruction[1]. Despite recent advances in the diagnosis and treatment of this disease, patient outcome remains poor. The high mortality rate arising from malignant biliary tract obstruction is due to the aggressiveness of tumors that are often discovered at a late stage of disease progression[2]. Palliative therapeutic approaches to endoscopic biliary drainage, such as the use of endoprosthesis stents, are generally recommended for these patients. The two major types of endoprosthesis stents are plastic or polyethylene (PE) stents and self-expanding metal stents (SEMS). Previous studies have demonstrated that partial or total occlusion of PE stents usually occurs 3-4 mo after insertion[3]. Four randomized controlled studies demonstrated that SEMS exhibited a significantly higher patency rate as compared with the PE stents (9 mo vs 1.5 mo)[4-7]; however, SEMS is much more expensive than PE stents (1500 USD vs 80 USD, in Thailand). A recent study indicated that patients who have a predicted survival duration of more than 4.5 mo should use SEMs for their palliative biliary drainage[8]. In this instance, the higher cost of the SEMs is balanced by a decreased need for repeat intervention that is often necessary in patients who have received PE stents. Therefore, identification of reliable prognostic factors that allow for an accurate prediction of survival duration in patients with advanced malignant biliary tract obstruction is extremely important.
One of the major mechanisms for tumor metastasis is the dissemination of tumor cells from the primary tumor into circulating blood[9]. Previous studies have indicated that detection of circulating tumor cells (CTCs) in the peripheral blood can be used in staging and prognosis stratification for breast and colon cancer patients[10-12]. Until now, however, there has been no study concerning the role of the detection of CTCs as a prognostic factor in patients with malignant biliary tract diseases.
To date, the most common CTCs detection method is quantitative real-time reverse transcription polymerase chain reaction (RT-PCR), a process that can detect mRNA expression levels of the genes coding for these tumor antigens[13]. A high-quality detection marker is required for efficient quantitative real-time RT-PCR-mediated detection of CTCs. Therefore, identification of a good target marker is of the utmost importance for CTC detection. Several gene markers, such as cytokeratin (CK) 19 and human telomerase reverse transcriptase (hTERT), have been evaluated as tumor-specific markers for the detection of CTCs in gastrointestinal cancers[14,15].
hTERT mRNA can be detected in 85% of all cancer cells, including cholangiocarcinoma cells[16]. This is in contrast to most normal cells, which exhibit little or no expression. Our previous study demonstrated that high levels of hTERT mRNA can be detected in the blood circulation of cholangiocarcinoma patients, and it has also been suggested that hTERT mRNA is a promising marker for the detection of cancer cells[17].
CK19 is generally expressed in ductal epithelium (bile ducts, pancreas, and renal collecting tubules) and in the mucosa of the gastrointestinal tract. CK19 immunohistochemistry is used in diagnostic pathology mainly to confirm epithelial immunophenotype in undifferentiated tumors or to establish biliary, pancreatic or renal ductular origin[18]. Most adenocarcinomas arising from the gastrointestinal tract are CK19 positive, including cholangiocarcinoma and pancreatic cancer[18]. Many investigators have used the detection of CK19 mRNA in peripheral blood as a target gene to investigate CTCs[14,19,20]; however, until now there has been no study focusing on the detection of hTERT and CK19 in the peripheral blood of patients with malignant biliary tract diseases.
This study was aimed to evaluate if the levels of CTCs could be used to predict the overall survival of patients with advanced malignant biliary tract obstruction. CK-19 and hTERT were selected as the target genes for CTCs. In addition, this study was performed in accordance with the REporting recommendations for tumor MARKer prognostic studies[21] to ensure the standardization and transparency of the study.
We prospectively included the patients with advanced malignant biliary tract diseases who underwent palliative endoscopic retrograde cholangiopancreatography or percutaneous transhepatic biliary drainage at Department of Surgery, Rajavithi Hospital from January 2008 to December 2009. The cutoff date for data analysis was December 31, 2010. The inclusion requirements included patients present with malignant bile duct obstruction that was not amenable to curative resection and patients who were followed up for at least one month after biliary tract drainage. All blood and clinical information was obtained with patient informed consent after approval by the Rajavithi Hospital Ethics Committee.
Pre-treatment fasting blood samples were collected from the peripheral vein into ethylenediaminetetraacetic acid-containing tubes. The first 3 mL blood was discarded to prevent epidermal contamination (2-syringe technique). Sample processing was performed within 1 h of blood withdrawal. Blood was transferred into a 30-mL falcon tube and centrifuged at 1800 r/min at room temperature for 20 min. Plasma was removed, and the peripheral blood mononuclear cell (PBMC) fraction was stored at -80 °C until use.
The total RNA of PBMC fraction samples was extracted using the RNeasy mini kit (Qiagen, GmbH, Germany) following the protocol provided by the manufacturer. RNA integrity was checked by electrophoresis and quantified by absorption at 260 and 280 nm using a spectrophotometer (Beckman Coulter Du® 800, Fullerton, CA). Total RNA was reversely transcribed using random primers and the IscriptTM cDNA synthesis kit (Bio-Rad, Hercules, CA, United States) following the protocol provided by the manufacturer. cDNA was stored at -80 °C until use.
Expression of CK19 and hTERT genes was analyzed using specific primers (Table 1). In this assay, the housekeeping gene β-actin was used as an internal control to normalize variations in integrity and the total amount of cDNA. Quantitative real-time PCR assays were performed in triplicate using SYBR Green master mix (Superarray, Frederick, MD, United States) on the Chromo 4™ System (MJ Opticon Monitor ver. 3.1) (Bio-Rad, United States) for 20 min at 50 °C. After this, 42 cycling steps for amplification of PCR products were performed (15 s, 94 °C for denaturation; 30 s, 60 °C for annealing; and 30 s, 72 °C for extension). Melting curve analysis was used to assess the specificity of the amplified products. The expression levels of CK19 and hTERT genes from the cDNA were measured by quantitative real-time PCR using the relative quantification method (2-ΔΔCt method)[22]. The fold-change in gene expression was normalized to a housekeeping gene (β-actin) and relative to a calibrator sample. A pool of cDNA derived from the PBMCs of 30 cases of benign (common bile duct stone and gall stone) biliary tract diseases was used as the calibrator source[23]. Evaluation of the 2-ΔΔCt indicates the fold change in gene expression relative to the calibrator. In this study, we set the positive value as a fold change in gene expression that was greater than 1.5 times relative to the calibrator and the negative value was set as a fold change in gene expression that was lesser than or equal to 1.5 times relative to the calibrator.
| Primer | Forward | Reverse |
| hTERT | GCGGAAGACAGTGGTGAACT | AGC TGGAGTAGT CGCTCT GC |
| CK19 | CCCGCGACTACAGCCACTA | GCTCATGCGCAGAGCCT |
| β-Actin | GTGGGGCGCCCCAGGCACCA | GTCCTTAATGTCACGCACGATTTC |
Biochemical studies of serum samples, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), total and direct bilirubin, alkaline phosphatase, carcinoembryonic antigen (CEA) and cancer antigen (CA)19-9, were measured using routine automated methods in the Pathological Laboratory at Rajavithi Hospital.
The human cholangiocarcinoma cell line RMCCA1[24] was incubated in Ham’s F12 medium (Invitrogen-Gibco, Carlsbad, CA, United States) containing 10% fetal calf serum (Euroclone-Celbio, Pero, MI) at 37 °C in 5% CO2. To determine the sensitivity of quantitative real-time PCR for detecting cancer cells in PBMCs, cell spiking experiments was performed. The PBMCs obtained from healthy volunteers were counted and diluted in Ham’s F12 medium. RMCCA1 cells were serially diluted from 1 × 106 cells/mL to 1 cell/mL and added to the PBMCs. Quantitative real-time PCR was then performed to detect CK19 and hTERT mRNA.
The primary endpoint of this study was the overall survival of the patients. Survival curves were estimated using the Kaplan-Meyer method, and univariable survival comparisons were calculated according to the log rank test. Multivariable survival analysis was performed using the Cox proportional hazards regression model. The quantitative variables were compared using Mann-Whitney U or Student’s t test, as appropriate. Qualitative variables were reported as counts, and comparisons between independent groups were performed using the Pearson χ2 test. All tests of significance were two sided and P < 0.05 was considered statistically significant.
Forty-two patients with malignant biliary tract disease were included in this study. Two patients were excluded because of the poor quality of the RNA extracted from their peripheral blood. The average age of these patients was 62 years (range, 41-82 years). With regard to cancer type, 6 (15.0%) were pancreatic head cancer, 2 (5.0%) were ampullary cancer, 2 (5.0%) were gall bladder cancer, 2 (5.0%) were middle and distal common bile duct cancer and 28 (70.0%) were hilar cholangiocarcinoma. The clinical characteristics of the patients are shown in Table 2.
| Parameters | n (%) | |
| Gender | Male | 23 (57.5) |
| Female | 17 (42.5) | |
| Age (yr) | < 60 | 18 (45.0) |
| > 60 | 22 (55.0) | |
| Type of cancers | Hilar cholangiocarcinoma | 28 (70.0) |
| Pancreatic cancer | 6 (15.0) | |
| Common bile duct cancer | 2 (5.0) | |
| Ampullary cancer | 2 (5.0) | |
| Gall bladder cancer | 2 (5.0) |
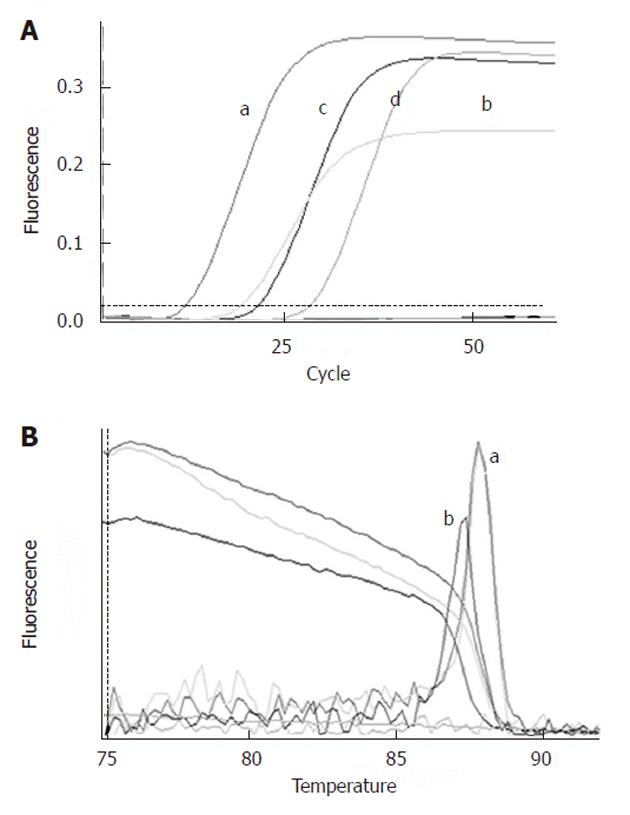
CK19 and hTERT mRNA levels were elevated in the RMCCA1 cell line (Figure 1); therefore, we decided to use this cell line as a positive control for our study. Detection sensitivity of the quantitative real-time PCR assay was determined by serial 10-fold dilutions of RMCCA1 cells in PBMCs. The results demonstrated that CK19 and hTERT mRNA could be detected at levels up to 1000 cells per 1010 PBMC dilutions.
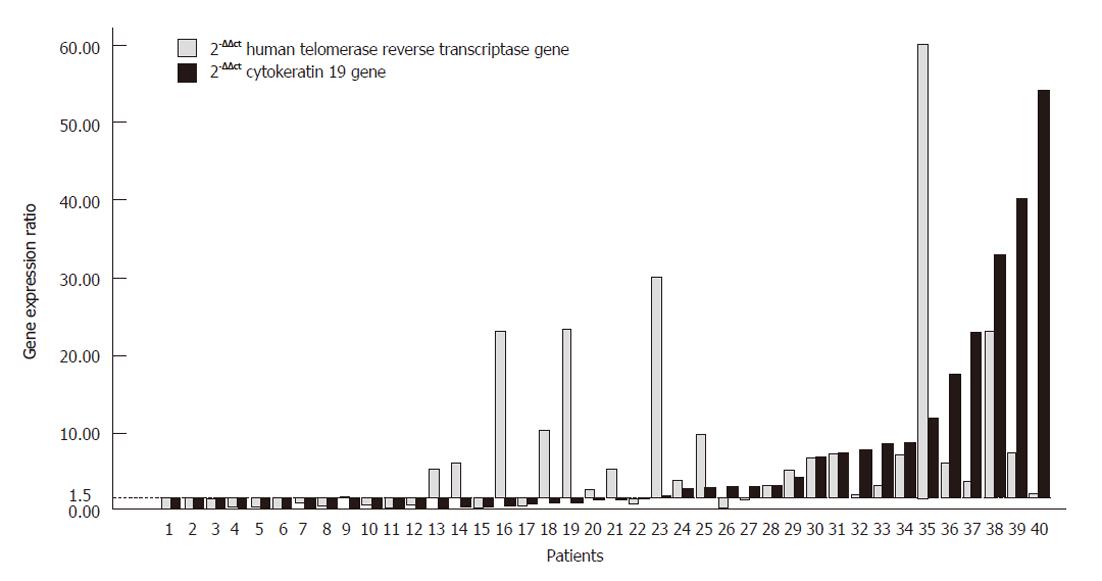
PBMC samples from 40 patients were evaluated for the expression of hTERT and CK19 mRNA. The expression was positive (gene expression more than 1.5 times relative to the calibrator) in 45% (18/40) of samples for CK19 mRNA and 60% (24/40) of samples for hTERT. Figure 2 illustrates the distribution of CK19 mRNA and hTERT mRNA expression in the peripheral blood of these patients.
No statistically significant difference was found among the data obtained from the patients considered as negative or those who were positive for hTERT or CK19 mRNA expression in PBMCs. Factors evaluated included gender, age, serum albumin, globulin bilirubin AST and ALT and alkaline phosphatase levels (Table 3).
| CK19 gene | P value | hTERT gene | P value | |||
| Negative | Positive | Negative | Positive | |||
| Age (yr) | 63.80 | 61.38 | 0.42 | 65.26 | 60.88 | 0.16 |
| Sex (male: female) | 11:11 | 12:6 | 0.351 | 8:8 | 15:9 | 0.521 |
| Total bilirubin (mg/dL) | 16.21 | 16.42 | 0.95 | 17.73 | 15.47 | 0.53 |
| Albumin (g/dL) | 2.88 | 2.97 | 0.62 | 2.83 | 2.99 | 0.36 |
| Globulin (g/dL) | 3.98 | 3.76 | 0.42 | 3.92 | 3.84 | 0.79 |
| AST (U/L) | 84.57 | 112.80 | 0.23 | 69.27 | 117.48 | 0.12 |
| ALT (U/L) | 42.47 | 65.85 | 0.12 | 34.87 | 66.68 | 0.28 |
| ALP (IU/L) | 449.68 | 528.85 | 0.56 | 436.88 | 421.91 | 0.98 |
| BUN (mg/dL) | 13.77 | 26.58 | 0.13 | 12.25 | 23.52 | 0.18 |
| Creatinine (mg/dL) | 0.75 | 1.30 | 0.20 | 0.61 | 1.19 | 0.22 |
| CA19-9 (U/mL) | 570.20 | 594.30 | 0.622 | 1818.00 | 259.15 | 0.112 |
| CEA (ng/mL) | 7.47 | 5.68 | 0.502 | 7.21 | 5.82 | 0.232 |
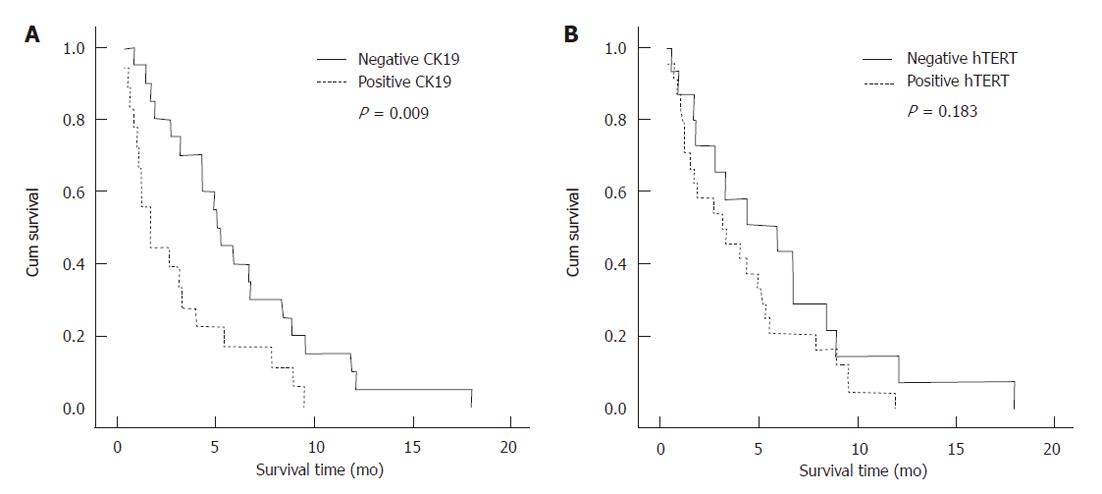
At the time of data analysis, only 1 patient was alive and 39 patients had died. The median overall survival for these patients was 4.0 mo (95% CI: 2.56-4.56). The median survival time was 1.7 mo in patients with positive CK19 mRNA expression, whereas the survival time was 5.3 mo in patients with negative CK19 mRNA expression (Log Rank, P = 0.009). We found that the median survival time in patients with a negative hTERT mRNA expression was not significantly different from patients with positive hTERT mRNA expression (5.9 mo vs 3.2 mo, Log Rank, P = 0.183) (Figure 3).
To identify variables that could be of potential prognostic significance in patients with advanced malignant biliary tract disease, univariable and multivariable analyses were performed using the Cox proportional hazard model to compare the impact of the mRNA expression levels of CK19 and hTERT. Other clinical parameters such as positive or negative hTERT expression, CK19 expression (positive or negative), CEA (cut-off value = 5 ng/mL), CA19-9 (cut-off value = 500 U/mL), total bilirubin (cut-off value = 15 mg/dL) and albumin (cut-off value = 3 g/dL) were also examined. Univariable analysis indicated that only CK19 mRNA expression showed significance as a prognostic factor. Multivariable analysis demonstrated that CK19 mRNA expression (P = 0.024), age (P = 0.026) and serum bilirubin (P = 0.002) were all independent risk factors for survival. The relative risk for CK19 mRNA positive patients was 3.2 times greater than that for CK19 mRNA negative patients (Table 4).
| Variables | Univariable analysis | Multivariate analysis | ||
| P value | Hazard ratio | P value | Hazard ratio | |
| (95% CI) | (95% CI) | |||
| CK19 expression | 0.0111 | 2.42 (1.22-4.78) | 0.0241 | 3.20 (1.17-8.75) |
| hTERT expression | 0.188 | 1.60 (0.80-3.22) | 0.580 | 1.34 (0.47-3.82) |
| Age | 0.541 | 0.82 (0.42-1.57) | 0.0261 | 0.38 (0.16-0.89) |
| Total bilirubin | 0.106 | 1.72 (0.89-3.31) | 0.0021 | 3.97 (1.69-9.36) |
| Albumin | 0.360 | 0.73 (0.37-1.35) | 0.213 | 0.59 (0.26-1.35) |
| CA19-9 | 0.374 | 0.73 (0.37-1.43) | 0.478 | 0.74 (0.32-1.70) |
| CEA | 0.381 | 1.39 (0.68-2.88) | 0.124 | 1.86 (0.84-4.12) |
The highest incidence of cholangiocarcinoma occurs in Thailand, and the majority of patients included in this study were diagnosed with this disease[1]. In this study, the median survival was 4.0 mo, a finding that is not significantly different from the survival time (3.6-5.0 mo) observed in previous studies of advanced malignant biliary tract disease where the majority of patients were diagnosed with pancreatic cancer[7,8,25]. These results indicate that all cancers that lead to malignant biliary tract obstruction are highly lethal. Most advanced malignant biliary tract disease patients can only be treated with palliative biliary tract drainage.
The choice of stents (PE or SEMs) for endoscopic palliation of jaundice due to malignant biliary tract obstruction is dependent upon the estimation of patient survival[8]. Therefore, there is a need for more accurate tests to predict the survival of patients with advanced malignant biliary tract diseases, as this could significantly improve the treatment outcome for these patients. This is the first cohort paper that studies the level of CTCs as a prognostic factor for overall survival of patients with advanced malignant biliary tract obstruction.
We used quantitative real-time RT-PCR to detect CTCs. As a result of the PCR-based methods, we cannot identify exactly the cell source of the measured markers. Quantitative real-time RT-PCR assesses the expression of target genes from mRNA extracted from the lysates of cells harvested from the peripheral blood of patients. As such, these samples contain not only CTC but also PBMC, circulating endothelial cells and skin cells (e.g., keratinocytes, fibroblasts and melanocytes) that contaminate the sample during blood withdrawal and provide alternate potential sources for the PCR-detected genes[13,26]. Therefore, strict selection of target genes for detection of CTCs is very important. In this study, we used CK19 and hTERT genes as targets for the detection of CTCs. Previous studies have suggested that CTCs are likely to be the principal cell source for CK19 gene expression, as CK19 expression is mainly restricted to epithelial cells and is limited in normal peripheral blood cells[20,27]. Additionally, we used the 2-syringe technique during blood collection to avoid epithelium contamination from injected site.
In our study, the patients with positive CK19 expression exhibited significantly shorter overall survival compared with the patients with negative CK19 expression (5.3 mo vs 1.7 mo; P = 0.009). Additionally, multivariable analysis using the Cox regression model also demonstrated that the levels of CK19 expression in peripheral blood, the levels of serum total bilirubin and the age of the patients can all function as independent prognostic factors in patients with advanced malignant biliary tract disease. This is consistent with previously published studies that reported that positive CK19 mRNA expression in peripheral blood was independently associated with a reduction in disease-free survival in patients with breast cancer[20]. In addition, positive CK19 mRNA expression in peripheral blood following chemoradiation was an independent, unfavorable prognostic factor for both overall survival and progression-free survival in patients diagnosed with non-small cell lung cancer[19].
In this study, there were more patients with positive hTERT mRNA expression in peripheral blood than the patients with positive CK19 mRNA expression (60% vs 45%); however, detection of hTERT mRNA in the peripheral blood was not identified as an independent prognostic factor in this study. We suggested that the detection of hTERT mRNA expression levels was not a good candidate as a prognostic factor for patients with advanced malignant biliary tract disease. It may be suitable for the distinction between malignant and benign biliary tract diseases in combination with other tumor specific markers.
In this study, neither univariable nor multivariable analysis indicated that serum levels of CA19-9 could be used as a prognostic factor for patients with advanced malignant biliary tract disease. This finding is inconsistent with a previous study that indicated that the levels of serum CA19-9 are of prognostic relevance in patients with biliary tract cancer[28,29]. We suggested that differences among the patients should be considered. Overall survival in a previous study was 16.1 mo[29], whereas the overall survival in our study was 4.0 mo. In addition, the majority of patients in the previous study received chemotherapy, while only two patients in our study received this treatment. This finding reflects a higher disease severity in the patients examined in our study.
Our study demonstrated that the expression of CK19 mRNA in PBMCs prior to palliative procedures was significantly associated with overall survival of the patients with advanced malignant biliary tract disease. We therefore recommend PE stents for patients with positive CK19 mRNA expression in their peripheral blood. The more expensive SEMS should be reserved for patients with negative peripheral blood expression of CK19 mRNA. Further cost-effectiveness studies should be conducted to evaluate the benefit of using CK19 mRNA in helping the physician make decisions regarding the selection of stent-type and the need for endoscopic repeat intervention in patients with advanced malignant biliary tract disease.
In Thailand, cholangiocarcinoma is the most common cause of malignant biliary tract obstruction. Despite recent advances in the diagnosis and treatment of this disease, patient outcome remains poor. Palliative therapeutic approaches to endoscopic biliary drainage, such as the use of endoprosthesis stents, are generally recommended for these patients. Identification of reliable prognostic factors that allow for an accurate prediction of survival duration in patients suffering from advanced malignant biliary tract obstruction is extremely important.
This study demonstrated that the levels of circulating tumor cells (CTCs) could be used to predict the overall survival of patients with advanced malignant biliary tract obstruction.
The expression of cytokeratin (CK)19 mRNA in peripheral blood mononuclear cells prior to palliative procedures was significantly associated with overall survival of patients with advanced malignant biliary tract disease.
This study recommends polyethylene stents for patients with positive CK19 mRNA expression in their peripheral blood. The more expensive self-expanding metal stents should be reserved for patients with negative peripheral blood expression of CK19 mRNA.
CTCs is the dissemination of tumor cells from the primary tumor into circulating blood. The detection of CTCs can be used in staging and prognosis stratification for cancer patients.
This study provides evidences that high levels of CTCs in advanced malignant biliary tract obstruction patients might be used as a prognostic factor. This is a well performed and clearly presented study.
Peer reviewers: Beata Jolanta Jablońska, MD, PhD, Depart-ment of Digestive Tract Surgery, University Hospital of Medical University of Silesia, Medyków 14 St., 40-752 Katowice, Poland; Antonio Basoli, Professor, General Surgery “Paride Stefanini”, Università di Roma-Sapienza, Viale del Policlinico 155, Roma 00161, Italy
S- Editor Sun H L- Editor Ma JY E- Editor Li JY
| 1. | Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 2. | Chu D, Adler DG. Malignant biliary tract obstruction: evaluation and therapy. J Natl Compr Canc Netw. 2010;8:1033-1044. [PubMed] [Cited in This Article: ] |
| 3. | Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, Choury AD, Buffet C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 346] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 386] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 753] [Cited by in F6Publishing: 736] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 8. | Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | Botteri E, Sandri MT, Bagnardi V, Munzone E, Zorzino L, Rotmensz N, Casadio C, Cassatella MC, Esposito A, Curigliano G. Modeling the relationship between circulating tumour cells number and prognosis of metastatic breast cancer. Breast Cancer Res Treat. 2010;122:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Allen JE, El-Deiry WS. Circulating Tumor Cells and Colorectal Cancer. Curr Colorectal Cancer Rep. 2010;6:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Negin BP, Cohen SJ. Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol. 2010;11:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Wülfing P, Borchard J, Buerger H, Heidl S, Zänker KS, Kiesel L, Brandt B. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12:1715-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Ghossein RA, Carusone L, Bhattacharya S. Review: polymerase chain reaction detection of micrometastases and circulating tumor cells: application to melanoma, prostate, and thyroid carcinomas. Diagn Mol Pathol. 1999;8:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Gradilone A, Gazzaniga P, Silvestri I, Gandini O, Trasatti L, Lauro S, Frati L, Aglianò AM. Detection of CK19, CK20 and EGFR mRNAs in peripheral blood of carcinoma patients: correlation with clinical stage of disease. Oncol Rep. 2003;10:217-222. [PubMed] [Cited in This Article: ] |
| 15. | Leelawat K, Leelawat S, Ratanachu-Ek T, Trubwongchareon S, Wannaprasert J, Tripongkaruna S, Chantawibul S, Tepaksorn P. Circulating hTERT mRNA as a tumor marker in cholangiocarcinoma patients. World J Gastroenterol. 2006;12:4195-4198. [PubMed] [Cited in This Article: ] |
| 16. | Udomchaiprasertkul W, Narong S, Kongsema M, Leelawat K. Detection of hTERT mRNA in gastrointestinal tract cancer specimens. Southeast Asian J Trop Med Public Health. 2008;39:324-327. [PubMed] [Cited in This Article: ] |
| 17. | Li H, Diao TY, Zhou ZY, Yang FY, Ma Q, Li QH. Relationship between the expression of hTERT and EYA4 mRNA in peripheral blood mononuclear cells with the progressive stages of carcinogenesis of the esophagus. J Exp Clin Cancer Res. 2009;28:145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Stroescu C, Herlea V, Dragnea A, Popescu I. The diagnostic value of cytokeratins and carcinoembryonic antigen immunostaining in differentiating hepatocellular carcinomas from intrahepatic cholangiocarcinomas. J Gastrointestin Liver Dis. 2006;15:9-14. [PubMed] [Cited in This Article: ] |
| 19. | Chen TF, Jiang GL, Fu XL, Wang LJ, Qian H, Wu KL, Zhao S. CK19 mRNA expression measured by reverse-transcription polymerase chain reaction (RT-PCR) in the peripheral blood of patients with non-small cell lung cancer treated by chemo-radiation: an independent prognostic factor. Lung Cancer. 2007;56:105-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Zou TN, Wu ZP, Zhou YC, Gu YL, Liu X, Jin CG, Wang XC. Detection of cytokeratin 19, human mammaglobin, and carcinoembryonic antigen-positive circulating tumor cells by three-marker reverse transcription-PCR assay and its relation to clinical outcome in early breast cancer. Int J Biol Markers. 2010;25:59-68. [PubMed] [Cited in This Article: ] |
| 21. | McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol. 2005;2:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [PubMed] [Cited in This Article: ] |
| 23. | Bertazza L, Mocellin S, Marchet A, Pilati P, Gabrieli J, Scalerta R, Nitti D. Survivin gene levels in the peripheral blood of patients with gastric cancer independently predict survival. J Transl Med. 2009;7:111. [PubMed] [Cited in This Article: ] |
| 24. | Rattanasinganchan P, Leelawat K, Treepongkaruna SA, Tocharoentanaphol C, Subwongcharoen S, Suthiphongchai T, Tohtong R. Establishment and characterization of a cholangiocarcinoma cell line (RMCCA-1) from a Thai patient. World J Gastroenterol. 2006;12:6500-6506. [PubMed] [Cited in This Article: ] |
| 25. | Kim HS, Lee DK, Kim HG, Park JJ, Park SH, Kim JH, Yoo BM, Roe IH, Moon YS, Myung SJ. Features of malignant biliary obstruction affecting the patency of metallic stents: a multicenter study. Gastrointest Endosc. 2002;55:359-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Ghossein RA, Bhattacharya S. Molecular detection and characterisation of circulating tumour cells and micrometastases in solid tumours. Eur J Cancer. 2000;36:1681-1694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Khair G, Monson JR, Greenman J. Epithelial molecular markers in the peripheral blood of patients with colorectal cancer. Dis Colon Rectum. 2007;50:1188-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Ali CW, Kaye TF, Adamson DJ, Tait IS, Polignano FM, Highley MS. CA 19-9 and survival in advanced and unresectable pancreatic adenocarcinoma and cholangiocarcinoma. J Gastrointest Cancer. 2007;38:108-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Harder J, Kummer O, Olschewski M, Otto F, Blum HE, Opitz O. Prognostic relevance of carbohydrate antigen 19-9 levels in patients with advanced biliary tract cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2097-2100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |